Abstract
Mechanical signals are important both as environmental and endogenous developmental cues in plants. Among the quickest measurable responses to mechanical stimulation (MS) in plants is the up-regulation of specific genes, including TCH3, in Arabidopsis. Little is known about the signaling events and components that link perception of mechanical signals to gene expression in plants. Calcium has been identified previously as being potentially involved, and a role for ethylene has also been suggested. Using the protein kinase inhibitor staurosporine, we determined that MS up-regulation of TCH3 expression requires protein kinase activity in young Arabidopsis seedlings. Our data from studies on the Arabidopsis ein6 mutant demonstrate that the EIN6 protein is also required, but that its role in mechanically induced TCH3 expression appears to be independent of ethylene. Challenge of seedlings with protein phosphatase inhibitors calyculin A and okadaic acid stimulated TCH3 expression even in the absence of MS, implying protein phosphatase activity acting to negatively regulate TCH3 gene expression. This phosphatase activity acts either downstream or independently of EIN6. EIN6 and protein kinase activity, on the other hand, operate downstream of calcium to mediate mechanically stimulated TCH3 expression.
Plant development is receptive to and influenced by mechanical signals, both internal and external. It has been speculated that cellular tension and compression within tissues during normal plant cell growth may act as a signal to alter the direction planes of cell division and possibly affect cell differentiation (Biro et al., 1980; for summary, see Trewavas and Knight, 1994). Environmental cues such as wind, touching, rubbing, and growth against objects are perceived by plants, and induce specific responses. These involve alterations in the growth of plants to cope and compensate for these mechanical variables in processes known as thigmomorphogenesis (Jaffe and Forbes, 1993) and thigmotropism (Okada and Shimura, 1990). Plant growth regulators, e.g. auxin, abscisic acid, and ethylene, have been implicated as being involved in the processes of thigmomorphogenesis (Jaffe and Biro, 1979; Biro and Jaffe, 1984; Erner and Jaffe, 1982) and thigmotropism (Okada and Shimura, 1990). However, knowledge of the signaling pathways leading from perception (the nature of which is itself not understood) of mechanical signals to such growth responses is very limited.
Calcium has been postulated to be involved in mechanical stimulation (MS) signaling by several strands of evidence. The treatment of soybean (Glycine max) plants with calcium antagonists has been shown to inhibit the growth responses during thigmomorphogenesis (Jones and Mitchell, 1989). In addition, MS in the form of touch and wind has been shown to cause rapid elevations in cytosolic-free calcium concentration ([Ca2+]cyt) in several plant species, including Arabidopsis (Knight et al., 1991, 1992, 1995; Haley et al., 1995). As well as these rapid changes in [Ca2+]cyt, one of the earliest of responses of plants (apart from specialized plants such as the Venus flytrap [Dionaea muscipula] and Mimosa pudica) to mechanical signals, often measurable after just a few minutes of stimulation (Braam and Davis, 1990), is the up-regulation of specific genes (Braam and Davis, 1990; Botella et al., 1995; Mizoguchi et al., 1996). In Arabidopsis, for instance, several touch (TCH) genes have been identified, including TCH3 (Braam and Davis, 1990). TCH3 is greatly up-regulated in response to a variety of mechanical signals, with peak expression (as measured by steady-state transcript levels) occurring 30 min after stimulation (Braam and Davis, 1990). It is interesting that calcium again has been implicated in the expression of these genes. Addition of exogenous calcium to Arabidopsis cell suspension cultures (Braam, 1992) causes induction of TCH3 expression in the absence of a primary (mechanical) signal. Additionally, TCH3 expression is inhibited in the presence of calcium antagonists when the gene is induced in response to cold (Polisensky and Braam, 1996). In addition to calcium, there is the potential for ethylene to be involved in MS signaling. It is interesting that ethylene can induce the expression of TCH3 in the absence of a primary (mechanical) signal (Sistrunk et al., 1994). However, some other evidence would suggest that ethylene is not actually used in planta to mediate MS up-regulation of TCH3 (Johnson et al., 1998).
This current study was aimed at determining whether there was evidence of components other than calcium in the signaling pathway(s) leading from perception of MS to TCH3 gene up-regulation specifically in Arabidopsis seedlings. Having identified such potential signaling components, this study also aimed to determine where they acted (upstream/downstream) relative to calcium and to each other in these signal transduction pathway(s).
