Abstract
Auxin is the mobile signal controlling the rate of growth and specific aspects of the development of plants. It has been known for over a century that auxins act as the messenger linking plant development to specific environmental changes. An often overlooked aspect of how this is accomplished is the effect of the environment on metabolism of the major plant auxin, indole-3-acetic acid (IAA). We have studied the metabolism of IAA in relation to one environmental variable, growth temperature. The model system used was an inbred line of the aquatic monocot Lemna gibba G-3, 3F7-11 grown at temperatures ranging from 5°C to 35°C. IAA levels, the rate of IAA turnover, and the patterns of label incorporation from IAA precursors were measured using stable isotope-mass spectrometric techniques and were evaluated relative to growth at the experimental temperatures. IAA levels exhibited unusually high variability in plants grown at 15°C and 20°C. Turnover rates were quite rapid throughout the range of experimental temperatures except at 25°C, where IAA turnover was notably slower. These results suggest that a transition occurred over these temperatures for some aspect of IAA metabolism. Analysis of [15N]anthranilate and [2H5]tryptophan (Trp) incorporation into IAA showed that Trp-dependent biosynthesis predominated at 15°C; however, Trp-independent biosynthesis of IAA was the major route to IAA at 30°C. The effects of growth temperature on auxin levels have been reported previously, but no prior studies correlated these effects with which pathway becomes the primary one for IAA production.
Plants, unlike most animals, cannot move to avoid environmental changes and thus, they must rely on other adaptive strategies to cope with a changing and often adverse habitat (Darwin, 1892; Basra, 1994). The perception of environmental signals triggers the specific modulation of metabolic and physical characteristics for optimal growth and survival. Plant hormone metabolism provides plants with a means by which they can transduce signals and adjust growth and development. The response of plants to drought stress mediated through changes in abscisic acid levels is one of the better documented cases of hormone metabolism regulated by environmental conditions (Cornish and Zeevaart, 1985). A long recognized but less well understood response of plants to environmental stress is the repression of growth rate (Berry and Raison, 1981). Temperature-dependent changes in growth can be mediated, in part, by the ability of the plant to respond to auxin (Rayle and Cleland, 1972). In addition, temperature modulation of the rate of plant growth has been correlated to changes in the level of auxin (Gray et al., 1998). Such changes in auxin level could result from a temperature-dependent effect on auxin transport or auxin metabolism.
It is important to recognize that analysis of phytohormone levels only gives a measurement of the steady-state conditions prevailing at the time of analysis, and this measured level should not be viewed as static. Any changes in hormone level must result from modulation of the rates of biosynthetic and/or catabolic events (Epstein et al., 1980). Biosynthetic and catabolic events change in a concerted manner, so the dynamic nature of indole-3-acetic acid (IAA) metabolism is not evident unless one looks at hormone turnover as well as hormone levels. It has been proposed that it may be the flux through the pathway that is important for growth rather than the absolute level of the hormone (Tam et al., 1995).
Early studies that measured the rate of IAA turnover showed that IAA turnover has a turnover time (t1/2) that falls within the range of 1 to 10 h in a variety of plants and tissues ranging from maize (Zea mays) endosperm (Epstein et al., 1980) and tomato (Lycopersicon esculentum) leaves (Nonhebel and Cooney, 1990) to intact Lemna gibba plants (Tam et al., 1995). Recent studies of developmental regulation of IAA turnover in Scots pine (Pinus sylvestris) revealed that there are more substantial differences in IAA t1/2 during various stages of seedling growth (Ljung et al., 2001). Likewise, light conditions appear to have significant effects on IAA turnover (Tam et al., 1998). We show in this paper that yet another environmental variable, temperature, affects IAA turnover. Turnover of IAA in L. gibba was impacted in a temperature-dependent manner from 5°C to 15°C, and in a unique way at higher temperatures.
