Abstract
Bacterial Nod factors trigger a number of cellular responses in root hairs of compatible legume hosts, which include periodic, transient increases in cytosolic calcium levels, termed calcium spiking. We screened 13 pharmaceutical modulators of eukaryotic signal transduction for effects on Nod factor-induced calcium spiking. The purpose of this screening was 2-fold: to implicate enzymes required for Nod factor-induced calcium spiking in Medicago sp., and to identify inhibitors of calcium spiking suitable for correlating calcium spiking to other Nod factor responses to begin to understand the function of calcium spiking in Nod factor signal transduction. 2-Aminoethoxydiphenylborate, caffeine, cyclopiazonic acid (CPA), 2,5-di-(t-butyl)-1,4-hydroquinone, and U-73122 inhibit Nod factor-induced calcium spiking. CPA and U-73122 are inhibitors of plant type IIA calcium pumps and phospholipase C, respectively, and implicate the requirement for these enzymes in Nod factor-induced calcium spiking. CPA and U-73122 inhibit Nod factor-induced calcium spiking robustly at concentrations with no apparent toxicity to root hairs, making CPA and U-73122 suitable for testing whether calcium spiking is causal to subsequent Nod factor responses.
Members of the legume family can enter into a symbiotic relationship with bacteria of the genus Rhizobium. Bacteria invade the root and are released into cells of the developing root nodule, a specialized organ in which the bacteria reduce atmospheric dinitrogen to ammonia and where reduced nitrogen is absorbed by the plant. Initial events in the establishment of the symbiosis entail reciprocal signaling between the prospective plant and bacterial partners (Long, 1996). Legume roots exude a variety of flavonoid compounds that activate the transcription of bacterial nod genes. The products of many nod genes direct the synthesis of a class of modified lipochitooligosaccharide signaling molecules, termed Nod factors. Perception of Nod factors by the prospective host elicits a range of responses in the root epidermis that include periodic, transient increases in cytosolic calcium levels, termed calcium spiking (Ehrhardt et al., 1996).
Nod factor-induced calcium spiking was initially characterized in alfalfa (Medicago sativa), and has subsequently been observed in additional legume species from four genera including Medicago truncatula, pea (Pisum sativum), vetch (Vicia sativa), and Lotus japonicus (Wais et al., 2000; Walker et al., 2000; D.W. Ehrhardt, K.E. Wilson, I.A. Downie, and S.R. Long, unpublished data; J.M. Harris, unpublished data). Activation of calcium spiking shows specificity for Nod factor structures produced by compatible symbiotic bacteria and is not observed in several non-nodulating plant mutants (Ehrhardt et al., 1996; Wais et al., 2000; Walker et al., 2000; Oldroyd et al., 2001). These observations are consistent with a role for calcium spiking in legume signal transduction pathways that regulate nodulation.
In alfalfa and M. truncatula, calcium spiking is initiated after a lag of 3 to 30 min after application of purified Nod factor (RmIV Ac, S). A typical “spike” is characterized as a rapid (1–4 s) increase in cytosolic calcium, of an average magnitude of 500 nm, followed immediately by a more gradual (approximately 30 s) return to resting calcium levels. Once initiated, spikes occur repeatedly with an average frequency of 60 s between spike initiations (Ehrhardt et al., 1996). These characteristics are essentially identical to those of the behavior termed “calcium spiking” in mammalian cell systems, as distinct from transient non-periodic calcium increases or sinusoidal oscillations in calcium levels (Thomas et al., 1996).
Calcium spiking has been most extensively studied in mammalian cell systems. Experiments in a number of cell types from a range of species have demonstrated that the cellular mechanisms generating calcium spiking are likely to be diverse, although a majority of nonexcitable cell types appears to possess the common requirements of second messenger-activated calcium release, often via inositol 1,4,5-trisphosphate (IP3), and calcium acting in a positive feedback loop to regulate calcium release (Meyer and Stryer, 1991; Fewtrell, 1993; Thomas et al., 1996). In plant systems, calcium spiking has been observed in legume root hairs responding to Nod factors, in stomatal guard cells responding to application of calcium or abscisic acid (ABA), and in Eremosphaera viridis, a unicellular green alga, after application of strontium or caffeine to the growth medium (McAinsh et al., 1995; Bauer et al., 1997; Staxen et al., 1999). Periodic calcium elevations are sufficient to induce stomatal closure in Arabidopsis (Allen et al., 2000). Intracellular release of caged IP3 in Commelina communis guard cells also induces partial stomatal closure (Gilroy et al., 1990). ABA-induced calcium spiking in C. communis guard cells is inhibited by U-73122, an inhibitor of plant phospholipase Cs, enzymes that activate the conversion of phosphatidylinositol 4,5-bisphosphate into diacylglycerol and IP3 (Staxen et al., 1999). These results suggest that IP3-mediated calcium release is a conserved feature of calcium spiking in both mammalian and plant systems. Pharmacological analysis of strontium-induced calcium spiking in E. viridis has suggested the requirement of calcium ATPases homologous to the mammalian sarcoplasmic/endoplasmic reticulum (SERCA) class and calcium channels homologous to the mammalian ryanodine receptor class (Bauer et al., 1998, 1999).
We screened a variety of compounds that modulate the activity of enzymes known to be components of calcium signaling in other systems, for the ability to inhibit or reproducibly alter Nod factor-induced calcium spiking. For simplicity, we collectively refer to both inhibitors and agonists as pharmaceuticals. The purpose of this study is 2-fold: to identify candidate enzymes required for Nod factor-induced calcium spiking in M. truncatula, and to identify inhibitors of calcium spiking suitable for correlating calcium spiking to other Nod factor responses. We report inhibition of Nod factor-induced calcium spiking in M. truncatula by 2-aminoethoxydiphenylborate (2-APB), a recently described inhibitor of both IP3-mediated and store depletion-mediated calcium release; by caffeine, an inhibitor of IP3-receptor calcium channels and an agonist of ryanodine receptor calcium channels; by cyclopiazonic acid (CPA), an inhibitor of type IIA calcium ATPases in plants; by 2,5-di-(t-butyl)-1,4-hydroquinone (BHQ), an inhibitor of mammalian SERCA calcium ATPases; and by the phospholipase C inhibitor U-73122.
RESULTS
Selection and Screening of Pharmaceuticals
The first phase of a calcium spike consists of a rapid increase in cytosolic calcium levels, implicating the opening of calcium channels and movement of calcium down its electrochemical gradient into the cytosol from either the extracellular space or an internal source. Therefore, we tested pharmaceuticals that modulate the activity of calcium channels for effects on Nod factor-induced calcium spiking (Table I). The second phase of a calcium spike consists of the gradual return of cytosolic calcium to resting levels, implicating the activity of a calcium ATPase or calcium antiporter to mediate the movement of calcium against its electrochemical gradient out of the cytosol and into an internal store or extracellular space. We therefore tested pharmaceuticals that inhibit calcium ATPases for effects on Nod factor-induced calcium spiking (Table I). Experiments have suggested a role for phospholipase C in Nod factor signal transduction and in ABA-induced calcium spiking (Pingret et al., 1998; Staxen et al., 1999; den Hartog et al., 2001). We therefore tested an inhibitor of phospholipase C for effects on Nod factor-induced calcium spiking (Table I).
Table I.