RESULTS
Protein Kinase and Protein Phosphatase Inhibitors Affect Mechanically Stimulated Expression of TCH3
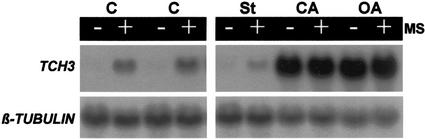
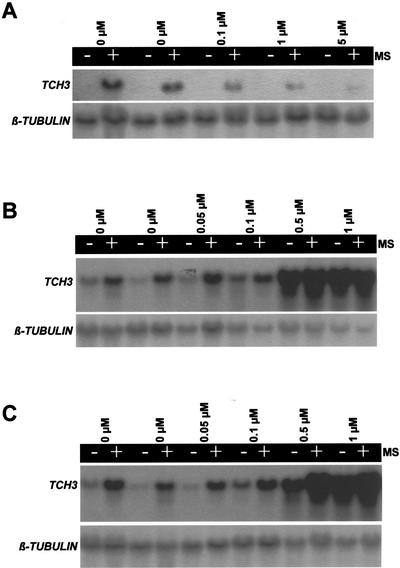
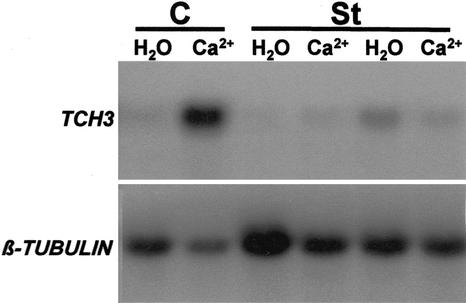
To test for the potential involvement of protein phosphorylation events in the pathway leading from MS to TCH3 gene expression, we tested the effect of the protein kinase inhibitor, staurosporine, and the protein phosphatase inhibitors, okadaic acid and calyculin A, on mechanically stimulated TCH3 expression in Arabidopsis seedlings (Fig. 1). At a concentration of 10 μm, staurosporine significantly inhibited mechanically stimulated TCH3 expression. Okadaic acid and calyculin A (both at 1 μm) produced significantly enhanced TCH3 expression in both mechanically stimulated and control (non-stimulated) samples. The induction of TCH3 expression by okadaic acid and calyculin A did not seem to depend at all on the involvement of the primary signal, i.e. MS. To examine the dose dependency of these inhbitors, the effect of different concentrations of staurosporine, okadaic acid, and calyculin A on TCH3 expression was tested (Fig. 2). Staurosporine showed inhibition of mechanically stimulated TCH3 expression at all the concentrations tested (0.1, 1, and 5 μm), with the severity of inhibition increasing with increasing staurosporine concentration (Fig. 2A). The induction of TCH3 expression by calyculin A was first detectable at a concentration of 0.1 μm, where significantly greater expression of TCH3 was detected in the inhibitor-treated, but nonmechanically stimulated, sample than in the corresponding unstimulated control sample (Fig. 2B). This effect became very clear at 0.5 μm calyculin A, where it appeared to be maximal (there was no greater effect at 1 μm; Fig. 2B), suggesting that the calyculin A effect saturates at 0.5 μm. The situation with okadaic acid (Fig. 2C) was very similar as for calyculin A, except that TCH3 induction in noninduced plants at 0.5 μm okadaic acid was significantly less than for 0.5 μm calyculin A. As a consequence, for okadaic acid, 1 μm produced a greater effect on TCH3 expression than did 0.5 μm. This implies that the okadaic acid effect saturates at 1 μm or greater concentrations of this particular inhibitor.
Figure 1.
Effect of protein kinase and phosphatase inhibitors on mechanically stimulated TCH3 gene expression in Arabidopsis seedlings. Seedlings in flasks were treated for 4 h in either 10 μm staurosporine (St), 1 μm calyculin A (CA), 1 μm okadaic acid (OA), or 1% (v/v) dimethyl sulfoxide (DMSO) as a control in duplicate (C), after which time flasks were shaken for 60 s (+MS) or left undisturbed (−MS). Thirty minutes after shaking, tissue was harvested, total RNA extracted, and TCH3 and β-tubulin mRNA levels detected by RNA-blot hybridization.
Figure 2.
Dose dependence of the effect of staurosporine, calyculin A and okadaic acid on TCH3 gene expression in Arabidopsis seedlings. Seedlings were treated and experiments performed exactly as described in the figure legend for Figure 1 with 0, 0.1, 1, and 5 μm staurosporine (A); 0, 0.05, 0.1, 0.5, and 1 μm calyculin A (B); and 0, 0.05, 0.1, 0.5, and 1 μm okadaic acid (C) being added. All 0 μm controls are presented in duplicate.