L. gibba, like other plant species that have been studied, utilizes two different precursors, indole or Trp, for the production of IAA (Rapparini et al., 1999; Slovin et al., 1999). These results, coupled with our earlier results (Baldi et al., 1991; Tam et al., 1998), led us to the hypothesis that temperature-induced changes in turnover might be related to a change in the primary pathway for IAA biosynthesis. Using stable isotope-labeled precursors and following the kinetics of label incorporation into Trp and IAA, we have found that temperature does alter the predominant pathway for IAA biosynthesis. Changes from Trp-dependent to Trp-independent IAA production had previously only been observed as a consequence of normal developmental changes (Michalczuk et al., 1992; Sitbon et al., 2000; Ljung et al., 2001) and following mechanical wounding (Sztein et al., 2002).
RESULTS
Growth Rates at Different Temperatures
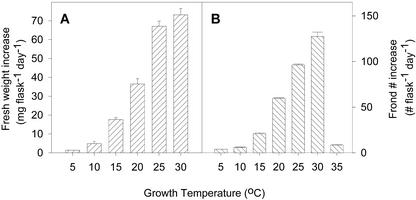
The growth rate of L. gibba increased with temperature from 5°C to 30°C (Fig. 1). Growth rate was expressed as the average increase in fresh weight per day over a period of 18 d for cultures growing at 15°C or above and a period of 38 d for cultures growing at 5°C and 10°C. Likewise, the average increase in number of fronds per flask per day over the same time periods exhibited a similar relationship with temperature. At the lowest temperatures (5°C and 10°C), the plants grew very slowly. When transferred back to room temperature (25°C), plants grown at these low temperatures recovered and grew at the normal rate of growth (data not shown). At 35°C, L. gibba plants grew for about 4 d and then stopped growing. Plants died within 2 d at 40°C.
Figure 1.
A, The effect of growth temperature on fresh weight increase of the 3F7-11 inbred line of L. gibba growing on E medium. Data are expressed as average daily weight increase as determined after 38 d (5°C and 10°C) or 18 d (>10°C). B, The average daily increase in frond number at the different growth temperatures after 12 d of growth at the indicated temperature. Data shown are the mean for four replicate experiments, and bars indicate se.
Free IAA Levels in L. gibba Grown from 5°C to 35°C
Free IAA levels were influenced by growth temperature (Table I), and from 5°C to 15°C, there appears to be a correlation between measured IAA levels and growth rate at the various temperatures. Worth noting is the large amount of variability in the levels of free IAA at two temperatures, 15°C and 20°C, with individual samples showing almost a 400-fold variation. Although there was wide variation among samples at these temperatures, IAA levels tended to group into two or three different classes differing by an order of magnitude, as indicated in Table I. This high degree of variability was seen in each of three replicate experiments. Above 20°C, the levels of IAA are low and do not seem to change significantly in relation to temperature.
Table I.
The effect of different growth temperatures on free IAA levels in L. gibba
| Temperature | Mean | Data Subset |
|---|---|---|
| °C | ng g−1 fresh weight | |
| 5 | 7.8 ± 1.6 (n = 4) | |
| 10 | 11 ± 1 (n = 4) | |
| 15 | 20 ± 8.9 (n = 10) | 58 ± 12 (n = 3)3.5 ± 0.4 (n = 7) |
| 20 | 267 ± 166 (n = 9) | 1,100 ± 1 (n = 2)36 ± 21 (n = 3) |
| 2.8 ± 0.1 (n = 4) | ||
| 25 | 3.4 ± 0.7 (n = 4) | |
| 30 | 2.2 ± 0.1 (n = 4) | |
| 35 | 4.1 ± 0.7 (n = 4) | |
Free IAA levels were measured by gas chromatography/mass spectrometry (GC-MS) analysis using [13C6]IAA as the internal standard. se is given and “n” is the number of samples analyzed. Values obtained at 15°C and 20°C showed a high se resulting from the fact that the levels grouped into two or three highly different subsets of median values, as shown in the third column.