Pharmaceuticals tested for inhibition of Nod factor-induced calcium spiking in M. truncatula and/or alfalfa root hairs
| Name | Principle Target(s) | Structure | References |
|---|---|---|---|
| TMB-8 | IP3 receptor calcium channels | 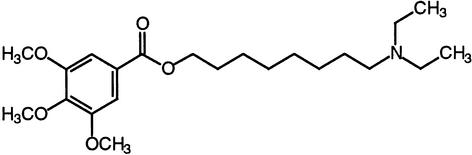 |
Saunders and Hepler (1983),Schumaker and Sze (1987) |
| Xestospongin C | IP3 receptor calcium channelsSERCA calcium ATPases | 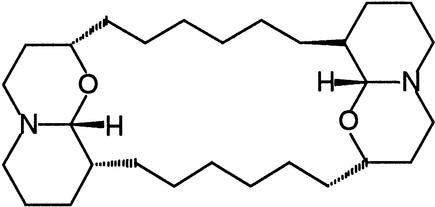 |
Gafni et al. (1997),De Smet et al. (1999) |
| 2-APB | IP3 receptor calcium channelsStore-operated calcium channels | 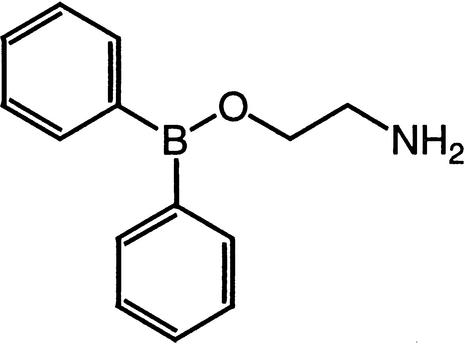 |
Maruyama et al. (1997),Ma et al. (2001) |
| Caffeine | IP3 receptor calcium channelsRyanodine receptor calcium channels | 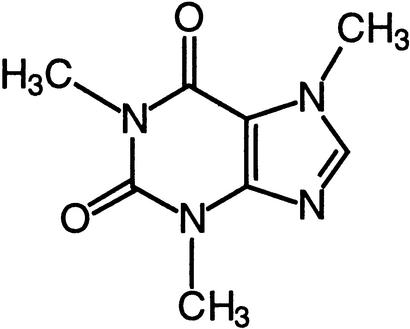 |
Tlalka and Fricker (1999),Pierson et al. (1996),Subbaiah et al. (1994) |
| Ryanodine | Ryanodine receptor calcium channels | 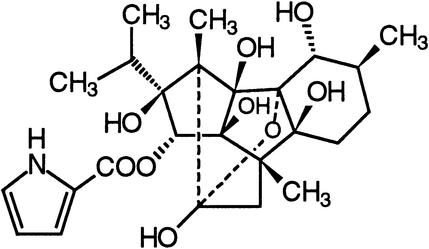 |
Grabov and Blatt (1999),Muir et al. (1997),Lai et al. (1988) |
| Verapamil | Voltage-operated calcium channels | 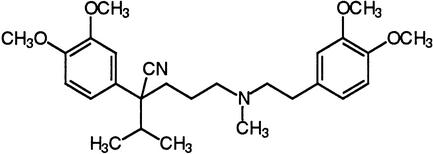 |
White (1998),Pineros and Tester (1997),Wymer et al. (1997) |
| Nifedipine | Voltage-operated calcium channels | 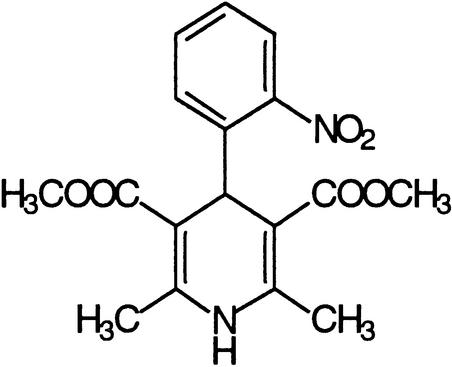 |
Felle et al. (1998),Schumaker and Gizinski (1996) |
| Gadolinium | Voltage-operated calcium channelsStore-operated calcium channels | White (1998),Kluesener et al. (1995),Marshall et al. (1994) | |
| Lanthanum | Voltage-operated calcium channelsPlasma membrane calcium ATPases | Grant et al. (2000),Lewis and Spalding (1998),Kwan et al. (1990) | |
| Cyclopiazonic acid (CPA) | SERCA calcium ATPases Type IIA calcium ATPases | 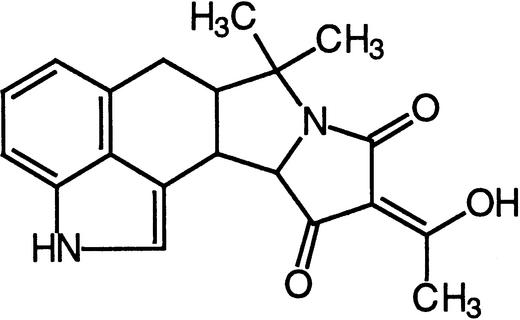 |
Inesi and Sagara (1994),Seidler et al. (1989),Liang and Sze (1998) |
| BHQ | SERCA calcium ATPasesVoltage-operated calcium channels | 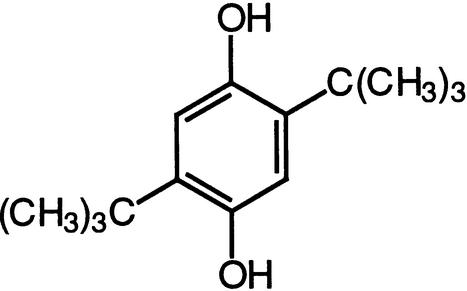 |
Inesi and Sagara (1994),Thomson et al. (1994),Nelson et al. (1994) |
| Thapsigargin | SERCA calcium ATPasesVoltage-operated calcium channels | 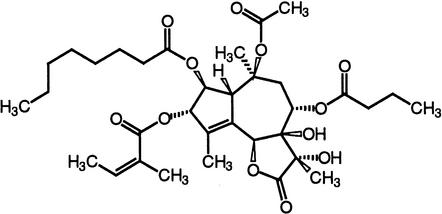 |
Inesi and Sagara (1994),Thomson et al. (1994) |
| U-73122 | Phospholipase C | 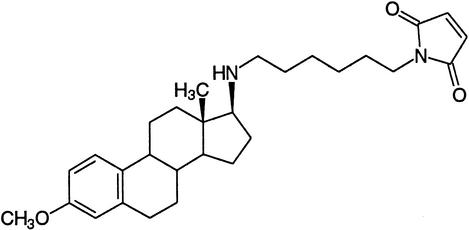 |
Bleasdale et al. (1990),Staxen et al. (1999) |
Root hairs of M. truncatula and/or alfalfa were challenged with Nod factor (NodRm-IV Ac, S) and assayed for calcium spiking as detailed in “Materials and Methods.” Fluorescence intensity measurements were taken from a region drawn around the cell nucleus. After a stable pattern of calcium spiking had been established, root hairs were challenged with a pharmaceutical (concentrations indicated in Table II). Cessation of spiking within 30 min after application of the pharmaceutical was scored as inhibition (Table II). While the pharmaceutical was applied, we assayed root hairs for redistribution of the calcium indicator dye, indicating active cytoplasmic streaming and cell vitality. With the exceptions of 2-APB and U-73122 treatments, all root hairs reported in Table II continued to undergo cytoplasmic streaming throughout application of pharmaceutical (data not shown). After application of 2-APB and U-73122, cytoplasmic streaming was not detectable as assayed by redistribution of the calcium indicator dye under fluorescence microscopy or redistribution of the cytoplasm under differential-interference-contrast microscopy (Figs. 1, B and C, and 2).