Mechanically Stimulated Expression of TCH3 Is Reduced in the ein6 Mutant of Arabidopsis
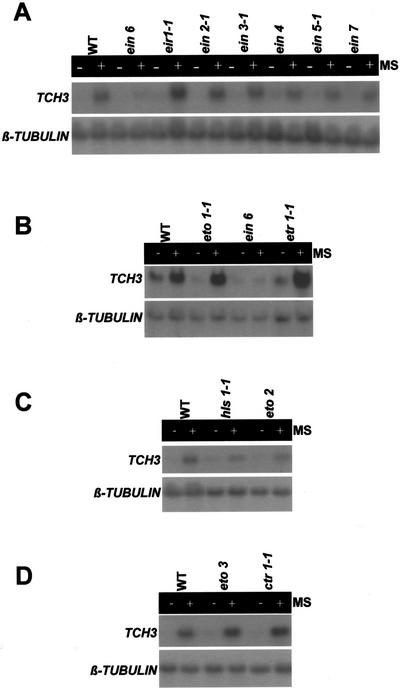
Ethylene has for some time been strongly implicated in thigmomorphogenesis (Jaffe and Biro, 1979; Biro and Jaffe, 1984) and TCH3 expression specifically has been shown to be inducible by the application of exogenous ethylene gas (Sistrunk et al., 1994). However, even taking these data together, it cannot be concluded that in planta, ethylene is actually used as a component of a chain of events leading from MS to TCH3 expression. The fact that the molecular (including TCH gene expression) and physiological responses to MS have been reported to be unaffected in ein2 and etr1 ethylene-insensitive mutants of Arabidopsis would support this view (Johnson et al., 1998). To test whether Arabidopsis actually uses ethylene in planta to mediate MS induction of TCH3, we examined the mechanically stimulated TCH3 expression in a number of ethylene-related mutants of Arabidopsis. These mutants included ethylene-insensitive mutants eir1-1, ein2-1, ein3-1, ein4, ein5-5, ein6, ein7, hls1-1, and etr1-1 (Chao et al., 1993, 1997; Roman et al., 1995; Alonso et al., 1999; Raz and Ecker, 1999); ethylene overproducing mutants eto1-1, eto2, and eto3 (Guzman and Ecker, 1990; Woeste et al., 1999); and the ethylene-constitutive mutant ctr1-1 (Kieber et al., 1993; Roman et al., 1995). The uninduced levels of TCH3 transcript appeared similar in all mutants, even the ctr1 and the ethylene-overproducing mutants. All mutants showed significant mechanically stimulated TCH3 expression comparable with wild type (Fig. 3), except for ein6 (Fig. 3, A and B). The ein6 mutant consistently showed a greatly reduced, or no, mechanically stimulated TCH3 expression.
Figure 3.
Mechanically stimulated TCH3 gene expression in seedlings of ethylene mutants of Arabidopsis. Wild-type and mutant seedlings were grown on agar plates and replicate plates either mechanically stimulated for 10 s (+MS) or left undisturbed (−MS). Thirty minutes after shaking, tissue was harvested, total RNA extracted, and TCH3 and β-tubulin mRNA levels detected by RNA-blot hybridization. Mutants analyzed included ethylene-insensitive mutants eir1-1, ein2-1, ein3-1, ein4, ein5-1, ein6, and ein7 (all A); hls1-1 (C), and etr1-1 (B); ethylene-overproducing mutants eto1-1 (B), eto2 (C), and eto3 (D); and the ethylene-constitutive mutant ctr1-1 (D).
Calcium-Induced TCH3 Expression Is Inhibited by Staurosporine and the ein6 Mutation
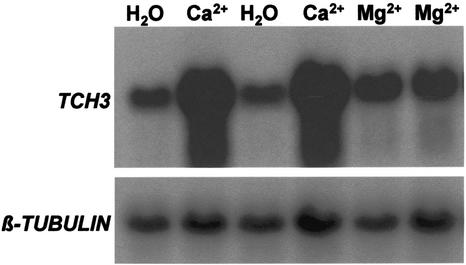
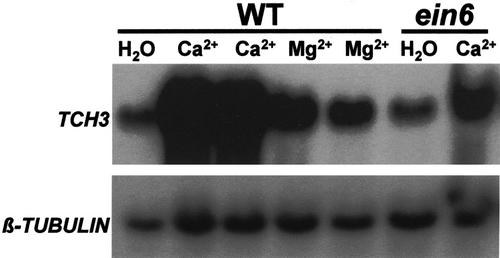
Calcium has been implicated as a second messenger involved in signaling pathways leading to TCH3 gene expression. In Arabidopsis cell suspension cultures, the addition of extracellular calcium induces the expression of TCH3 (Braam, 1992) and mechanically stimulated transient elevations in [Ca2+]cyt have been detected in plants including Arabidopsis (Knight et al., 1991, 1992, 1995; Haley et al., 1995). To see if the inhibitory effects of staurosporine and ein6 were either upstream or downstream of calcium, it was desirable to measure calcium-induced TCH3 expression in whole seedlings. First of all, we tested whether externally added calcium chloride could in fact induce TCH3 in whole seedlings in our experimental system (Fig. 4). In these experiments, we mechanically desensitized the seedlings overnight as described in “Materials and Methods.” This was necessary to allow the observation of the effect of extracellular calcium addition, which would not be possible with the high background of TCH3 expression because of MS provoked by the addition itself. Magnesium chloride (isoosmotic to the calcium chloride) was used as a control for the calcium ion, and also to control for the possible effect of osmotic shock on TCH3 expression. The addition of 100 mm MgCl2 caused a relatively small elevation of TCH3 expression. In contrast, 100 mm CaCl2 caused a much more substantial induction of TCH3 expression. Thus, the inducing effect specifically attributable to the calcium ion was clear in this experimental setup. The small induction caused by the MgCl2 compared with the water control was most likely because of an osmotic response. Using this experimental system of calcium-induced TCH3 expression in whole Arabidopsis seedlings, we tested the effects of staurosporine and the ein6 mutation. The data in Figure 5 show that at a concentration of 10 μm, staurosporine inhibited calcium-induced TCH3 expression in Arabidopsis seedlings, similar to its effect on mechanically stimulated TCH3 expression (Figs. 1 and 2). Treating seedlings with both calcium and phosphatase inhibitor did not produce TCH3 expression in excess of levels seen when seedlings were given these two treatments separately (data not shown). The data in Figure 6 show that the ein6 mutation also inhibits calcium-induced TCH3 expression, similar to its effect on mechanically stimulated TCH3 expression (Fig. 3, A and B). It is interesting that with ein6, there is still a small amount of induction of TCH3 after treatment with CaCl2, the level of which is very similar to that obtained with MgCl2 in the wild type.