Measurement of the Rate of IAA Turnover
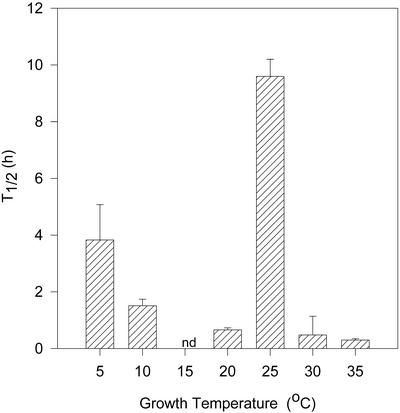
Growth temperature also correlated with the rate of IAA turnover in L. gibba grown at 5°C to 15°C (Fig. 2). At 5°C, the measured turnover rate for IAA in line 3F7-11 varied over a 10-fold range as indicated by the large se. The turnover rate could not be determined at 15°C due to the extremely low levels of labeled [13C6]IAA recovered relative to the endogenous unlabeled IAA pool. These results suggest an extremely rapid rate of turnover at 15°C (t1/2 ≤ 0.1 h) because IAA levels were not significantly different at 15°C from that measured at 10°C or 20°C (Table I). At higher temperatures (30°C and 35°C), IAA turnover rates were also less than 1 h. At 25°C, the turnover time for IAA increased to almost 10 h, indicating enhanced IAA stability in vivo at this growth temperature. Although the t1/2 at 25°C indicated a much slower rate of IAA turnover compared with that measured at all other temperatures, it is comparable with IAA turnover rates reported in other plants (Epstein et al., 1980; Nonhebel and Cooney, 1990; Ljung et al., 2001). The reason for the slower turnover time at 25°C is not known. It is interesting that we had found 25°C to be the optimal growth temperature for long-term culturing of the original L. gibba G-3 parent line, and the inbred line 3F7-11 was isolated from seeds germinated and grown at this temperature.
Figure 2.
The t1/2 of IAA in the 3F7-11 line of L. gibba. Turnover was determined by GC-MS analysis of the endogenous IAA pool enrichment at T0 and after a 1-h incubation of plants that had been prelabeled for 1 h in 10−7 m [13C6]IAA (see “Materials and Methods”). Data shown are the mean for four to 16 replicate experiments, and bars indicate se.
Labeling Studies with [15N]Anthranilate and [2H5]Trp
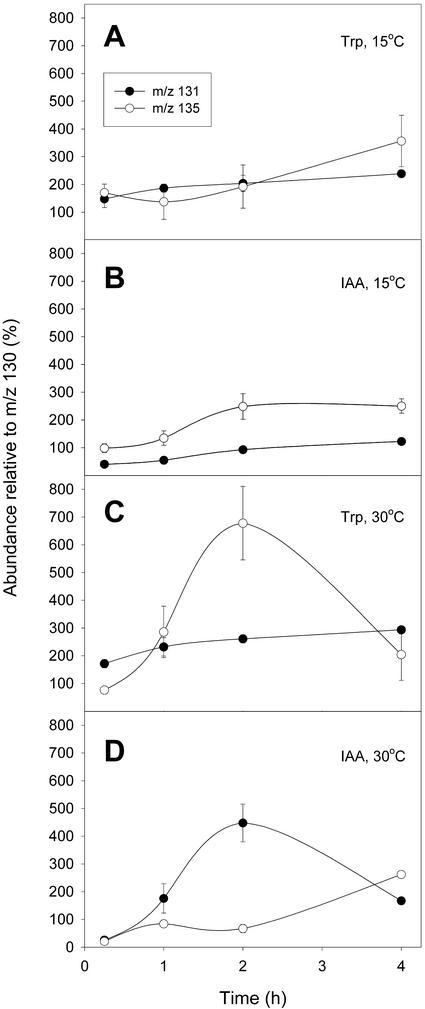
The endogenous pool of Trp was rapidly labeled by [15N]anthranilate or [2H5]Trp at 15°C or 30°C (Fig. 3), with measurable incorporation seen within 15 min of exposure to label. The IAA pool, on the other hand, was more slowly labeled at both growth temperatures. At 30°C, minimal labeling of IAA from either precursor was observed in the first sample period, and incorporation was not significantly above background until 1 h. At 30°C, the IAA pool was more enriched in [15N] from anthranilate after 2 h of incubation and was less enriched in [2H] from Trp than was the Trp pool, indicating that IAA synthesis was predominantly Trp independent. In contrast, at 15°C, there is no clear preferential labeling of the IAA pool prior to precursor labeling of the Trp pool, indicating that Trp-dependent IAA biosynthesis was the predominant pathway utilized. The Trp pool was about as enriched in [15N] from anthranilate after 2 h as the IAA pool. Thus, different pathways for IAA synthesis appear to operate at different temperature conditions. A Trp-dependent pathway to IAA appears to be active when the plant is exposed to the lower temperature (15°C), whereas based on the higher [15N] enrichment of IAA relative to Trp, a Trp-independent pathway to IAA is the principal biosynthetic route involved at the higher temperature (30°C).