Table II.
Results of inhibition assays
| Species | Pharmaceutical | Frequency of Calcium Spiking Inhibitionab | Frequency of Root Hair Lethality |
|---|---|---|---|
| M. truncatula | TMB-8 (200 μm) | 0 /12 | – |
| Xestospongin C (1 μm) | 0 /6 | – | |
| 2-APB (50 μm) | 7 /7 | 30% (n = 128) | |
| Caffeine (50 mm) | 9 /9 | 19% (n = 206) | |
| Ryanodine (200 μm) | 0 /7 | – | |
| Verapamil (100 μm) | 0 /10 | – | |
| Gadolinium chloride (1 mm) | 0 /9 | – | |
| Lanthanum chloride (1 mm) | 0 /7 | – | |
| CPA (5 μm) | 17 /17 | 1% (n = 187) | |
| BHQ (10 μm) | 6 /6 | 0% (n = 145) | |
| Thapsigargin (1 μm) | 0 /8 | – | |
| U-73122 (20 μm) | 13 /14 | 2% (n = 210) | |
| U-73433 (10 μm) | 1 /11 | – | |
| Alfalfa | Caffeine (10 mm) | 6 /6 | – |
| Nifedipine (10 μm) | 0 /6 | – | |
| U-73122 (10 μm) | 21 /21 | – | |
| U-73433 (10 μm) | 0 /13 | – |
Frequency of inhibition is presented as the ratio (no. of cells inhibited/no. of cells tested).
Data for each inhibitor are derived from at least three individual plants.
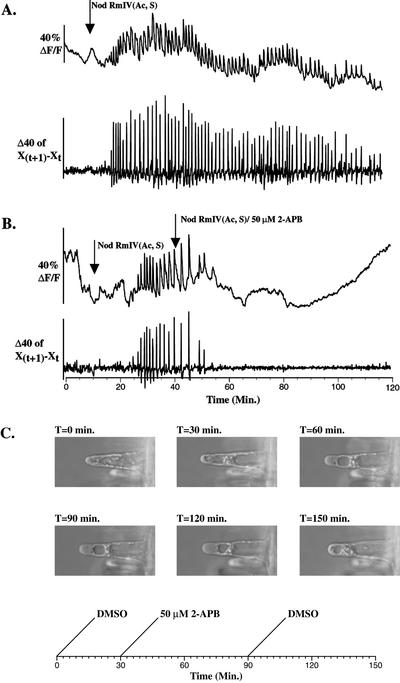
Figure 1.
2-APB inhibits Nod factor-induced calcium spiking in M. truncatula root hairs at concentrations that slow or stop cytoplasmic streaming and alter the distribution of root hair cytoplasm. Root hairs of M. truncatula were injected with Oregon green and fluorescence intensity was recorded as described in “Materials and Methods.” Relative change in average fluorescence intensity is plotted versus time as ΔF/F, designating the value of the first time point subtracted from the value of a given time point and dividing the difference by the value of the first timepoint. A derivatized version of these data is plotted as X(t+1) − Xt, designating the value of each time point subtracted from the value of the preceding time point. This derivation accentuates the rising phase of the calcium spikes relative to fluorescence changes attributable to cytoplasmic dynamics or changes in excitation fluorescence intensity. The points at which Nod factors and pharmaceuticals were added are designated with labeled arrows. A, Nod factor-induced calcium spiking. B, 2-APB (50 μm) inhibits Nod factor-induced calcium spiking. The smaller average changes in the calcium indicator fluorescence baseline after application of 2-APB relative to before the initiation of calcium spiking reflect the slowing or cessation of cytoplasmic streaming. C, 2-APB (50 μm) alters the distribution of root hair cytoplasm. A 2-d-old M. truncatula seedling was placed in a bath constructed on a glass coverslip as described in “Materials and Methods” and allowed to equilibrate without liquid medium perfusion for 30 min. Perfusion of buffered nodulation medium with 1mm α-aminoisobutyric acid (BNM-AIB) medium containing 0.005% (v/v) dimethyl sulfoxide (DMSO) as a solvent control and 1 pm Nod factor (NodRmIV Ac, S) was initiated (T = 0 min). After 30 min, perfusion was switched to BNM-AIB medium containing 50 μm 2-APB and 1 pm Nod factor (T = 30 min). After 1 h of perfusion with medium containing 2-APB, perfusion was returned to medium with solvent control (T = 90 min). Photographs were taken of a root hair magnified 400× at 30-min intervals from T = 0 min to T = 150 min.
We used propidium iodide, a nucleic acid stain that is excluded from living root hairs, to test the toxicity to root hairs of pharmaceuticals that inhibit Nod factor-induced calcium spiking (Van Den Berg et al., 1995). Roots of M. truncatula seedlings were challenged with Nod factor and pharmaceutical for 30 min, as detailed for calcium spiking experiments, and then stained with propidium iodide. The percentage of dead root hairs in the observable field of root hairs at 200× magnification was scored as the frequency of root hair lethality (Table II).
2-APB and Caffeine, Modulators of Ligand-Operated Calcium Channels, Inhibit Nod Factor-Induced Calcium Spiking at Concentrations Toxic to Growing Root Hairs
2-APB inhibits Nod factor-induced calcium spiking when applied at 50 μm (Table II; Fig. 1B). Inhibition occurs within 5 to 20 min after application and is preceded by a gradual decrease in spike amplitude (Fig. 1B). Once inhibited, calcium spiking does not reinitiate within 80 min after 2-APB application (Fig. 1B). 2-APB also induces a dramatic decrease or cessation of cytoplasmic streaming, which is reflected in a dampening of fluorescence intensity baseline noise (Fig. 1B). Coincident with the disruption of cytoplasmic streaming, the distribution of cytosol in the root hair shaft is altered. Cytosol accumulates at the root hair tip and nuclear regions of the root hair shaft, and in many cells there is an absence of cytosolic strands that overlap the vacuole in untreated root hairs (Fig. 1C). This change in the organization of the cytoplasm is reversible; cells revert to their normal appearance and reinitiate cytoplasmic streaming within 1 h after removal of 2-APB from the bath (Fig. 1C). Within 30 min after application of 50 μm 2-APB, 30% of root hairs are killed as assessed by propidium iodide staining (Table II).