Figure 4.
Calcium induction of TCH3 expression in Arabidopsis seedlings. Seedlings in flasks were desensitized to MS by maintaining shaking up to and beyond the point of addition of compounds. Ca2+, Mg2+ (both added to a final concentration of 100 mm) or the same volume of water was added. Thirty minutes after addition, tissue was harvested, RNA extracted, and TCH3 and β-tubulin mRNA levels detected by RNA-blot hybridization.
Figure 5.
Staurosporine inhibition of calcium-induced TCH3 expression in Arabidopsis seedlings. Seedlings in flasks were treated exactly as described in the legend to Figure 4, except that 4 h before addition of Ca2+ or water, some samples were treated with 10 μm staurosporine (St; in duplicate) and others with 1% (v/v) DMSO as a control (C). Thirty minutes after addition of Ca2+/water, tissue was harvested, total RNA extracted, and TCH3 and β-tubulin mRNA levels detected by RNA-blot hybridization.
Figure 6.
Inhibition of calcium induction of TCH3 expression in the ein6 mutant. Seedlings in flasks were treated exactly as described in the legend to Figure 4. Both wild-type (WT) and ein6 seedlings were treated with either 100 mm Ca2+, 100 mm Mg2+, or water. Thirty minutes after addition of Ca2+/Mg2+/water, tissue was harvested, RNA extracted, and TCH3 and β-tubulin mRNA levels detected by RNA-blot hybridization.
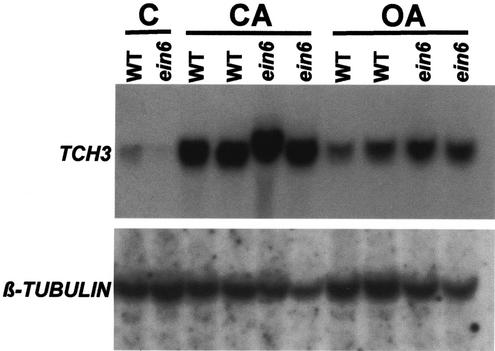
We also examined whether the okadaic acid and calyculin A induction of TCH3 was affected by the ein6 mutation (Fig. 7). The data show that both okadaic acid and calyculin A at concentrations of 0.5 μm caused similar levels of induction of TCH3 in ein6 as in the wild type. These data also confirmed the finding (Fig. 2, B and C) that at a concentration of 0.5 μm, calyculin A is more potent than okadaic acid in terms of its effect on TCH3 expression.
Figure 7.
Protein phosphatase inhibitor-induced TCH3 expression in seedlings of the ein6 mutant of Arabidopsis. Seedlings in flasks were treated for 4.5 h in either 0.5 μm calyculin A (CA), 0.5 μm okadaic acid (OA), or 1% (v/v) DMSO as a control (C), during which time the samples were left undisturbed. Both wild-type (WT) and ein6 (ein6) seedlings were used. Tissue was harvested, RNA extracted, and TCH3 and β-tubulin mRNA levels detected by RNA-blot hybridization.
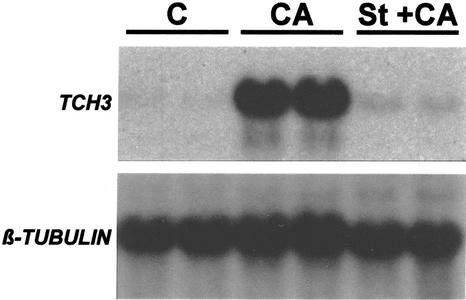
Finally, we examined the effect of kinase inhibition, using staurosporine, upon the calyculin A-mediated induction of TCH3 expression (Fig. 8). As can be seen, treatment with staurosporine under the same conditions that inhibited MS-induced TCH3 expression also inhibited the induction of TCH3 expression caused by calyculin A addition.
Figure 8.
Inhibition of calyculin A induction of TCH3 expression by staurosporine. Seedlings in flasks were treated exactly as described in the legend to Figure 4, except that 4 h before addition of calyculin A or 1% (v/v) DMSO, some samples were treated with 10 μm staurosporine (St+CA) (in duplicate) and others with 1% (w/v) DMSO as a control (CA and C). Four hours after addition of 0.5 μm calyculin A (St+CA and CA) or 1% (v/v) DMSO (C), tissue was harvested, total RNA extracted, and TCH3 and β-tubulin mRNA levels detected by RNA-blot hybridization.
DISCUSSION
The signal transduction of MS in plants is a poorly understood process. Primary stimuli (touching, rubbing, shaking etc.) lead to a battery of growth responses, which collectively form the process known as thigmomorphogenesis (Jaffe and Forbes, 1993) and the specialized thigmotropic responses in organs such as tendrils and roots (Okada and Shimura, 1990; Klüsener et al., 1995). Many plant genes are up-regulated in response to MS, and the best characterized of these are the TCH genes of Arabidopsis (Braam and Davis, 1990). One of these genes, TCH3, encodes a calmodulin-like protein, of as yet undetermined function, which is rapidly (peak of expression 30 min after MS) induced (Braam and Davis, 1990; Sistrunk et al., 1994) by MS. It is known that this gene is also induced by ethylene, auxin, cold, and extracellular calcium (Braam, 1992a; Sistrunk et al., 1994; Antosiewicz et al., 1995; Polisensky and Braam, 1996).
