Abstract
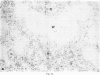
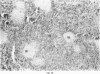
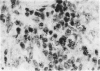
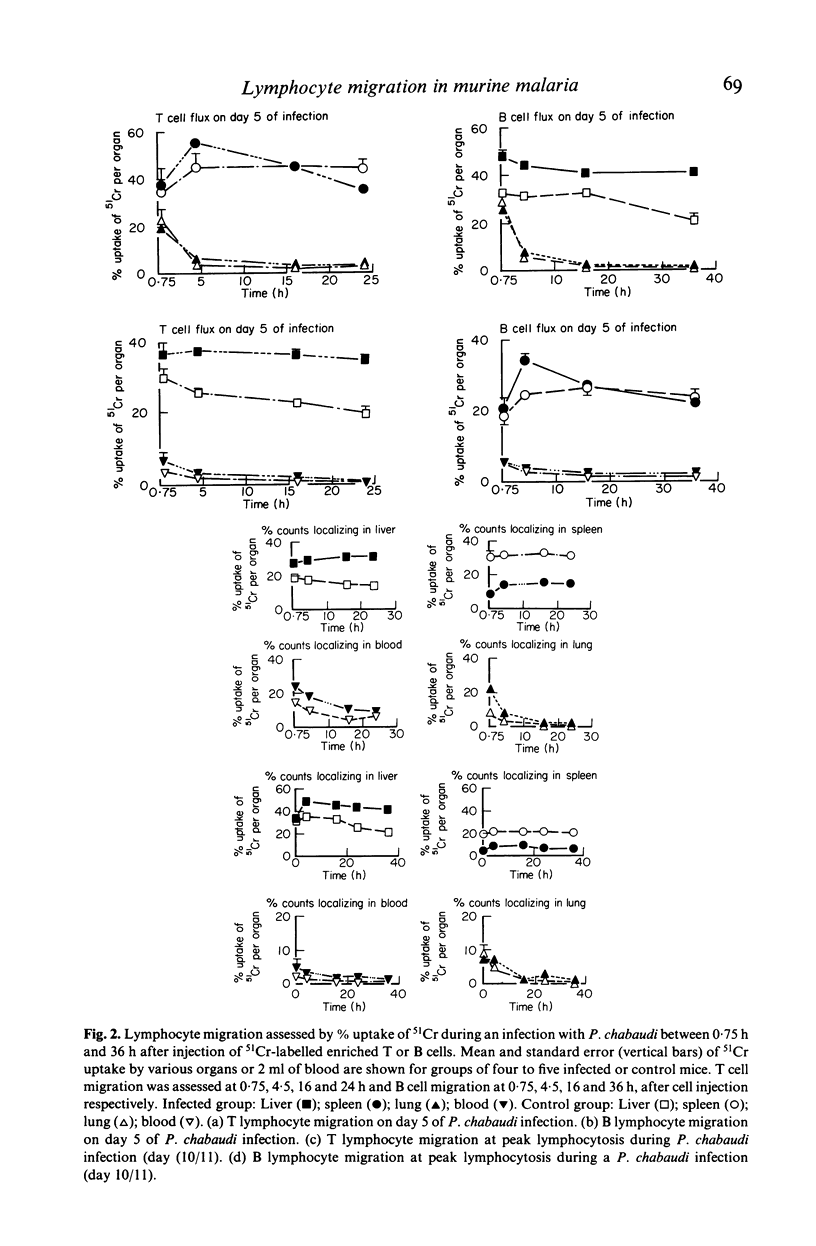
Inoculation of adult C57/BC mice with 10(6) red cells infected with Plasmodium chabaudi induces an acute primary parasitaemia peaking around the 8th or 9th day and lasting 10-14 days. Concomitantly, the spleen enlarges to reach 6-7 times its normal weight by the 11th day. The major component of this increase is between day 9 and 11, due primarily to an increase in erythropoietic cells in the red pulp. Although initially the white pulp increases in size, by day 11 it shows partial lymphocyte depletion which coincides with the occurrence of massive absolute lymphocytosis in the peripheral blood. 3H-Thymidine labelling in vivo suggests that this lymphocytosis is not due to lymphocytopoiesis. Collectively, these findings suggest a redistribution of lymphocytes. Lymphocyte migration was investigated around peak parasitaemia, using enriched populations of T and B cells labelled with 51Cr. The traffic patterns of these cells were followed over 36 h. These studies show decreased uptake (or decreased retention) of T and B cells by spleens of infected mice. Concomitantly, there is increased retention of T and B cells in the liver and lungs of infected mice, suggesting a complex redistribution of these cells. Lymphocyte migration to lymph nodes was unimpaired in these animals. Similar changes in T and B cell migration do not occur in Babesia microti infections in C57/BL mice. We relate our findings to histological and histochemical changes in the liver and spleen of malarious mice and discuss the significance of these findings to immunosuppression in malaria and to the development of parasiticidal immunity.