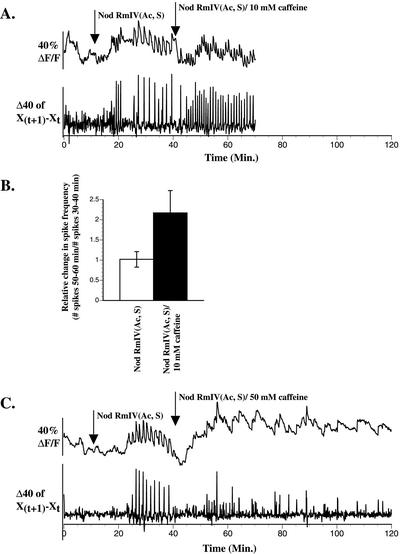
Caffeine inhibits Nod factor-induced calcium spiking in alfalfa root hairs when applied at 10 mm (Table II). When caffeine was applied to M. truncatula root hairs at 10 mm, Nod factor-induced calcium spiking was inhibited in only one of 11 cells tested (data not shown). However, an increase in the frequency of calcium spikes, of an average magnitude of 2-fold, is observed in uninhibited root hairs after treatment with 10 mm caffeine (Fig. 3, A and B). When caffeine is applied to M. truncatula root hairs at 50 mm, Nod factor-induced calcium spiking is inhibited (Table II; Fig. 3C). Inhibition occurs within 5 min after application of caffeine (Fig. 3C). Once inhibited, calcium spiking does not reinitiate within 80 min after application of 50 mm caffeine (Fig. 3C). A subset of root hairs shows significant increases in baseline fluorescence after application of 50 mm caffeine (Fig. 3C). The fluorescence baseline becomes noticeably more variable after application of 50 mm caffeine relative to the baseline before spiking initiation. Large increases in fluorescence, some of a comparable amplitude to calcium spikes, are observed, though these occur with irregular amplitude and frequency (Fig. 3C). Within 30 min after application of 50 mm caffeine, 19% of root hairs are killed as assessed by propidium iodide staining (Table II).
Figure 3.
Ten millimolar caffeine alters the frequency of Nod factor-induced calcium spiking and 50 mm caffeine inhibits Nod factor-induced calcium spiking in M. truncatula root hairs. A, Caffeine (10 mm) increases the frequency of Nod factor-induced calcium spiking. B, Relative change in the frequency of Nod factor-induced calcium spiking after application of 10 mm caffeine. Nod factor [NodRmIV (Ac, S)] was applied at 10 min as shown in A. The number of calcium spikes occurring in the interval from 50 to 60 min was divided by the number of calcium spikes occurring in the interval from 30 to 40 min for cells for which Nod factor application was constant [Nod RmIV (Ac, S), n = 6] and for cells for which 10 mm caffeine was applied at 40 min [Nod RmIV (Ac, S)/10 mm caffeine, n = 6]. C, Caffeine (50 mm) inhibits Nod factor-induced calcium spiking.
Application of the calcium channel antagonists TMB-8, xestospongin C, ryanodine, verapamil, nifedipine, lanthanum, and gadolinium had no apparent effects on Nod factor-induced calcium spiking (Table II).
CPA and BHQ, Inhibitors of Calcium ATPases, Rapidly Inhibit Nod Factor-Induced Calcium Spiking
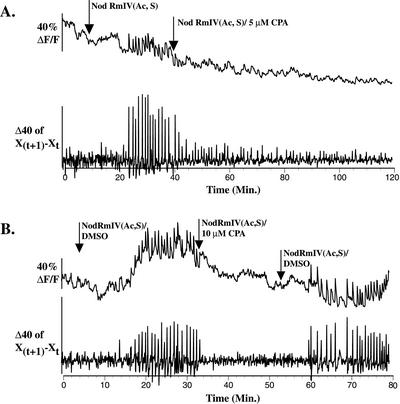
CPA inhibits Nod factor-induced calcium spiking in M. truncatula root hairs when applied at 5 μm (Table II; Fig. 4A). Inhibition of Nod factor-induced calcium spiking occurs within 5 min after CPA application (Fig. 4, A and B). Once inhibited, calcium spiking does not reinitiate for up to 80 min after CPA application (Fig. 4A). Within 30 min after application of 5 μm CPA, only 1% of root hairs are killed as assessed by propidium iodide staining (Table II). To further assess the health of root hairs during application of CPA, we assayed for reversibility of calcium spiking inhibition after removal of CPA. Inhibition of Nod factor-induced calcium spiking by CPA is reversible at CPA concentrations as high as 10 μm with calcium spiking reinitiating within 5 to 10 min after removal of CPA in eight of nine root hairs tested (Fig. 4B).
Figure 4.
CPA reversibly inhibits Nod factor-induced calcium spiking in M. truncatula root hairs. A, Application of 5 μm CPA inhibits Nod factor-induced calcium spiking. B, Inhibition of Nod factor-induced calcium spiking by 10 μm CPA is reversible within 10 min after removal of CPA (n = 9).
To determine the minimum effective dose of CPA for inhibition of Nod factor-induced calcium spiking in M. truncatula, we applied CPA at concentrations spanning 2.5 orders of magnitude. One-tenth micromolar CPA fails to inhibit Nod factor-induced calcium spiking, whereas 1 μm CPA inhibited Nod factor-induced calcium spiking in eight of 13 root hairs tested (Table III). CPA (5 μm) is the lowest concentration that inhibited Nod factor-induced calcium spiking in all cells tested (Table III).
Table III.
| Pharmaceutical | Concentration | Frequency of Calcium Spiking Inhibitiona |
|---|---|---|
| μm | ||
| CPA | 0.1 | 0 /10 |
| 1 | 8 /13 | |
| 5 | 17 /17 | |
| U-73122 | 0.2 | 2 /15 |
| 2 | 13 /16 | |
| 20 | 13 /14 |
Frequency of inhibition is presented as the ratio (no. of cells inhibited/no. of cells tested).
Data for each inhibitor are derived from at least three individual plants.
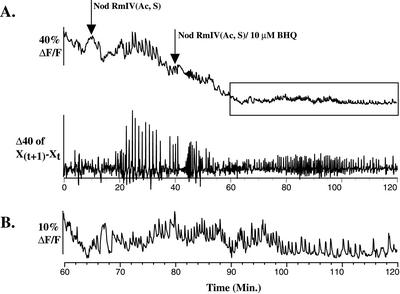
BHQ inhibits Nod factor-induced calcium spiking in M. truncatula root hairs when applied at 10 μm (Table II; Fig. 5). The characteristics of BHQ inhibition are markedly different from those of CPA. Inhibition is rapid, occurring within 5 min after BHQ application (Fig. 5A). This rapid inhibition is transient, lasting from 4 to 10 min, at which point the cells reinitiate calcium spiking (Fig. 5, A and B). Once reestablished, the pattern of calcium spiking is variable with some cells spiking with an irregular frequency and most spiking with a greatly reduced spike amplitude of one-half to one-fifth that observed before application of BHQ (Fig. 5, A and B; data not shown). Within 30 min after application of 10 μm BHQ, 0% of root hairs are killed as assessed by propidium iodide staining (Table II). Application of 50 μm BHQ resulted in inhibition of Nod factor-induced calcium spiking of the same character as application of 10 μm BHQ (data not shown).
Figure 5.
BHQ transiently inhibits Nod factor-induced calcium spiking in M. truncatula root hairs. A, BHQ (10 μm) transiently inhibits Nod factor-induced calcium spiking. B, The final 60 min of the trace shown in A is shown enlarged. The root hair is undergoing calcium spiking of increased frequency and decreased amplitude relative to spiking before application of BHQ.
Application of the calcium ATPase antagonist thapsigargin had no apparent effects on Nod factor-induced calcium spiking (Table II).