In terms of MS signaling, the second messenger calcium is as yet the only potential component identified. The evidence available includes the fact that MS provokes rapid [Ca2+]cyt increases in plants including Arabidopsis (Knight et al., 1991, 1995) and addition of extracellular calcium in the absence of MS in Arabidopsis cell suspension cultures induces TCH3 expression (Braam, 1992). Furthermore, in response to cold, TCH3 expression is inhibited by the calcium channel blockers lanthanum and gadolinium and the calcium chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (Polisensky and Braam, 1996).
The research described in this paper was aimed at obtaining evidence of new components in MS signal transduction, leading to TCH3 expression, and placing these (upstream/downstream) relative to calcium in a signal transduction pathway in Arabidopsis seedlings.
Staurosporine has been shown to inhibit other signal transduction pathways in plants, e.g. the calcium-regulated expression of CAB in response to red light in soybean suspension cells and tomato (Lycopersicon esculentum) hypocotyl cells (Bowler et al., 1994a, 1994b) and the calcium-regulated expression of KIN2 in response to abscisic acid in Arabidopsis hypocotyl cells (Wu et al., 1997). The data presented here are consistent with the involvement of staurosporine-sensitive protein kinase activity in the transduction of MS leading to TCH3 expression. This activity may be the result of one or more kinases. It appears that this kinase activity is necessary for MS induction of TCH3 expression. MAP kinase cascade activation has been demonstrated in response to MS (Bogre et al., 1996), so it is possible that the target for staurosporine is one or more of the kinase components of these cascades.
Because inhibition of protein phosphatase activity (Figs. 1 and 2, B and C) results in increased expression of TCH3, it seems that protein phosphatase activity is negatively regulating TCH3 expression. This phosphatase activity must act upstream of, or in concert with, the staurosporine-sensitive kinase activity (Fig. 8). One possible mechanism could be that this protein phosphatase is acting antagonistically to a protein kinase that positively regulates TCH3 expression, in a similar way as is proposed for kinase induction of EIN3 activity in ethylene signaling in Arabidopsis (Bowler and Chua, 1994). If the effect of okadaic acid and calyculin A inhibition of protein phosphatase activity is to release the activity of such a reciprocal kinase, then this implies (as in the ethylene/EIN3 example) that the MS signaling pathway leading to TCH3 gene expression is constitutively switched on, and is inhibited from acting when there is no MS. In such a scheme, MS would release the inhibition (by inhibiting the protein phosphatase activity) of the signal transduction pathway and greater flux would occur through the pathway leading to TCH3 expression. This may be possible, but our present study only allows the conclusion that protein phosphatase activity may be involved as a negative regulator of the signaling pathway leading from MS to TCH3 expression. The staurosporine-sensitive kinase activity is required for mechanically stimulated TCH3 expression; therefore, we conclude that this kinase activity is part of the signaling pathway leading from MS to TCH3 up-regulation. It is still possible that the protein phosphatase activity, whereas definitely negatively regulating TCH3 expression (as shown in Figs. 1 and 2), is not involved in MS signal transduction specifically, i.e. this activity is not inhibited by MS to lead to induced TCH3 expression, but is used by the plant for induction of TCH3 gene expression in response to another factor. However, the fact that the effect of inhibiting this phosphatase activity can be blocked by staurosporine gives credence to the idea that this phosphatase activity is actually involved in MS signaling.
Ethylene is strongly implicated in the mechanical responses of plants leading to thigmomorphogenesis (Biro et al., 1984; Jaffe and Forbes, 1993). The artificial application of ethylene gas has also been shown to induce TCH3 (Sistrunk et al., 1994). All ethylene mutants we tested, apart from ein6, showed a wild-type TCH3 response to MS when RNA loading was taken into account by examining constitutive tubulin expression (Fig. 3). It is notable that the dominant ethylene-insensitive mutants etr1-1 and ein4 showed normal expression because these genes encode parts of the ethylene receptor in Arabidopsis, and the ein4 and etr1-1 mutants show reductions in all other ethylene responses tested (Chang et al., 1993; Roman et al., 1995; Hua et al., 1998). Therefore, if ethylene were used in planta to mediate mechanically stimulated TCH3 up-regulation, one would expect reduced, or no, TCH3 induction in ein4 and etr1-1 (but this is clearly not the case; Fig. 3). Our data are consistent with the observation by Johnson et al. (1998) that ein2 and etr1 mutants show wild-type MS responses. In addition, it can be seen in Figure 3 that the basal levels of TCH3 expression in the ethylene-overproducing mutants (eto 1-1, eto2, and eto3) and the constitutive ethylene signaling mutant ctr1 are not elevated, despite the fact that such mutants show elevated levels of expression of bona fide ethylene-regulated genes (Kieber et al., 1993; Ecker, 1995). Taken together, this seems to be strong evidence that in planta, ethylene is not used to mediate mechanically stimulated TCH3 gene expression, even though application of exogenous ethylene can induce TCH3 gene expression (Sistrunk et al., 1994).