Figure 3.
[2H5]Trp and [15N]anthranilic acid labeling of IAA (B and D) and Trp (A and C) pools in L. gibba at low and high temperatures. When L. gibba was grown at 30°C, the IAA pool became enriched in [15N] from [15N]anthranilic acid (as shown by the enrichment at m/z 131) well before conversion of [2H5]Trp to [2H5]IAA could be observed (at m/z 135). These data show that during normal growth at 30°C, the Trp-independent pathway predominates in these plants. However, when L. gibba plants were grown at 15°C, label from exogenous [2H5]Trp labeled the IAA pool at the same time as label from [15N]anthranilic acid, and to a greater extent. This suggests that the Trp-dependent pathway is active under these conditions. Data are the mean from four or more replicate experiments, and error bars, when greater than the marked point, indicate se.
DISCUSSION
Environmental factors such as temperature are known to affect many processes involved in the growth and development of plants (Fowden et al., 1993; Basra, 1994). Enzymatic and nonenzymatic reactions in plant cells, as well as physical structures such as membranes, can be directly affected by temperature. The net result of temperature change is often seen as a change in growth rate, although a number of physiological aberrations can also result. Hormones have long been known to play a role in growth and physiological processes, and have been postulated to exhibit control over basic metabolism (Bandurski, 1980; Xiao et al., 2000) as well as altering patterns of development. Therefore, a major goal in studies of environmental physiology is to understand the link between environment and phytohormone signaling. Advances in methodology (Rapparini et al., 1999) as well as an improved understanding of auxin biochemistry have enabled us to use the L. gibba model system (Slovin and Cohen, 1988; Tam et al., 1998) for detailed analysis of the interaction of environment and auxin metabolism. The results reported in this paper show that the temperature of the growth environment alters two aspects of IAA metabolism, turnover and biosynthetic route.
Changes in temperature affected growth rate over the entire range of growth temperatures studied. It is often assumed, as a simple model for plant growth control, that under conditions where auxin is regulating growth, the higher the concentration of IAA, the greater the growth rate. Thus, the increased growth rate noted with increasing growth temperature would suggest, based on this simple model, that the levels of free IAA should also go up with growth temperature. However, the levels of free IAA in L. gibba at higher growth temperatures did not increase and were actually somewhat less than at 20°C or lower, even though the growth rate increased more than 10-fold between 5°C and 30°C (Fig. 1; Table I).
IAA levels can vary greatly over the culture cycle even though the growth rate in L. gibba remains constant (Slovin and Cohen, 1988). These observations may be related to changing culture conditions such as population density. In the studies presented here, great care was taken to measure IAA levels and turnover at the same stage in the culture cycle. However, large variations in IAA levels were measured in repeated analyses of plants grown at 15°C and 20°C.
The level of free IAA in plants is the net result of the balance between IAA biosynthesis and catabolism. Thus, it would be expected that only when the temperature differentially affects the rate of biosynthesis or catabolism of IAA will free IAA levels show a difference. Over a range of growth temperatures, we detected changes in rates of IAA production and catabolism as measured by t1/2. This is particularly evident at 15°C and 25°C, where turnover is much more rapid or much slower, respectively, than at other temperatures (Fig. 2). Although IAA levels exhibited great variability at 15°C and 20°C, the measured rates of turnover did not show a similar variation at these temperatures, suggesting that the rates of turnover and absolute levels of IAA were not directly correlated under these conditions.