U-73122, an Inhibitor of Phospholipase C, Inhibits Calcium Spiking at Concentrations That Impair Cytoplasmic Streaming
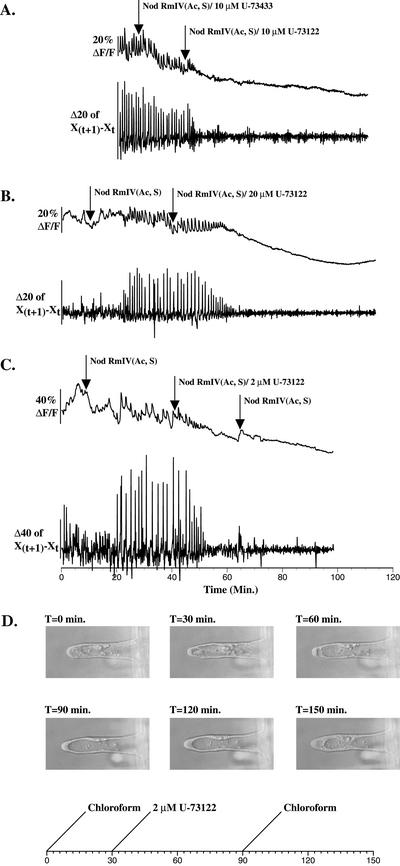
U-73122 inhibits Nod factor-induced calcium spiking in alfalfa when applied at 10 μm (Table II; Fig. 2A) and in M. truncatula when applied at 20 μm (Table II; Fig. 2B). Inhibition occurs within 30 min after application and is preceded by a gradual decrease in spike amplitude (Fig. 2B). Once inhibited, calcium spiking does not reinitiate for up to 70 min after U-73122 application (Fig. 2B). Within 30 min after application of 20 μm U-73122, only 2% of root hairs are killed as assessed by propidium iodide staining (Table II). However, inhibition of Nod factor-induced calcium spiking by U-73122 is not reversible within 30 min after removal of U-73122 at U-73122 concentrations as low as 2 μm (Fig. 2C). U-73433, a less potent structural analog of U-73122, has no effect on Nod factor-induced calcium spiking in alfalfa and M. truncatula when applied at 10 μm (Table II; Fig. 2A; data not shown). In addition to inhibiting Nod factor-induced calcium spiking, U-73122 induces a dramatic decrease or cessation of cytoplasmic streaming as assessed by changes in the distribution of the calcium indicator dye (Fig. 2, A–C). Coincident with the inhibition of cytoplasmic streaming, cytosol accumulates at the root hair tip (Fig. 2D). Disruption of cytoplasmic streaming is not reversible within 1 h after removal of U-73122 at concentrations as low as 2 μm, although root hairs do recover a normal distribution of cytosol (Fig. 2D).
Figure 2.
U-73122 inhibits Nod factor-induced calcium spiking in alfalfa and M. truncatula root hairs at concentrations that slow or stop cytoplasmic streaming. A, U-73122 (10 μm) inhibits Nod factor-induced calcium spiking, whereas the structural analog, U-73433, does not in alfalfa root hairs. B, U-73122 (20 μm) inhibits Nod factor-induced calcium spiking in M. truncatula root hairs. The smaller average changes in the calcium indicator fluorescence baseline after application of U-73122 relative to before the initiation of calcium spiking reflect the slowing or cessation of cytoplasmic streaming. C, Inhibition of Nod factor-induced calcium spiking is not reversible within 30 min after removal of 2 μm U-73122 (n = 10). D, U-73122 (2 μm) reversibly alters the distribution of root hair cytoplasm. Experimental methods and image acquisition parallel those employed in Figure 1C. Chloroform (0.002% [v/v]) was used as a solvent control.
To determine the minimum effective dose of U-73122 for inhibition of Nod factor-induced calcium spiking in M. truncatula, we applied U-73122 at concentrations spanning three orders of magnitude. U-73122 (0.2 μm) inhibited Nod factor-induced calcium spiking in two of 15 cells tested, whereas 2 μm U-73122 inhibited Nod factor-induced calcium spiking in 13 of 16 cells tested (Table III). Twenty micromolar U-73122 was the highest concentration tested and inhibited Nod factor-induced calcium spiking in 13 of 14 cells tested (Table III).
DISCUSSION
This study lays groundwork for addressing two central questions regarding Nod factor-induced calcium spiking in legumes. First, what is the mechanism of Nod factor-induced calcium spiking, i.e. what enzymatic components are required and how are they coordinately regulated to generate the calcium spiking response? The present study allows inference of some enzymatic components, and is a prerequisite to future study of regulation. Second, what is the role of calcium spiking in Nod factor signal transduction? We consider the implications of the results presented here for addressing each of the questions in turn.
The assay used in this study, Nod factor-induced calcium spiking, permits us to use inferred enzymatic activities to link Nod factor perception to a rapidly induced (within 15 min) and discreet cellular event, calcium spiking. Our results complement those obtained from previous studies that employed pharmaceuticals to study Nod factor signaling by assaying events occurring several hours after Nod factor application such as gene induction or root hair deformation (Pingret et al., 1998; den Hartog et al., 2001). Pingret et al. (1998) observed U-73122 inhibition of Nod factor induction of an ENOD12 promoter-β-glucuronidase fusion in transgenic alfalfa 6 h after Nod factor application. Our empirical demonstration of U-73122 inhibition of Nod factor-induced calcium spiking now places a putative phopholipase C activity within approximately 15 min of Nod factor application and establishes its requirement for calcium spiking. Other pharmacological studies of Nod factor signaling have used mastoparan as a G-protein activator, ruthenium red as a ryanodine receptor antagonist, and neomycin as a phospholipase C antagonist (Pingret et al., 1998; den Hartog et al., 2001). Under the conditions of our experiments, 1 μm mastoparan rapidly kills all exposed root hairs, precluding our ability to assay mastoparan effects on calcium spiking (R.M. Mitra, unpublished data). Similarly, micromolar concentrations of ruthenium red induce a rapid quenching of the calcium indicator dye fluorescence immediately upon application (E.M. Engstrom, unpublished data). We elected not to employ neomycin in this study because of its wide range of established targets in mammalian cell systems.
What Enzymatic Components Are Required for Nod Factor-Induced Calcium Spiking?
Inferences from pharmaceutical studies as to the requirement of an enzyme or class of enzymes in a cell signaling process are constrained by the selectivity of the pharmaceuticals employed. Many pharmaceuticals target multiple enzymes or classes of enzymes, and it is not possible to define the complete set of enzymes modulated by a given pharmaceutical. This issue is accentuated in plants, where the cellular targets of most pharmaceuticals have not been established and mechanistic inferences must often depend upon characterization of the pharmaceutical in animal systems. Notwithstanding these limitations, pharmaceuticals are useful for identifying candidate components of signaling pathways that can then be examined with greater precision by further cellular and molecular studies. Two pharmaceuticals that inhibit Nod factor-induced calcium spiking, CPA and U-73122, are selective inhibitors with defined enzymatic targets in plants (see references in Table I).