In light of these observations, it seems that the (as yet uncloned) ein6 mutation is exerting its effect on TCH3 expression independently of the involvement of ethylene. In other words, EIN6 is involved in both ethylene signaling and MS signaling, similar to the way in which HLS1 affects both ethylene and auxin signaling (Ecker, 1995). The possibility that ein6 is simply a mutation in the TCH3 gene itself, thus leading to reduced expression, is discounted as in response to other stimuli TCH3 expression reaches wild-type levels in ein6 (e.g. Fig. 7). Also arguing against this possibility is the fact that EIN6 resides on chromosome III of Arabidopsis (Roman et al., 1995), whereas TCH3 resides on chromosome II (Lin et al., 1999). The data presented here (Fig. 3, A and B) suggest that in wild-type Arabidopsis seedlings, the EIN6 protein plays a role in the mediation of the MS signal leading to TCH3 expression.
As discussed above, calcium appears to be a component in MS signaling leading to TCH3 expression. To establish whether the effects of staurosporine and ein6 were upstream or downstream of calcium, the effect of either inhibitor or mutation, respectively, on calcium induction of TCH3 expression was measured. Addition of extracellular calcium chloride caused a significant induction of TCH3 expression in Arabidopsis seedlings, much greater than iso-osmolar magnesium chloride, suggesting this effect was largely calcium specific (Fig. 4). Our experiments were performed upon MS-desensitized plants for technical reasons outlined in “Results.” Thus, the data obtained relating to calcium activation of TCH3 expression may not be specific to MS signaling and may also relate to signaling from other stimuli that lead to TCH3 expression, and which involve calcium, e.g. low temperature. Calcium induction of TCH3 expression has been similarly demonstrated in Arabidopsis cell suspension cultures (Braam, 1992). It is interesting that magnesium caused a slight induction, likely to be as a result of osmotic stress. We have shown previously that this level of osmoticum can induce osmotically regulated genes (Knight et al., 1997). This slight induction appeared to be unaffected by the ein6 mutation (Fig. 6), suggesting that osmotically induced TCH3 expression occurs via an EIN6-independent pathway. Both staurosporine (Fig. 5) and ein6 (Fig. 6) significantly inhibited the calcium induction of TCH3. These data imply that the staurosporine-sensitive kinase and the EIN6 protein act downstream of calcium in MS signaling leading to TCH3 expression. Staurosporine treatment did not affect MS-induced [Ca2+]cyt responses in Arabidopsis seedlings (data not shown), also consistent with the kinase activity acting downstream of MS-induced [Ca2+]cyt. A combined treatment of calcium and phosphatase inhibitor did not show an amplified response in terms of TCH3 expression. This implies that increased flux through the calcium part of the signaling pathway does not lead to enhanced flux through the phosphatase-sensitive part of the pathway. This leads to the conclusion that either calcium is not upstream of the phosphatase activity, or that under these conditions the pathway has already achieved maximal flux. Thus, it is not possible to conclude the hierarchy of calcium and protein phosphatase activity in the MS signaling pathway. To investigate whether the okadaic acid- and calyculin A-sensitive protein phosphatase activity was potentially upstream or downstream of EIN6, we compared levels of TCH3 induction promoted by these two inhibitors in ein6 and wild type (Fig. 7). Our data suggest that the negatively regulating protein phosphatase activity acts either downstream of EIN6 in the MS signal transduction pathway leading to TCH3 expression in Arabidopsis seedlings or independently of EIN6.
In conclusion, TCH3 expression can be negatively regulated by protein phosphatase activity. This appears to be independent or downstream of EIN6, but whether it is specifically involved in MS up-regulation of TCH3 expression is not yet known. This study also indicates the necessity for both EIN6 and staurosporine-sensitive protein kinase activity to mediate mechanically stimulated TCH3 expression in Arabidopsis. Both of these components seem to act downstream of calcium, and may well be involved in transducing mechanically stimulated [Ca2+]cyt signals to effect TCH3 up-regulation. In the future, it will be very interesting to clone the EIN6 locus to be able to begin to understand the fundamentals of its role in mechanical signaling of Arabidopsis.
MATERIALS AND METHODS
Plant Materials and Chemicals
All experiments were performed using Arabidopsis seedlings grown on 0.8% (w/v) agar plates containing full-strength Murashige and Skoog nutrient medium (Murashige and Skoog, 1962) with a 16-h photoperiod as previously described (Knight et al., 1997). Arabidopsis wild-type seeds were supplied by Lehle Seeds (Round Rock, TX). Ethylene mutant seeds were supplied by the Nottingham Arabidopsis Stock Centre (Nottingham, UK), contacted via the Arabidopsis Information Management System (http://aims.cps.msu.edu/aims/aims.html), and were sown, selfed, and seeds harvested. Seedlings were either 5 or 11 d old at the beginning of experiments, depending on the particular experiment (see below). Calbiochem-Novabiochem Ltd. (Nottingham, UK) supplied staurosporine and calyculin A. LC Laboratories (Woburn, MA) supplied okadaic acid. These inhibitors were all dissolved in DMSO from Sigma (Poole, UK) to produce stock solutions (as described below). All other chemicals were obtained from BDH (Poole, UK).