The lack of a correlated change in IAA levels with changes in growth rate at different temperatures, the high degree of variation in IAA levels at moderate temperatures, and the shift in turnover time at 25°C (Fig. 2) suggests the possibility that some metabolic change is occurring at a transition point between 15°C and 25°C. The high variability in IAA levels in the transition between low temperatures and elevated temperatures in L. gibba was accompanied by a major change in the labeling pattern from [2H5]Trp and [15N]anthranilate into the free Trp and IAA pools. These results are diagnostic of a switch from Trp-dependent IAA production at lower temperatures to Trp-independent biosynthesis at elevated temperatures. Because of the large number of consistent samples necessary to determine the labeling pattern over time, it was not practical to attempt to capture the statistical phenomenon accounting for the variation seen in IAA levels at transition temperatures (20°C–25°C). However, based on the results of the labeling patterns seen at 15°C and 30°C, the activation of more than one pathway and its concomitant alteration in set point for IAA levels seems likely to account for the high degree of variation in IAA levels measured at 20°C and 25°C. This implies that in L. gibba, as was reported for seedlings of Scots pine (Ljung et al., 2001), both pathways are operational at moderate temperatures. This conclusion is further supported by the labeling patterns from [15N]anthranilate and [2H5]Trp reported in our previous study with L. gibba. At 25°C, the labeling of the IAA pool preceded label incorporation into the Trp pool by only a few minutes, suggesting that the Trp-dependent and -independent pathways were operational (Rapparini et al., 1999).
To measure the operation of two different IAA biosynthetic pathways in the same plant, it was necessary to design labeling studies that minimally perturbed endogenous auxin metabolism and also to then carefully interpret the resulting enrichments (Rapparini et al., 1999). When these conditions are not taken into account, serious misinterpretations may result. For example, in their studies, Müller et al. (1998) applied labeled Trp and indoleacetonitrile at levels that were 15 to 25 times higher than the endogenous levels of these compounds (Tam et al., 1995; Ilić et al., 1996). From these high-level feeding studies, the authors concluded that IAA was produced only from Trp. This result does not take into account the fact that IAA is a common catabolic product of excess Trp (Epstein et al., 1980). Nevertheless, carefully designed isotopic experiments have demonstrated the operation of only the Trp-dependent IAA biosynthesis pathway in maize kernels (Glawischnig et al., 2000). The results confirmed previous in vitro enzyme studies that indicated that the Trp-dependent and -independent pathways were active at different times in development in maize (Ilić et al., 1999; Östin et al., 1999).
Our results suggest several hypotheses that need further testing. Differential utilization of Trp-dependent and Trp-independent IAA biosynthesis pathways may be a component of growth control. Although no obvious developmental program in L. gibba correlates with the changes noted at moderate temperatures, the predominance of Trp-dependent IAA production at 15°C may reflect the low temperature induction of the formation of the resting forms of the fronds known as turions. Pathway specificity might result from changes in the subcellular localization or changes in the tissues where IAA is produced. Previous work suggested that Trp-dependent IAA biosynthesis occurs in the cytoplasm and that Trp-independent IAA production may be plastid localized (Rapparini et al., 1999). The results from studies on developmental changes in pathway (Michalzcuk et al., 1992; Sitbon et al., 2000; Ljung et al., 2001) would be consistent with specific tissues producing IAA at critical developmental stages.
Another hypothesis that requires further study is the idea that higher steady-state IAA levels correlate with Trp-dependent IAA production (Ribnicky et al., 2001). The results from carrot (Daucus carota) somatic embryos (Michalzcuk et al., 1992) and the data presented here for L. gibba plants grown at 5°C to 15°C compared with 25°C to 35°C are consistent with this idea. However, a change in IAA pathway occurs in tomato pericarp tissue during ripening (Epstein et al., 2002) and in Scots pine seedlings during germination (Ljung et al., 2001) without concomitant significant changes in free IAA level. Thus, the relationship between IAA levels and pathways does not appear to be a simple one.
