Abstract
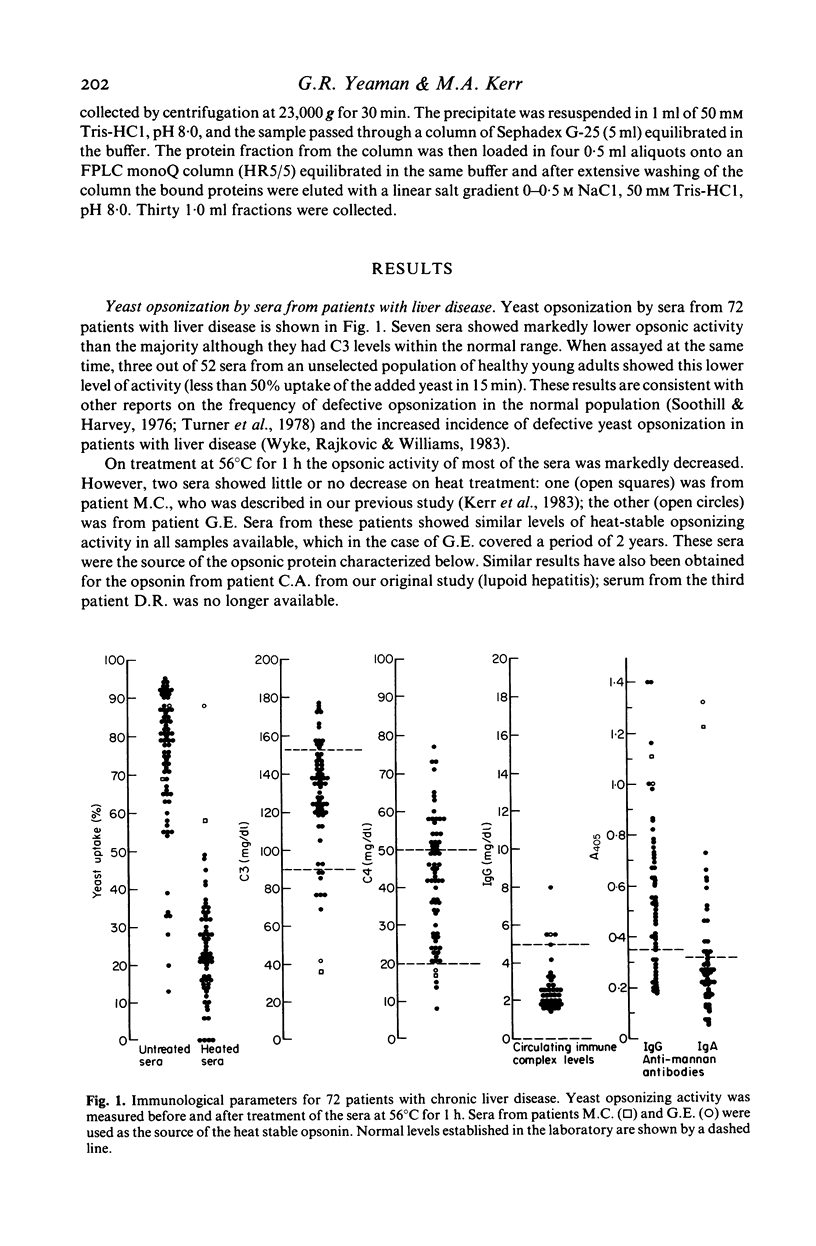
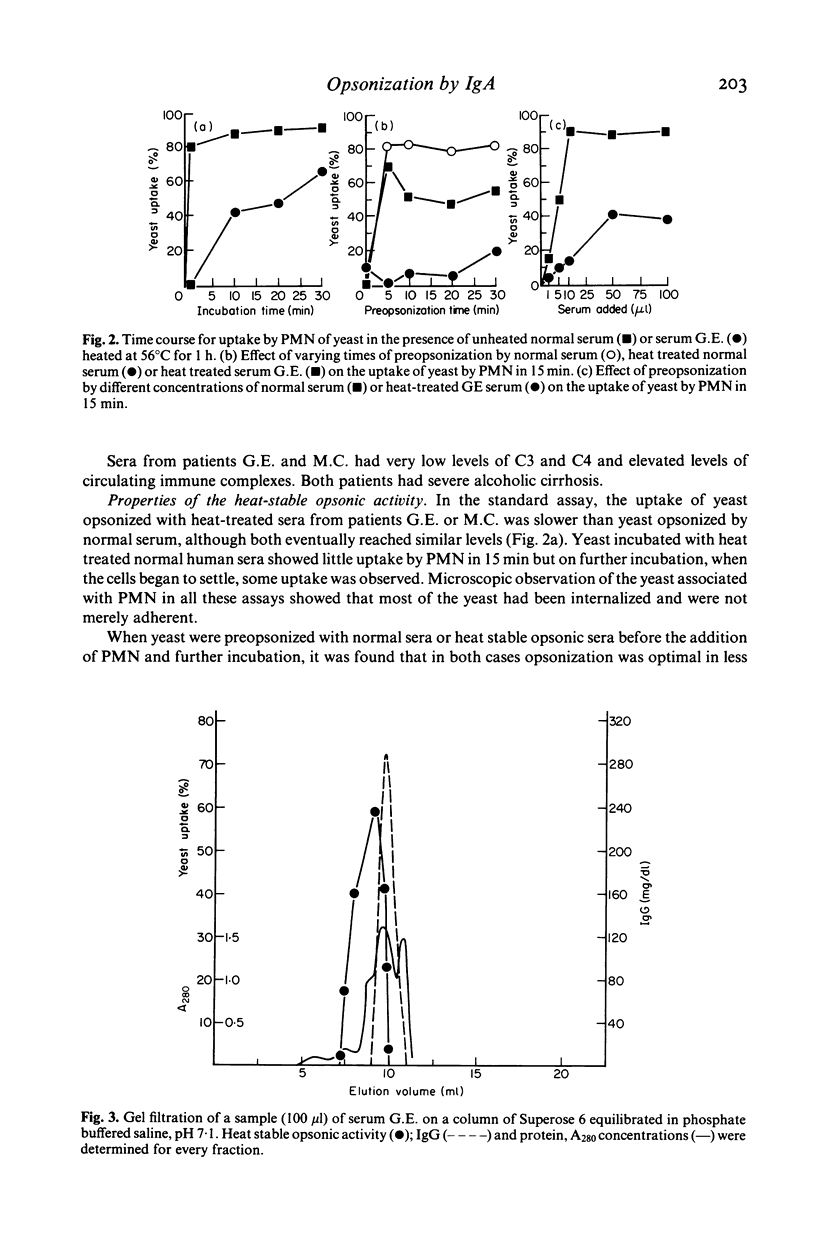
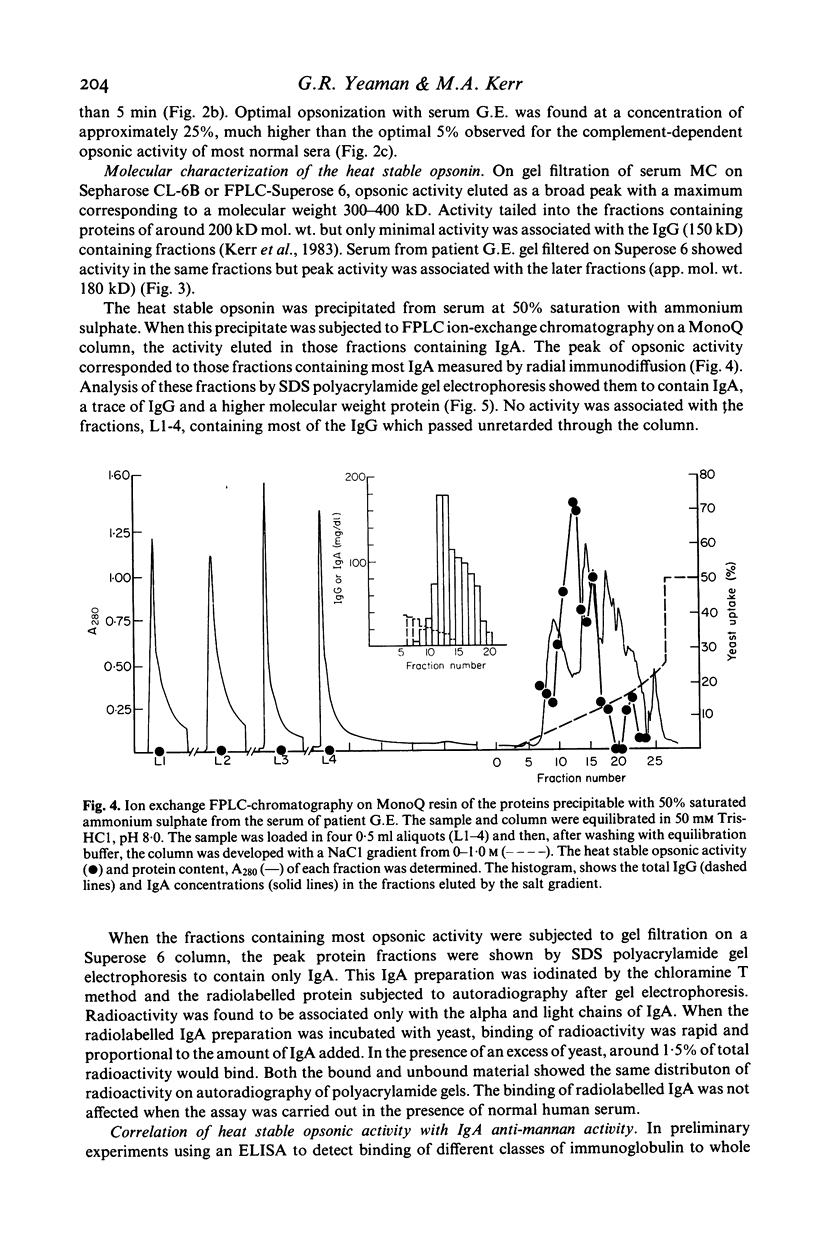
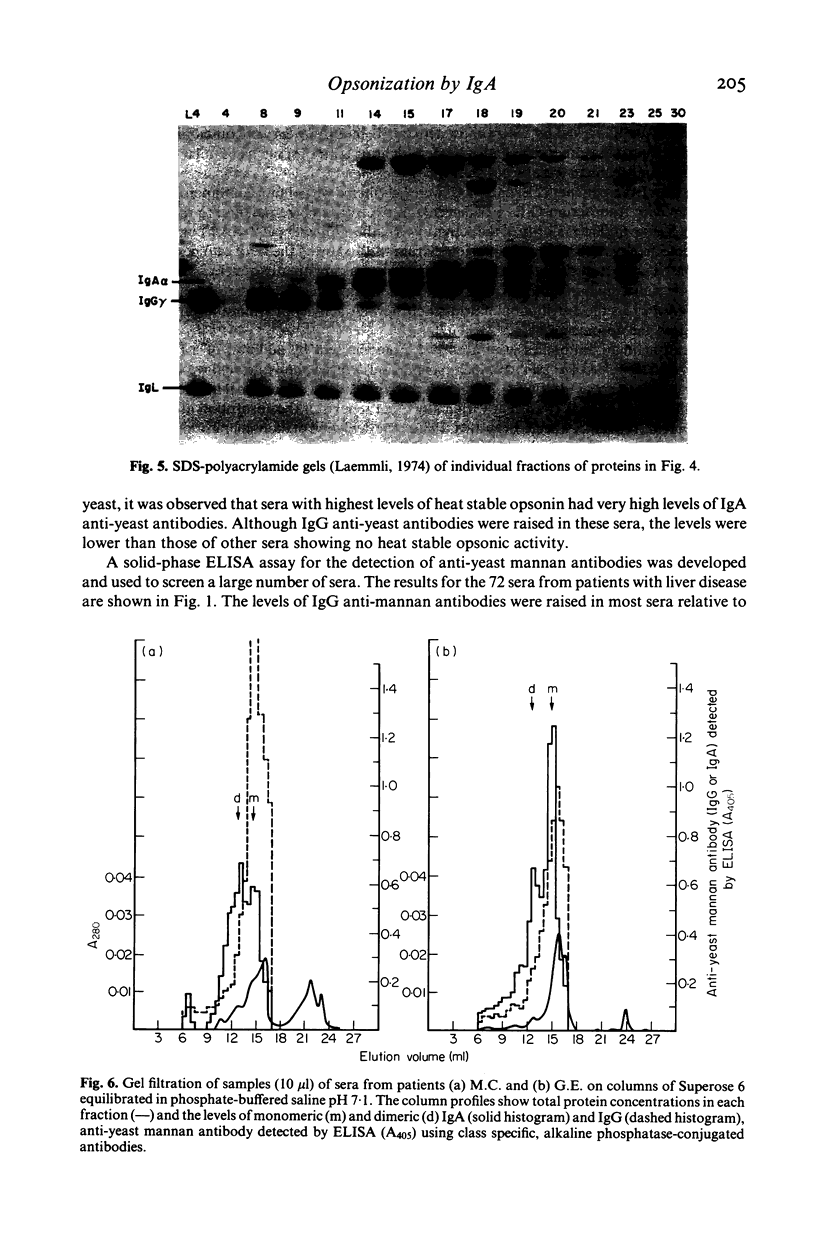
The ability of sera from 72 patients with liver disease to opsonize yeast for phagocytosis by normal polymorphonuclear leucocytes has been studied. Seven showed defective opsonization. The opsonic activity of all but two sera was decreased markedly by heating at 56 degrees C for 1 h. When the two sera with heat stable opsonic activity were fractionated by gel filtration and by ion exchange chromatography, the activity copurified with IgA, not with IgG. The purified IgA, radiolabelled with 125I was shown to bind in a saturable manner to the yeast. Both sera had high levels of anti-yeast mannan IgA detected by an ELISA. In one case most of the anti-mannan activity was due to monomeric IgA, in the other it was dimeric. This was consistent with the observation of an apparent molecular weight of the opsonin of approximately 180 kD in one serum and 300-400 kD in the other.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann A. J., Hong R. Selective IgA deficiency: presentation of 30 cases and a review of the literature. Medicine (Baltimore) 1971 May;50(3):223–236. [PubMed] [Google Scholar]