MS of Plants
For analysis of ethylene mutants, 11-d-old seedlings were stimulated by applying a whole agar plate of seedlings for 10 s to a vortex mixer at its maximum setting (Rotamixer, Hook and Tucker Instruments Ltd., Croydon, UK) for 10 s. For experiments involving inhibitors, seedlings aged 5 d were transferred to 4 mL of one-half-strength Murashige and Skoog nutrient medium in a 25-mL conical flask. The flasks were covered with foil and plants left to recover in the growth cabinet overnight. For calcium induction experiments, to achieve mechanical desensitization of the seedlings, after transfer into the liquid induction system, the seedlings were incubated overnight on an orbital shaker set at a speed of 120 rpm in the growth cabinet. The following day, calyculin A, staurosporine, or okadaic acid at the desired concentration were added to the system in a total volume of 40 μL of DMSO (1% [v/v] final). The system was mixed briefly (approximately 2–3 s) by manual shaking. The seedlings were then left free from MS to recover. Four hours later, the seedlings were mechanically stimulated by manually shaking the flask for 60 s. The seedlings were harvested a further 30 min after the stimulus (time point confirmed as having maximal TCH3 expression by RNA gel-blot hybridization; data not shown). The seedlings were blotted dry with tissue paper and frozen in liquid nitrogen. The entire harvesting process was designed to take less than 2 min to avoid any unwanted mechanically induced gene expression. For experiments with exogenous CaCl2, Arabidopsis seedlings aged 5 d were desensitized to MS by shaking (120 rpm) flasks overnight on a shaker in the growth room. The following day, the desired inhibitors were added to the flask and incubated for 4 h before the addition of 1 mL of 0.5 m CaCl2 or 0.5 m MgCl2. The seedlings were harvested 30 min after the addition of these latter solutions.
RNA Gel-Blot Hybridization
Approximately 20 to 25 mg of wild-type or mutant Arabidopsis seedlings were treated as described above, and harvested into microcentrifuge tubes. Total RNA was prepared from seedling tissue using RNeasy plant RNA minipreps (Qiagen, Dorking, UK). For RNA gel-blot hybridizations, total RNA samples (10 μg per lane) were electrophoresed through 1.0% (w/v) agarose (Life Technologies, Paisley, UK) formaldehyde gels (Sambrook et al., 1989). RNA was transferred to nylon membranes (Böehringer Mannheim, Mannheim, Germany) by capillary action. Blots were prehybridized and hybridized in 50% (v/v) formamide at 42°C. Blots were washed twice in each of the following successively: 2× SSC (1× SSC is 0.15 m NaCl and 0.015 m sodium citrate, pH 7.0) and 0.1% (w/v) SDS, followed by 1× SSC and 0.1% (w/v) SDS, and finally 0.1× SSC and 0.1% (w/v) SDS at 42°C. Probes for β-tubulin were prepared from the products of PCR using specific primers as described previously (Knight et al., 1999). Probe for TCH3 was prepared in the same way, using the primers TCH-L (5-TCAAGATAACAGCGCTTCGAA-3) and TCH-R (5-AACAATGGTGGATTATCAGCTC-3; Genosys, Cambridge, UK).
ACKNOWLEDGMENTS
A.J.W. would like to thank the Biotechnology and Biological Sciences Research Council for the funding of his PhD studentship. H.K. and M.R.K. would like to thank the Biotechnology and Biological Sciences Research Council for funding this research.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council and Royal Society (studentship to A.J.W.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010660.