The discovery of plant hormones owes its origins to the responses of plants to gravity and light (Darwin, 1892). Auxin in plants has now been closely tied to such interactions between plants and the environment for more than a century. With the finding of multiple pathways for the biosynthesis of IAA, questions arose regarding the need for pathway redundancy (Normanly and Bartel, 1999). The differential use of biosynthetic route illustrated in our current data, together with the finding that Trp-dependent IAA biosynthesis in germinating bean (Phaseolus vulgaris) axes is activated by wounding (Sztein et al., 2002), makes it clear that environmental as well as development signals for plant growth and development involve a far more complex process than previously envisioned. How the selective activation of specific pathways for IAA biosynthesis relates to the ability of plants to sense and respond remains an important question.
MATERIALS AND METHODS
Plant Materials
A near-isogenic line of Lemna gibba G-3, 3F7-11 (Tam et al., 1995) was grown aseptically on 50 mL of liquid E medium in 125-mL flasks (Cleland and Briggs, 1967). Cultures were kept under continuous light that was provided by a mixture of cool-white fluorescent and incandescent lamps at 25 μEm−2 s−1 (Slovin and Cohen, 1988).
Growth Rate Measurements
Cultures, started with two pairs of mother and daughter fronds of 3F7-11, were maintained on lighted wire racks in large walk-in chambers at the indicated temperatures. The number of visible fronds was determined every 2 d, and fresh weights were determined after 18 or 34 d of growth.
Growth of Plants for Measurement of IAA Levels, Turnover, and Biosynthesis
All cultures were kept at the experimental temperatures for 10 d, except for the 35°C treatment, which was given for 3 d. To adjust for the difference in growth rate at the different temperatures, two pairs of mother and daughter fronds of 3F7-11 were grown in 125-mL flasks with E medium at 25°C for various time periods of 6 to 14 d before transfer to the experimental temperature. Therefore, IAA analyses and turnover measurements were done on plants that had received a temperature treatment for the same amount of time and that were at the same stage in the culture cycle at the time of analysis. For example, for the 5°C treatment plants were grown at 25°C for 14 d and were then transferred to 5°C for 10 d. For the 30°C treatment, plants were grown at 25°C for 6 d, and were then transferred to 30°C for 10 d. However, plants were grown for 16 d at 25°C and were transferred to 35°C for only 3 d because we had determined that the plants stop growing after about 4 d at 35°C. These protocols were necessary because previous studies had shown that IAA levels were not constant throughout the culture period at 25°C (Slovin and Cohen, 1988).
Measurement of Free IAA Levels
Approximately 0.5 g of tissue (fresh weight) from cultures grown at each temperature was used for determination of free IAA levels. [13C6]IAA was used as internal standard and [3H]IAA was used as tracer for GC-MS analysis, as described in Tam et al. (1995).
Measurement of the Rate of IAA Turnover
The IAA turnover rates in L. gibba growing at the various temperatures were determined as previously described (Tam et al., 1995). In brief, the technique involved labeling fronds with [13C6]IAA and determining the enrichment of labeled IAA in the endogenous IAA pool after the initial labeling period (T0) and again after 1 or more hours of incubation (Tt). These studies used 370 ng of [13C6]IAA in 20 mL of E medium (10−7 m) with a 1-h labeling period followed by a 1-h period on E medium at the indicated temperatures.
Analysis of Stable Isotope Labeling Patterns
The samples were injected using the split-splitless mode into a 5890 gas chromatograph/5970 quadrupole mass spectrometer (Hewlett Packard, Palo Alto, CA) equipped with a 60 m, 0.25 mm i.d. capillary column (HP5; Hewlett Packard) coated with a 0.25-μm film of phenylmethylsiloxane. The injector temperature was 270°C. The column temperature was held at 90°C for 0.5 min, then increased by 20°C min−1 to 180°C, by 5°C min−1 to 220°C, and by 20°C min−1 to 280°C, and then held for 3 min. All other conditions for analysis of Trp and IAA labeling were as described previously (Rapparini et al., 1999).