Full text
PDF


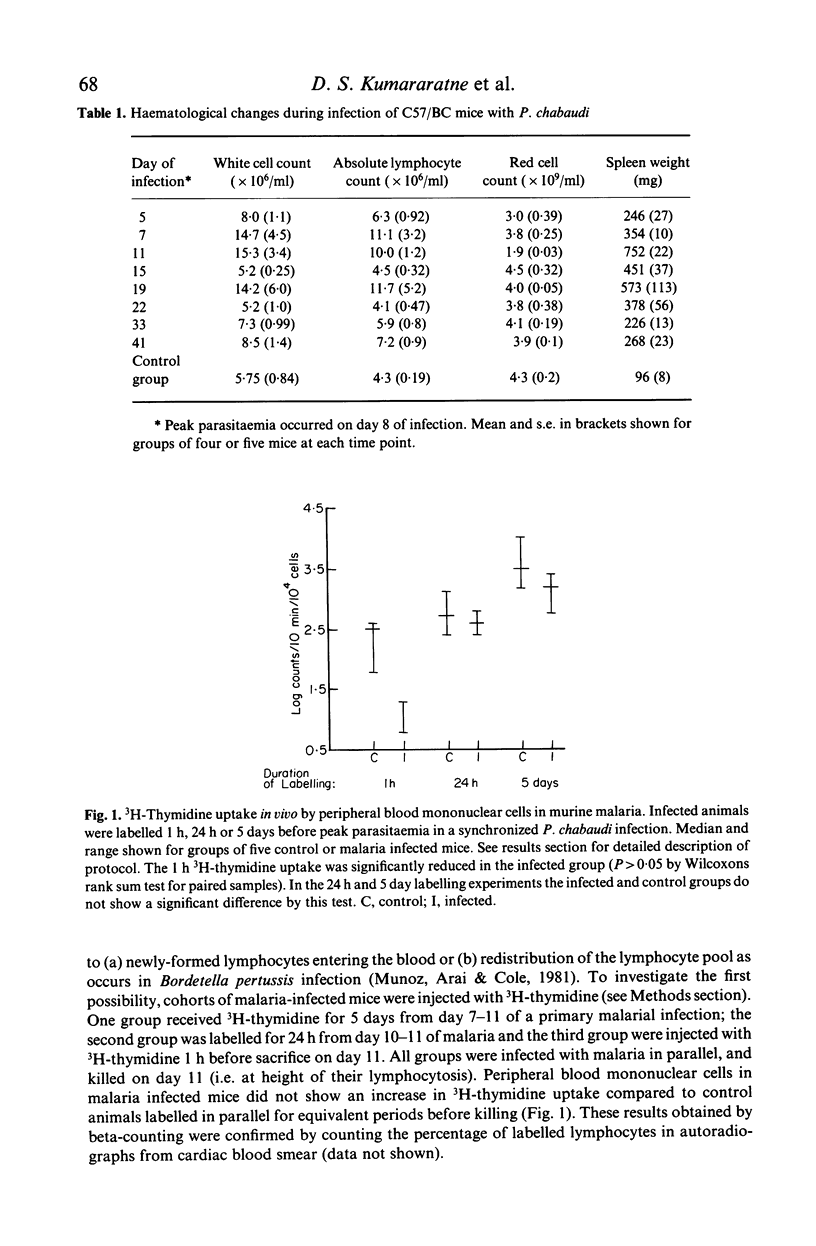






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Bitzan M., Spira D. T. Lymphocyte trapping and malaria in Plasmodium-berghei-infected mice. Isr J Med Sci. 1978 Jun;14(6):673–681. [PubMed] [Google Scholar]
- Brinkmann V., Kaufmann S. H., Simon M. M. T-cell-mediated immune response in murine malaria: differential effects of antigen-specific Lyt T-cell subsets in recovery from Plasmodium yoelii infection in normal and T-cell-deficient mice. Infect Immun. 1985 Mar;47(3):737–743. doi: 10.1128/iai.47.3.737-743.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette W. H., Coleman R. M., Rencricca N. J. Depressed splenic T lymphocyte numbers and thymocyte migratory patterns in murine malaria. Proc Soc Exp Biol Med. 1978 Nov;159(2):317–320. doi: 10.3181/00379727-159-40340. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Clouston W. M. Effects of endotoxin on the histology of intact and athymic mice infected with Plasmodium vinckei petteri. J Pathol. 1980 Jul;131(3):221–233. doi: 10.1002/path.1711310304. [DOI] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of Plasmodium yoelii by enzyme-induced products of the oxidative burst. Infect Immun. 1984 Feb;43(2):451–456. doi: 10.1128/iai.43.2.451-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Seymour G. J., Playfair J. H., Greenspan J. S. Cytochemical identification of T and B cells in situ in mouse lymphoid tissue and lymph nodes from the rat, gerbil and cat. Ann Immunol (Paris) 1978 Jul-Sep;129 100(5):617–633. [PubMed] [Google Scholar]
- Ford W. L. Lymphocyte migration and immune responses. Prog Allergy. 1975;19:1–59. doi: 10.1159/000313381. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Smith M. E. Experimental approaches to lymphocyte traffic: pitfalls of the tracer sample method. Adv Exp Med Biol. 1982;149:139–145. doi: 10.1007/978-1-4684-9066-4_19. [DOI] [PubMed] [Google Scholar]
- Gray G. D., Phillips R. S. Suppression of primary and secondary antibody responses and inhibition of antigen priming during Babesia microti infections in mice. Parasite Immunol. 1983 Mar;5(2):123–134. doi: 10.1111/j.1365-3024.1983.tb00730.x. [DOI] [PubMed] [Google Scholar]
- Gray G. D., Phillips R. S. Use of sorbitol in the cryopreservation of babesia. Res Vet Sci. 1981 May;30(3):388–389. [PubMed] [Google Scholar]
- Greenwood B. M., Bradley-Moore A. M., Bryceson A. D., Palit A. Immunosuppression in children with malaria. Lancet. 1972 Jan 22;1(7743):169–172. doi: 10.1016/s0140-6736(72)90569-7. [DOI] [PubMed] [Google Scholar]