Inhibition of Nod factor induced-calcium spiking by CPA is evidence for the requirement of a type IIA calcium pump. In animal systems, calcium ATPases are classified as belonging to one of two classes based upon their cellular localization, pharmacology, and regulation by calmodulin. Members of the SERCA class are localized to the sarcoplasmic or endoplasmic reticulum, are inhibited by CPA and thapsigargin, and are not regulated by calmodulin. Members of the plasma membrane class are localized to the plasma membrane, are insensitive to CPA and thapsigargin, and are regulated by calmodulin. Homologs of both classes have been identified in plants and are classified as type IIA, homologous to the SERCA class of calcium pumps in animals, or type IIB, homologous to the plasma membrane class of calcium pumps in animals (Sze et al., 2000). Calcium translocation in vitro by the Arabidopsis type IIA calcium pump ECA1/ACA3 is inhibited by CPA but not by thapsigargin (Liang and Sze, 1998). In contrast, calcium translocation in vitro by the Arabidopsis type IIB calcium pump ACA2 is unaffected by CPA (Hwang et al., 2000). Strontium-induced calcium spiking in the alga E. viridis is also inhibited by CPA, indicating that the requirement of type IIA calcium ATPase activity is a shared feature of calcium spiking in both organisms.
Inhibition of Nod factor induced-calcium spiking by U-73122 is evidence for the requirement of a phospholipase C. U-73122 inhibits the activity of a plant phospholipase C in vitro at concentrations comparable with those employed in this study (Staxen et al., 1999). U-73122 inhibits Nod factor-induced expression of an M. truncatula ENOD12 promoter-β-glucuronidase fusion in transgenic alfalfa, providing additional evidence for phospholipase C activity in Nod factor signaling (Pingret et al., 1998). U-73122 also inhibits ABA induced calcium oscillations in C. communis guard cells, indicating that phospholipase C activity may be a shared component of both Nod factor- and ABA-induced calcium signaling (Staxen et al., 1999).
That U-73122 inhibition of Nod factor-induced calcium spiking and cytoplasmic streaming is not reversible may indicate that inhibition does not result from the selective enzymatic inhibition of a calcium spiking component, but rather from irreversible structural damage. However, the structural analog U-73433, which differs from U-73122 only in the presence of a single double bond, has no effect on Nod factor-induced calcium spiking or cytoplasmic streaming. This result renders less likely the possibility that U-73122 exerts its inhibitory effects in a nonselective manner, such as by disruption of membrane integrity or chelating of an enzymatic substrate. It has been cautioned that inhibition of cytoplasmic streaming by U-73122 may indicate cell death, thus preventing any inferences based upon the action of the pharmaceutical applied (den Hartog et al., 2001). Our controls suggest that this is not the case in our trials because U-73122-treated root hairs, which exhibit inhibition of cytoplasmic streaming, remain viable as assessed by the heterogeneous distribution of the cytoplasm and the exclusion of propidium iodide. Inhibition of cytoplasmic streaming by U-73122 implicates the requirement of phospholipase C for cytoplasmic streaming.
To our knowledge, this study contains the first published use of 2-APB to study signal transduction in a plant. Our data demonstrate that plants possess a 2-APB target(s), whose function is required for Nod factor-induced calcium spiking and cytoplasmic streaming. Inhibition of Nod factor-induced calcium spiking and cytoplasmic streaming by 2-APB is strikingly similar in character to inhibition by U-73122. This may indicate that 2-APB and U-73122 inhibit enzymes in the same signal transduction pathway, and that this signaling pathway is required for cytoplasmic streaming as well as for Nod factor-induced calcium spiking. In mammalian cells, 2-APB inhibits IP3-mediated calcium release from internal calcium stores, whereas U-73122 inhibits production of IP3. It would be premature to conclude from the evidence presented here that 2-APB is inhibiting IP3-mediated calcium release required for Nod factor-induced calcium spiking, but the hypothesis is suggested by our data.
The character of 50 mm caffeine inhibition of Nod factor-induced calcium spiking in M. truncatula, with some cells exhibiting increases in the fluorescence baseline and all cells exhibiting frequent, non-periodic fluorescence increases, is suggestive of a disruption of root hair calcium homeostasis. However, the reproducible alteration in calcium spiking frequency after application of 10 mm caffeine is evidence for a caffeine-modulated component of Nod factor-induced calcium spiking. Cellular targets of caffeine in plants have not been identified, precluding prediction of a specific mechanism for caffeine inhibition of Nod factor-induced calcium spiking. Caffeine has been demonstrated to both induce and inhibit translocation of calcium into the cytosol of plant cells (Subbaiah et al., 1994; Pierson et al., 1996). These data are consistent with the hypothesis that caffeine directly or indirectly modulates plant calcium channels as it is known to do in mammalian cells. Our data provide additional evidence for the existence of caffeine sensitive enzymes in plants and their importance for calcium translocation in plant cells.
Application of 10 μm BHQ reproducibly caused a transient inhibition of Nod factor-induced calcium spiking and altered the character of calcium spiking upon recovery. BHQ has not been extensively applied to the study of signal transduction in plants and the cellular targets are unknown (Thomson et al., 1994). Our data demonstrate the existence of a BHQ sensitive enzyme in M. truncatula root hairs whose inhibition has dramatic effects on the character of Nod factor-induced calcium spiking. Given that separate results have implicated type IIA calcium pumps and phospholipase C as essential components of Nod factor-induced calcium spiking, it may be informative to assay these enzymes for sensitivity to BHQ.
It is interesting to note that of the pharmaceuticals tested that had no apparent effect on Nod factor-induced calcium spiking, the calcium channel inhibitors ryanodine, verapamil, gadolinium, and lanthanum all inhibited strontium-induced calcium spiking in E. viridis (Bauer et al., 1998). Nifedipine, which has no effect on Nod factor-induced calcium spiking in alfalfa, is effective in blocking calcium influx in alfalfa root hairs (Felle et al., 1998) Although the design of our experiments does not permit us to make confident inferences from negative results, it is notable that we have found no indications to date for the requirement of plasma membrane-localized calcium channels for Nod factor-induced calcium spiking.
In summary, our results implicate the requirement of a type IIA calcium pump and a phospholipase C for Nod factor-induced calcium spiking. Efforts are currently under way in this laboratory to isolate and characterize type IIA calcium pump and phospholipase C transcripts from M. truncatula roots, and to profile their expression, possible Nod factor induction, and pharmacology to further implicate specific enzymes required for Nod factor-induced calcium spiking.
What Is the Function of Nod Factor-Induced Calcium Spiking in Nod Factor Signal Transduction?
One approach to elucidating the function of Nod factor-induced calcium spiking is to inhibit calcium spiking and observe what other Nod factor-induced responses are altered, indicating that they reside downstream of calcium spiking in the signal transduction pathway. For this approach to be successful requires that the pharmaceutical employed inhibits calcium spiking robustly, i.e. with a high frequency and as long as the pharmaceutical is applied, and that inhibition occurs at pharmaceutical concentrations well below lethal levels. CPA and U-73122 fulfill both criteria. It is also desirable that inhibition of calcium spiking occurs with minimal disruption of other cellular processes. This criterion presents a problem, as one cannot comprehensibly ascertain the effects of any applied pharmaceutical. U-73122 inhibition of calcium spiking occurs only at concentrations that also affect cytoplasmic streaming and cytosol organization, potentially complicating interpretation of U-73122 effects on other Nod factor responses. We have noted that higher concentrations of CPA (5–10 μm) may inhibit anisotropic root hair growth (E.M. Engstrom, unpublished data). Even without such observations, it would be reasonable to assume that type IIA calcium ATPases and phospholipase Cs have multiple functions in root hairs that would be disrupted by application of inhibitor, and that some of these functions may occur coincident with Nod factor-induced calcium spiking. Correlation of Nod factor-induced calcium spiking with other Nod factor responses therefore are best performed with multiple pharmaceuticals and in conjunction with analysis of calcium spiking-deficient plant mutants and/or Nod factor structures that fail to elicit calcium spiking. We propose that CPA and U-73122 are useful tools for elucidating the function of Nod factor-induced calcium spiking in legume signal transduction. Studies are under way in this laboratory examining the effects of CPA and U-73122 on Nod factor-induced gene expression.