ACKNOWLEDGMENTS
We thank Dr. Jennifer Normanly (University of Massachusetts, Amherst), Dr. Jutta Ludwig-Müller (Technische Universitat Dresden, Dresden, Germany), and anonymous reviewers for helpful comments on the manuscript. This paper is dedicated to the memory of our colleague, teacher, and friend, Prof. David L. Rayle. His contagious enthusiasm for science and for all things related to auxin will be greatly missed.
Footnotes
This work was supported in part by the U.S. Department of Energy (grant DE–FG020–00ER15079), by funds from the U.S. Department of Agriculture-Agricultural Research Service, by the Minnesota Agricultural Experiment Station, and by the Gordon and Margaret Bailey Endowment for Environmental Horticulture.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011005.
LITERATURE CITED
- Baldi BG, Maher BR, Slovin JP, Cohen JD. Stable isotope labeling, in vivo, of d and l tryptophan pools in Lemna gibba and the low incorporation of label into indole-3-acetic acid. Plant Physiol. 1991;95:1203–1208. doi: 10.1104/pp.95.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski RS. Homeostatic control of concentration of indole-3-acetic acid. In: Skoog F, editor. Plant Growth Substances 1979. New York: Springer-Verlag; 1980. pp. 37–49. [Google Scholar]
- Basra AS. Stress-Induced Gene Expression in Plants. Chur, Switzerland: Harwood Academic Publishers; 1994. [Google Scholar]
- Berry JA, Raison JK. Responses of macrophytes to temperature. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Physiological Plant Ecology I: Responses to the Physical Environment. Encyclopedia of Plant Physiology, New Series. 12A. Berlin: Springer-Verlag; 1981. pp. 277–338. [Google Scholar]
- Cleland CF, Briggs WR. Flowering responses of the long-day plant Lemna gibba G-3. Plant Physiol. 1967;42:1553–1561. doi: 10.1104/pp.42.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Zeevaart JAD. Abscisic acid accumulation by roots of Xanthium strumarium L. and Lycopersicon esculentum Mill in relation to water stress. Plant Physiol. 1985;79:653–658. doi: 10.1104/pp.79.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The Power of Movement in Plants. D. New York: Appleton and Company; 1892. [Google Scholar]
- Epstein E, Cohen JD, Bandurski RS. Concentration and metabolic turnover of indoles in germinating kernels of Zea mays L. Plant Physiol. 1980;65:415–421. doi: 10.1104/pp.65.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Cohen JD, Slovin JP (2002) Studies of IAA biosynthesis in tomato fruit using stable isotope labeled anthranilic acid, indole and tryptophan. Plant Growth Regul (in press)
- Fowden L, Mansfield T, Stoddart J. Plant Adaptation to Environmental Stress. London: Chapman and Hall; 1993. [Google Scholar]
- Glawischnig E, Tomas A, Eisenreich W, Spiteller P, Bacher A, Gierl A. Auxin biosynthesis in maize kernels. Plant Physiol. 2000;123:1109–1119. doi: 10.1104/pp.123.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Östin A, Sandberg G, Romano CP, Estelle M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:7197–7202. doi: 10.1073/pnas.95.12.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilić N, Normanly J, Cohen JD. Quantification of free plus conjugated indole-3-acetic acid in Arabidopsis requires correction for the non-enzymatic conversion of indolic nitriles. Plant Physiol. 1996;111:781–788. doi: 10.1104/pp.111.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilić N, Östin A, Cohen JD. Differential inhibition of IAA and tryptophan biosynthesis by indole analogues: tryptophan-dependent IAA biosynthesis. Plant Growth Regul. 1999;27:57–62. [Google Scholar]