- Bruin G., Faber A., Biewenga J. Binding of human IgA fragments to protein A-Sepharose studied with an ELISA method. Scand J Immunol. 1985 Jan;21(1):49–54. doi: 10.1111/j.1365-3083.1985.tb01402.x. [DOI] [PubMed] [Google Scholar]
- Burton-Kee J., Morgan-Capner P., Mowbray J. F. Nature of circulating immune complexes in infective endocarditis. J Clin Pathol. 1980 Jul;33(7):653–659. doi: 10.1136/jcp.33.7.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. A., Callicoat P. A., Brenner N. A., Bradley C. A., Smith D. M., Jr Selective IgA deficiency in blood donors. Am J Clin Pathol. 1983 Aug;80(2):210–213. doi: 10.1093/ajcp/80.2.210. [DOI] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Fanger M. W., Goldstine S. N., Shen L. Cytofluorographic analysis of receptors for IgA on human polymorphonuclear cells and monocytes and the correlation of receptor expression with phagocytosis. Mol Immunol. 1983 Sep;20(9):1019–1027. doi: 10.1016/0161-5890(83)90043-3. [DOI] [PubMed] [Google Scholar]
- Fanger M. W., Pugh J., Bernier G. M. The specificity of receptors for IgA on human peripheral polymorphonuclear cells and monocytes. Cell Immunol. 1981 May 15;60(2):324–334. doi: 10.1016/0008-8749(81)90274-4. [DOI] [PubMed] [Google Scholar]