MATERIALS AND METHODS
Plant Growth and Preparation
Medicago truncatula cv Jemalong A-17 seeds were scarified by 7 min of exposure to sulfuric acid, rinsed twice with sterile water, sterilized by 3 min of exposure to household bleach, and rinsed with sterile water eight times to remove residual bleach. Seeds were allowed to imbibe in sterile water at 4°C for 12 h to 1 week. Imbibed seeds were transferred to 100- × 25-mm petri dishes. Excess water was removed. Plates were wrapped with parafilm and placed inverted in a drawer to germinate for 12 h. Germinated seedlings were transferred to BNM medium plates [2.0 mm Ca(SO4)2, 0.5 mm KH2PO4, 0.5 mm Mg(SO4)2, 50 μm Na2 EDTA, 50 μm FeSo4.7H20, 16 μm ZnSo4.7H2O, 50 μm H3BO3, 50 μm MnSO4, 1 μm Na2MoO4.H2O, 0.1 μm CuSO4, 0.1 μm CoCl2.6H20, and 2.0 mm MES.KOH (pH = 6.5)] containing 1 mm AIB or 0.1 μm aminoethoxyvinylglycine as an ethylene biosynthesis inhibitor, and grown under fluorescent lighting at 22°C. For microscopy studies, 1- to 2-d-old seedlings were mounted in silicon grease chambers constructed on glass coverslips as previously described (Ehrhardt et al., 1996), and submerged in BNM or BNM-AIB media.
Alfalfa (Medicago sativa) seedlings were prepared in a similar fashion, except seeds were sterilized for 35 min in 70% (v/v) ethanol, rinsed three times with sterile water, and further sterilized for 35 min in household bleach followed by eight rinses in sterile water. Agar plates used for alfalfa contained 1 μm silver chloride as an ethylene response inhibitor.
Perfusion of Liquid Medium
Liquid medium was applied to the chamber well by syringe pumping or gravity perfusion and removed by syringe pumping or aspiration. Syringe pumping was driven by a programmable syringe pump (model YA-12 Multi-Phaser, Yale Apparatus, Wontagh, NY). Flow rates ranged from approximately 0.5 to 1 mL min−1. The root was submerged in 1 to 2 mL of liquid.
Fluorescence Imaging Instrumentation
The quantitative fluorescence imaging system was built around a Diaphot inverted microscope (Nikon, Melville, NY) equipped with a 100-W mercury lamp or a 75-W xenon lamp, a dual filter wheel in the excitation path (Metaltek Instruments, Raleigh, NC), a Nikon B1-E filter set for Oregon green fluorescence (470–490-nm excitation, 520–560-nm emission), and a Nikon 40× long working distance differential-interference-contrast/Fluor objective (numerical aperture 0.7) or a Nikon 20× Fluor objective (numerical aperture 0.7). Fluorescence images were detected with a CCD camera (model 1338Y, Princeton Instruments, Trenton, NJ) or a silicon-intensified tube camera (model 2400–08, Hamamatsu Photonics Systems Corporation, Bridgewater, NJ) mounted on the side port of the microscope with a 1× video relay lens (Nikon). Control of microscope instrumentation, image digitizing, and quantitative image analysis was performed with the Image 1/Fluor Imaging System or the MetaFluor Imaging System (Universal Imaging Corporation, West Chester, PA). Video frames (200 ms) were acquired every 5 s. Eight video frames were averaged for each image acquired with the silicon-intensified tube camera. Excitation energy was attenuated with a neutral density filter (optical density 1.0).
Injection of Indicator Dye
Root hairs were injected with Oregon green 488 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid dextran (Mr 10,000 dextran, Molecular Probes, Eugene, OR) utilizing the method described previously for injection of calcium green and Fura-2 dextrans (Ehrhardt et al., 1996).
Photography
Photographs were taken with a Nikon model FE-2 camera loaded with Elite CHROME 160T film (Eastman-Kodak, Rochester, NY) and affixed to the camera port of the Nikon Diaphot inverted microscope.
Nod Factors
Calcium spiking in M. truncatula was elicited using a highly purified preparation of NodRm-IV(Ac, S; Ehrhardt et al., 1996).
Chemicals
CPA was diluted from a 50 mm stock solution in DMSO. BHQ was diluted from a 200 mm stock solution in ethanol. Thapsigargin was diluted from a 5 mm stock solution in DMSO. TMB-8 was diluted from a 100 mm stock solution in water. Xestospongin C was diluted from a 5 mm stock solution in DMSO. Caffeine was diluted from a 100 mm stock solution in BNM liquid medium. Ryanodine was diluted from a 25 mm stock solution in water. Lanthanum chloride and gadolinium chloride were diluted from 1 m solutions in water. Verapamil was diluted from a 100 mm stock solution in ethanol. U-73122 and U-73433 were diluted from a 10 or 100 mm stock solution in chloroform. Propidium iodide was diluted from a 1.0 mg mL−1 solution in water. All chemicals were obtained from Calbiochem except lanthanum chloride and gadolinium chloride (Sigma, St. Louis) and propidium iodide (Molecular Probes).
ACKNOWLEDGMENTS
We thank Robert Fisher for critical reading of the manuscript, Sidney Shaw for assistance with the fluorescence microscopy, and Jeanne Harris for sharing unpublished data.
Footnotes
This work was supported by the Howard Hughes Medical Foundation and by the Department of Energy (grant no. DE–FG03–90ER200120). R.M.M. was supported by a Howard Hughes Medical Foundation predoctoral fellowship.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010691.