- Grun J. L., Long C. A., Weidanz W. P. Effects of splenectomy on antibody-independent immunity to Plasmodium chabaudi adami malaria. Infect Immun. 1985 Jun;48(3):853–858. doi: 10.1128/iai.48.3.853-858.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. V., Fowler M. H. A repopulation assay for B and T lymphocyte stem cells employing radiation chimaeras. Cell Tissue Kinet. 1981 Jul;14(4):445–464. doi: 10.1111/j.1365-2184.1981.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Hussein H. S. The pathology of Babesia hylomysci infection in mice I. Clinical signs and liver lesion. J Comp Pathol. 1977 Apr;87(2):161–167. doi: 10.1016/0021-9975(77)90002-0. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F., Doenhoff M. J., Snajdr J. Lymphocyte activation. V. Quantitation of the proliferative responses to mitogens using defined T and B cell populations. Clin Exp Immunol. 1973 Aug;14(4):581–596. [PMC free article] [PubMed] [Google Scholar]
- KRETSCHMAR W., JERUSALEM C. MILZ UND MALARIA. DER INFEKTIONSVERLAUF (PLASMODIUM BERGHEI) IN SPLENEKTOMIERTEN NMRI-MAEUSEN UND SEINE DEUTUNG ANHAND DER HISTOPATHOLOGISCHEN VERAENDERUNGEN DER MILZ NICHTSPLENEKTOMIERTER MAEUSE. Z Tropenmed Parasitol. 1963 Oct;14:279–310. [PubMed] [Google Scholar]
- Kumararatne D. S., Gray D., MacLennan I. C., Lortan J., Platteau B., Bazin H. The paradox of high rates of B cell production in bone marrow and the longevity of most mature B cells. Adv Exp Med Biol. 1985;186:73–80. doi: 10.1007/978-1-4613-2463-8_9. [DOI] [PubMed] [Google Scholar]
- McDonald V., Phillips R. S. Plasmodium chabaudi in mice. Adoptive transfer of immunity with enriched populations of spleen T and B lymphocytes. Immunology. 1978 May;34(5):821–830. [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Cole R. L. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1981 Apr;32(1):243–250. doi: 10.1128/iai.32.1.243-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C. D., McLean S. A., Tetley K., Phillips R. S. Induction of secondary antibody responses to Plasmodium chabaudi in vitro. Clin Exp Immunol. 1983 Apr;52(1):121–128. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., de Souza J. B. Lymphocyte traffic and lymphocyte destruction in murine malaria. Immunology. 1982 May;46(1):125–133. [PMC free article] [PubMed] [Google Scholar]
- Strickland G. T., DeSilva S., Sayles P. C. Lymphocyte changes in murine and human malaria. Tropenmed Parasitol. 1979 Mar;30(1):35–42. [PubMed] [Google Scholar]
- Van Zon A., Eling W., Jerusalem C. Histo- and immunopathology of a malaria (Plasmodium berghei) infection in mice. Isr J Med Sci. 1978 Jun;14(6):659–672. [PubMed] [Google Scholar]
- Weidanz W. P. Malaria and alterations in immune reactivity. Br Med Bull. 1982 May;38(2):167–172. doi: 10.1093/oxfordjournals.bmb.a071754. [DOI] [PubMed] [Google Scholar]
- Wyler D. J. Malaria: host-pathogen biology. Rev Infect Dis. 1982 Jul-Aug;4(4):785–797. doi: 10.1093/4.4.785. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Quinn T. C., Chen L. T. Relationship of alterations in splenic clearance function and microcirculation to host defense in acute rodent malaria. J Clin Invest. 1981 May;67(5):1400–1404. doi: 10.1172/JCI110168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]