- Fanger M. W., Shen L., Pugh J., Bernier G. M. Subpopulations of human peripheral granulocyes and monocytes express receptors for IgA. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3640–3644. doi: 10.1073/pnas.77.6.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Goroff D. K. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J Immunol. 1983 Jun;130(6):2882–2885. [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Kemp A. S., Turner M. W. The role of opsonins in vacuolar sealing and the ingestion of zymosan by human neutrophils. Immunology. 1986 Sep;59(1):69–74. [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Falconer J. S., Bashey A., Beck J. S. The effect of C3 levels on yeast opsonization by normal and pathological sera: identification of a complement independent opsonin. Clin Exp Immunol. 1983 Dec;54(3):793–800. [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Highison B., Stratton C. J. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun. 1984 Feb;43(2):574–579. doi: 10.1128/iai.43.2.574-579.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magnusson K. E., Stendahl O., Stjernström I., Edebo L. Reduction of phagocytosis, surface hydrophobicity and charge of Salmonella typhimurium 395 MR10 by reaction with secretory IgA (SIgA). Immunology. 1979 Mar;36(3):439–447. [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Johnston R. B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979 Oct;123(4):1839–1846. [PubMed] [Google Scholar]
- Nolan J. P., DeLissio M. G., Camara D. S., Feind D. M., Gagliardi N. C. IgA antibody to lipid A in alcoholic liver disease. Lancet. 1986 Jan 25;1(8474):176–179. doi: 10.1016/s0140-6736(86)90652-5. [DOI] [PubMed] [Google Scholar]
- Quie P. G. Bactericidal function of human polymorphonuclear leukocytes. E. Mead Johnson Award Address. Pediatrics. 1972 Aug;50(2):264–270. [PubMed] [Google Scholar]
- Roos D., Bot A. A., van Schaik M. L., de Boer M., Daha M. R. Interaction between human neutrophils and zymosan particles: the role of opsonins and divalent cations. J Immunol. 1981 Feb;126(2):433–440. [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Soothill J. F., Harvey B. A. Defective opsonization. A common immunity deficiency. Arch Dis Child. 1976 Feb;51(2):91–99. doi: 10.1136/adc.51.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. W., Mowbray J. F., Harvey B. A., Brostoff J., Wells R. S., Soothill J. F. Defective yeast opsonization and C2 deficiency in atopic patients. Clin Exp Immunol. 1978 Nov;34(2):253–259. [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Brown S. L. Inhibition of formylmethionyl-leucyl-phenylalanine-stimulated neutrophil chemiluminescence by human immunoglobulin A paraproteins. Infect Immun. 