LITERATURE CITED
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF et al. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. doi: 10.1126/science.289.5488.2338. [DOI] [PubMed] [Google Scholar]
- Bauer CS, Plieth C, Bethmann B, Popescu O, Hansen U-P, Simonis W, Schoenknecht G. Strontium-induced repetitive calcium spikes in a unicellular green alga. Plant Physiol. 1998;117:545–557. doi: 10.1104/pp.117.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CS, Plieth C, Hansen UP, Sattelmacher B, Simonis W, Schoenknecht G. Repetitive Ca2+ spikes in a unicellular green alga. FEBS Lett. 1997;405:390–393. doi: 10.1016/s0014-5793(97)00231-7. [DOI] [PubMed] [Google Scholar]
- Bauer CS, Simonis W, Schoenknecht G. Different xanthines cause membrane potential oscillations in a unicellular green alga pointing to a ryanodine/cADPR receptor Ca2+ channel. Plant Cell Physiol. 1999;40:453–456. [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- De Smet P, Parys JB, Callewaert G, Weidema AF, Hill E, De Smedt H, Erneux C, Sorrentino V, Missiaen L. Xestospongin C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor and the endoplasmic-reticulum Ca2+ pumps. Cell Calcium. 1999;26:9–13. doi: 10.1054/ceca.1999.0047. [DOI] [PubMed] [Google Scholar]
- den Hartog M, Musgrave A, Munnik T. Nod factor-induced phosphatidic acid and diacylglycerol pyrophosphate formation: a role for phospholipase C and D in root hair deformation. Plant J. 2001;25:55–65. doi: 10.1046/j.1365-313x.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. The role of ion fluxes in Nod factor signalling in Medicago sativa. Plant J. 1998;13:455–463. [Google Scholar]
- Fewtrell C. Calcium oscillations in non-excitable cells. Ann Rev Physiol. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessach IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Read ND, Trewavas AJ. Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature. 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol. 1999;119:277–287. doi: 10.1104/pp.119.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 2000;23:441–450. doi: 10.1046/j.1365-313x.2000.00804.x. [DOI] [PubMed] [Google Scholar]
- Hwang I, Harper JF, Liang F, Sze H. Calmodulin activation of an endoplasmic reticulum-located calcium pump involves an interaction with the N-terminal autoinhibitory domain. Plant Physiol. 2000;122:157–167. doi: 10.1104/pp.122.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G, Sagra Y. Specific inhibitors of intracellular Ca2+ transport ATPases. J Membr Biol. 1994;141:1–6. doi: 10.1007/BF00232868. [DOI] [PubMed] [Google Scholar]
- Kluesener B, Boheim G, Liss H, Engelberth J, Weiler EW. Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J. 1995;14:2708–2714. doi: 10.1002/j.1460-2075.1995.tb07271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan CY, Takemura H, Obie JF, Thastrup O, Putney JWJ. Effects of methacholine, thapsigargin, and lanthanum ion on plasmalemmal and intracellular calcium ion transport in lacrimal acinar cells. Am J Physiol. 1990;258:C1006–C1015. doi: 10.1152/ajpcell.1990.258.6.C1006. [DOI] [PubMed] [Google Scholar]
- Lai FA, Erickson HP, Rousseau E, Liu QY, Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988;331:315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lewis BD, Spalding EP. Nonselective block by La3+ of Arabidopsis ion channels involved in signal transduction. J Membr Biol. 1998;162:81–90. doi: 10.1007/s002329900344. [DOI] [PubMed] [Google Scholar]
- Liang F, Sze H. A high-affinity Ca2+ pump, ECA1, from the endoplasmic reticulum is inhibited by cyclopiazonic acid but not by thapsigargin. Plant Physiol. 1998;118:817–825. doi: 10.1104/pp.118.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SR. Rhizobium symbiosis: Nod factors in perspective. Plant Cell. 1996;8:1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H-T, Venkatachalam K, Li H-S, Montell C, Kurosaki T, Patterson RL, Gill DL. Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J Biol Chem. 2001;276:18888–18896. doi: 10.1074/jbc.M100944200. [DOI] [PubMed] [Google Scholar]
- Marshall J, Corzo A, Leigh RA, Sanders D. Membrane potential-dependent calcium transport in right-side-out plasma membrane vesicles from Zea mays L. roots. Plant J. 1994;5:683–694. [Google Scholar]
- Maruyama T, Kanaji T, Kakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Webb AAR, Taylor JE, Hetherington AM. Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Stryer L. Calcium spiking. Ann Rev Biophys Chem. 1991;20:153–174. doi: 10.1146/annurev.bb.20.060191.001101. [DOI] [PubMed] [Google Scholar]
- Muir SR, Bewell MA, Sanders D, Allen GJ. Ligand-gated Ca2+ channels and Ca2+ signalling in higher plants. J Exp Bot. 1997;48:589–597. doi: 10.1093/jxb/48.Special_Issue.589. [DOI] [PubMed] [Google Scholar]
- Nelson EJ, Li CCR, Bangalore R, Benson T, Kass RS, Hinkle PM. Inhibition of L-type calcium-channel activity by thapsigargin and 2,5-t-butylhydroquinone, but not by cyclopiazonic acid. Biochem J. 1994;302:146–154. doi: 10.1042/bj3020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GED, Mitra RM, Wais RJ, Long SR. Evidence for structurally specific negative feedback in the Nod factor signal transduction pathway. Plant J. 2001;28:191–200. doi: 10.1046/j.1365-313x.2001.01149.x. [DOI] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, Van Aken J, Hackett G, Hepler PK. Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol. 1996;174:160–173. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- Pineros M, Tester M. Calcium channels in higher plant cells: selectivity, regulation and pharmacology. J Exp Bot. 1997;48:551–577. doi: 10.1093/jxb/48.Special_Issue.551. [DOI] [PubMed] [Google Scholar]
- Pingret J-L, Journet E-P, Barker DG. Rhizobium Nod factor signaling: evidence for a G protein-mediated transduction mechanism. Plant Cell. 1998;10:659–671. doi: 10.1105/tpc.10.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders MJ, Helper PK. Calcium antagonists and calmodulin inhibitors block cytokinin-induced bud formation in Funaria hygrometrica. Dev Biol. 1983;99:41–49. doi: 10.1016/0012-1606(83)90252-x. [DOI] [PubMed] [Google Scholar]
- Schumaker KS, Gizinski MJ. G proteins regulate dihydropyridine binding to moss plasma membranes. J Biol Chem. 1996;271:21292–21296. doi: 10.1074/jbc.271.35.21292. [DOI] [PubMed] [Google Scholar]
- Schumaker KS, Sze H. Inositol 1,4,5-trisphosphate releases calcium ions from vacuolar membrane vesicles of oat roots. J Biol Chem. 1987;262:3944–3946. [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the calcium-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- Staxen I, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc Natl Acad Sci USA. 1999;96:1779–1784. doi: 10.1073/pnas.96.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension-cultured cells. Plant Cell. 1994;6:1747–1762. doi: 10.1105/tpc.6.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H, Liang F, Hwang I, Curran AC, Harper JF. Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Ann Rev Plant Physiol Plant Mol Biol. 2000;51:433–462. doi: 10.1146/annurev.arplant.51.1.433. [DOI] [PubMed] [Google Scholar]
- Thomas AP, Bird GSJ, Hajnoczky G, Robb-Gaspers LD, Putney JW., Jr Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- Thomson LJ, Hall JL, Williams LE. A study of the effect of inhibitors of the animal sarcoplasmic/endoplasmic reticulum-type calcium pumps on the primary Ca2+-ATPases of red beet. Plant Physiol. 1994;104:1295–1300. doi: 10.1104/pp.104.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlalka M, Fricker M. The role of calcium in blue-light-dependent chloroplast movement in Lemna trisulca L. Plant J. 1999;20:461–473. doi: 10.1046/j.1365-313x.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Van Den Berg C, Willemsen V, Hage W, Weisbeek P, Scheres B. Cell fate in the Arabidopsis root meristem determined by directional signaling. Nature. 1995;378:62–65. doi: 10.1038/378062a0. [DOI] [PubMed] [Google Scholar]
- Wais RJ, Galera C, Oldroyd G, Catoira R, Penmetsa RV, Cook D, Gough C, Denarie J, Long SR. Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc Natl Acad Sci USA. 2000;97:13407–13412. doi: 10.1073/pnas.230439797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SA, Viprey V, Downie JA. Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by Nod factors and chitin oligomers. Proc Natl Acad Sci USA. 2000;97:13413–13418. doi: 10.1073/pnas.230440097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. Calcium channels in the plasma membrane of root cells. Ann Bot. 1998;81:173–183. [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 1997;12:427–439. doi: 10.1046/j.1365-313x.1997.12020427.x. [DOI] [PubMed] [Google Scholar]