LITERATURE CITED
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Antosiewicz DM, Polisensky DH, Braam J. Cellular localization of the Ca2+ binding TCH3 protein of Arabidopsis. Plant J. 1995;8:623–636. doi: 10.1046/j.1365-313x.1995.08050623.x. [DOI] [PubMed] [Google Scholar]
- Biro RL, Hunt ER, Erner Y, Jaffe MJ. Thigmomorphogenesis: changes in cell division and elongation in the internodes of mechanically perturbed or etherel-treated bean plants. Ann Bot. 1980;45:655–664. [Google Scholar]
- Biro RL, Jaffe MJ. Thigmomorphogenesis: ethylene evolution and its role in the changes observed in mechanically perturbed bean plants. Physiol Plant. 1984;62:289–296. doi: 10.1111/j.1399-3054.1984.tb05925.x. [DOI] [PubMed] [Google Scholar]
- Bogre L, Ligterink W, Heberlebors E, Hirt H. Mechanosensors in plants. Nature. 1996;383:489–490. doi: 10.1038/383489a0. [DOI] [PubMed] [Google Scholar]
- Botella JR, Arteca RN, Frangos JA. A mechanical strain-induced 1-aminocyclopropane-1-carboxylic acid synthase gene. Proc Natl Acad Sci USA. 1995;92:1595–1598. doi: 10.1073/pnas.92.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Chua NH. Emerging themes of plant signal transduction. Plant Cell. 1994;6:1529–1541. doi: 10.1105/tpc.6.11.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Neuhaus G, Yamagata H, Chua NH. Cyclic-GMP and calcium mediate phytochrome phototransduction. Cell. 1994a;77:73–81. doi: 10.1016/0092-8674(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Bowler C, Yamagata H, Neuhaus G, Chua Nam H. Phytochrome signal transduction pathways are regulated by reciprocal control mechanisms. Genes Dev. 1994b;8:2188–2202. doi: 10.1101/gad.8.18.2188. [DOI] [PubMed] [Google Scholar]
- Braam J. Regulated expression of the calmodulin-related TCH genes in cultured Arabidopsis cells: induction by calcium and heat-shock. Proc Natl Acad Sci USA. 1992;89:3213–3216. doi: 10.1073/pnas.89.8.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J, Davis RW. Rain-induced, wind-induced, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleeker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chao QM, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Ecker JR. The ethylene signal transduction pathway in plants. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- Erner Y, Jaffe MJ. Thigmomorphogenesis - the involvement of auxin and abscisic-acid in growth-retardation due to mechanical perturbation. Plant Cell Physiol. 1982;23:935–941. [Google Scholar]
- Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley A, Russell AJ, Wood N, Allan AC, Knight M, Campbell AK, Trewavas AJ. Effects of mechanical signaling on plant cell cytosolic calcium. Proc Natl Acad Sci USA. 1995;92:4124–4128. doi: 10.1073/pnas.92.10.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleeker AB, Ecker JR, Meyerowitz EM. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M, Biro R. Thigmorphogenesis. In: Mussel H, Staples R, editors. Stress Physiology. New York: Wiley; 1979. pp. 25–59. [Google Scholar]
- Jaffe MJ, Forbes S. Thigmomorphogenesis: the effect of mechanical perturbation on plants. Plant Growth Regul. 1993;12:313–324. doi: 10.1007/BF00027213. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Sistrunk ML, Polisensky DH, Braam J. Arabidopsis thaliana responses to mechanical stimulation do not require ETR1 or EIN2. Plant Physiol. 1998;116:643–649. doi: 10.1104/pp.116.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RS, Mitchell CA. Calcium ion involvement in growth inhibition of mechanically stressed soybean (Glycine max) seedlings. Physiol Plant. 1989;76:598–602. doi: 10.1111/j.1399-3054.1989.tb05485.x. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the RAF family of protein-kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Klüsener B, Boheim G, Liss H, Engelberth J, Weiler EW. Gadolinium-sensitive, voltage-dependent calcium-release channels in the endoplasmic-reticulum of a higher-plant mechanoreceptor organ. EMBO J. 1995;14:2708–2714. doi: 10.1002/j.1460-2075.1995.tb07271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12:1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- Knight H, Veale E, Warren GJ, Knight MR. The sfr6 mutation in Arabidopsis suppresses low-temperature induction of genes dependent on the CRT/DRE sequence motif. Plant Cell. 1999;11:875–886. doi: 10.1105/tpc.11.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Knight MR, Knight H, Watkins NJ. Calcium and the generation of plant form. Philos Trans R Soc Lond Ser B Biol Sci. 1995;350:83–86. doi: 10.1098/rstb.1995.0141. [DOI] [PubMed] [Google Scholar]
- Knight MR, Smith SM, Trewavas AJ. Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci USA. 1992;89:4967–4971. doi: 10.1073/pnas.89.11.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Kaul S, Rounsley S, Shea TP, Benito M, Town CD, Fujii CY, Mason T, Bowman CL, Barnstead M et al. Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature. 1999;402:761–768. doi: 10.1038/45471. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, Shinozaki K. A gene encoding a mitogen activated protein-kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein- kinase and an S6 ribosomal-protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:765–769. doi: 10.1073/pnas.93.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962;15:473–497. [Google Scholar]
- Okada K, Shimura Y. Reversible root-tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science. 1990;250:274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- Polisensky DH, Braam J. Cold-shock regulation of the Arabidopsis TCH genes and the effects of modulating intracellular calcium levels. Plant Physiol. 1996;111:1271–1279. doi: 10.1104/pp.111.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz V, Ecker JR. Regulation of differential growth in the apical hook of Arabidopsis. Development. 1999;126:3661–3668. doi: 10.1242/dev.126.16.3661. [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal-transduction in Arabidopsis thaliana: 5 novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sistrunk ML, Antosiewicz DM, Purugganan MM, Braam J. Arabidopsis TCH3 encodes a novel Ca2+ binding protein and shows environmentally induced and tissue-specific regulation. Plant Cell. 1994;6:1553–1565. doi: 10.1105/tpc.6.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A, Knight M. Mechanical signaling, calcium and plant form. Plant Mol Biol. 1994;26:1329–1341. doi: 10.1007/BF00016478. [DOI] [PubMed] [Google Scholar]
- Woeste KE, Ye C, Kieber JJ. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 1999;119:521–530. doi: 10.1104/pp.119.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Kuzma J, Maréchal E, Graeff R, Lee HC, Foster R, Chua N-H. Abscisic acid signaling through cyclic ADP-ribose in plants. Science. 1997;278:2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]