1981 Dec;34(3):864–870. doi: 10.1128/iai.34.3.864-870.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Strickland R. G., Williams R. C., Jr Inhibitors of leukocyte chemotaxis in alcoholic liver disease. Am J Med. 1975 Aug;59(2):200–207. doi: 10.1016/0002-9343(75)90354-x. [DOI] [PubMed] [Google Scholar]
- Walsh G. M., Kay A. B. Binding of immunoglobulin classes and subclasses to human neutrophils and eosinophils. Clin Exp Immunol. 1986 Feb;63(2):466–472. [PMC free article] [PubMed] [Google Scholar]
- Wilson I. D. Studies on the opsonic activity of human secretory IgA using an in vitro phagocytosis system. J Immunol. 1972 Mar;108(3):726–730. [PubMed] [Google Scholar]
- Wilton J. M. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1978 Dec;34(3):423–428. [PMC free article] [PubMed] [Google Scholar]
- Wyke R. J., Rajkovic I. A., Williams R. Impaired opsonization by serum from patients with chronic liver disease. Clin Exp Immunol. 1983 Jan;51(1):91–98. [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]
- Zipursky A., Brown E. J., Bienenstock J. Lack of opsonization potential of 11S human secretory A. Proc Soc Exp Biol Med. 1973 Jan;142(1):181–184. doi: 10.3181/00379727-142-36983. [DOI] [PubMed] [Google Scholar]