- Ljung K, Östin A, Lioussanne L, Sandberg G. Developmental regulation of indole-3-acetic acid turnover in Scots pine seedlings. Plant Physiol. 2001;125:464–475. doi: 10.1104/pp.125.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczuk L, Ribnicky DM, Cooke TJ, Cohen JD. Regulation of indole-3-acetic acid biosynthesis in carrot cell cultures. Plant Physiol. 1992;100:1346–1353. doi: 10.1104/pp.100.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Hillebrand H, Weiler EW. Indole-3-acetic acid is synthesized from l-tryptophan in roots of Arabidopsis thaliana. Planta. 1998;206:362–369. doi: 10.1007/s004250050411. [DOI] [PubMed] [Google Scholar]
- Nonhebel HM, Cooney TP. Pharis RP, Rood SB, eds, Plant Growth Substances 1988. Berlin: Springer-Verlag; 1990. Measurement of the in vivo rate of indole-3-acetic acid turnover; pp. 333–340. [Google Scholar]
- Normanly J, Bartel B. Redundancy as a way of life: IAA metabolism. Curr Opin Plant Biol. 1999;2:207–213. doi: 10.1016/s1369-5266(99)80037-5. [DOI] [PubMed] [Google Scholar]
- Östin A, Ilić N, Cohen JD. An in vitro system for tryptophan-independent indole-3-acetic acid biosynthesis from Zea mays seedlings. Plant Physiol. 1999;119:173–178. doi: 10.1104/pp.119.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapparini F, Cohen JD, Slovin JP. Indole-3-acetic acid biosynthesis in Lemna gibba studied using stable isotope labeled anthranilate and tryptophan. Plant Growth Regul. 1999;27:139–144. [Google Scholar]
- Rayle DL, Cleland R. Carr DJ, ed, Plant Growth Substances 1970. Berlin: Springer-Verlag; 1972. Rapid growth responses in the Avena coleoptile: a comparison of the action of hydrogen ions, CO2, and auxin; pp. 44–51. [Google Scholar]
- Ribnicky DM, Cohen JD, Hu W-S, Cooke TJ. An extraordinary auxin surge following fertilization in carrot: its significance for plant totipotency. Planta. 2002;214:505–509. doi: 10.1007/s004250100639. [DOI] [PubMed] [Google Scholar]
- Sitbon F, Astot C, Edlund A, Crozier A, Sandberg G. The relative importance of tryptophan-dependent and tryptophan-independent biosynthesis of indole-3-acetic acid in tobacco during vegetative growth. Planta. 2000;211:715–721. doi: 10.1007/s004250000338. [DOI] [PubMed] [Google Scholar]
- Slovin JP, Bandurski RS, Cohen JD. Control of hormone synthesis and metabolism: auxins. In: Hooykaas PJJ, Hall MA, Libbenga KR, editors. Biochemistry and Molecular Biology of Plant Hormones. Amsterdam, The Netherlands: Elsevier; 1999. pp. 115–140. [Google Scholar]
- Slovin JP, Cohen JD. Levels of indole-3-acetic acid in Lemna gibba G-3 and in a large Lemna mutant regenerated from tissue culture. Plant Physiol. 1988;86:522–526. doi: 10.1104/pp.86.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztein AE, Cohen JD, Cooke TJ (2002) Indole-3-acetic acid biosynthesis in isolated axes from germinating bean seeds: the effect of wounding on the biosynthetic pathway. Plant Growth Regul (in press)
- Tam YY, Slovin JP, Cohen JD. Selection and characterization of α-methyltryptophan-resistant lines of Lemna gibba showing a rapid rate of indole-3-acetic acid turnover. Plant Physiol. 1995;107:77–85. doi: 10.1104/pp.107.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam YY, Slovin JP, Cohen JD. Continuous light alters indole-3-acetic acid metabolism in Lemna gibba. Phytochemistry. 1998;49:17–21. [Google Scholar]
- Xiao W, Sheen J, Jang JC. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol. 2000;44:451–461. doi: 10.1023/a:1026501430422. [DOI] [PubMed] [Google Scholar]