Abstract
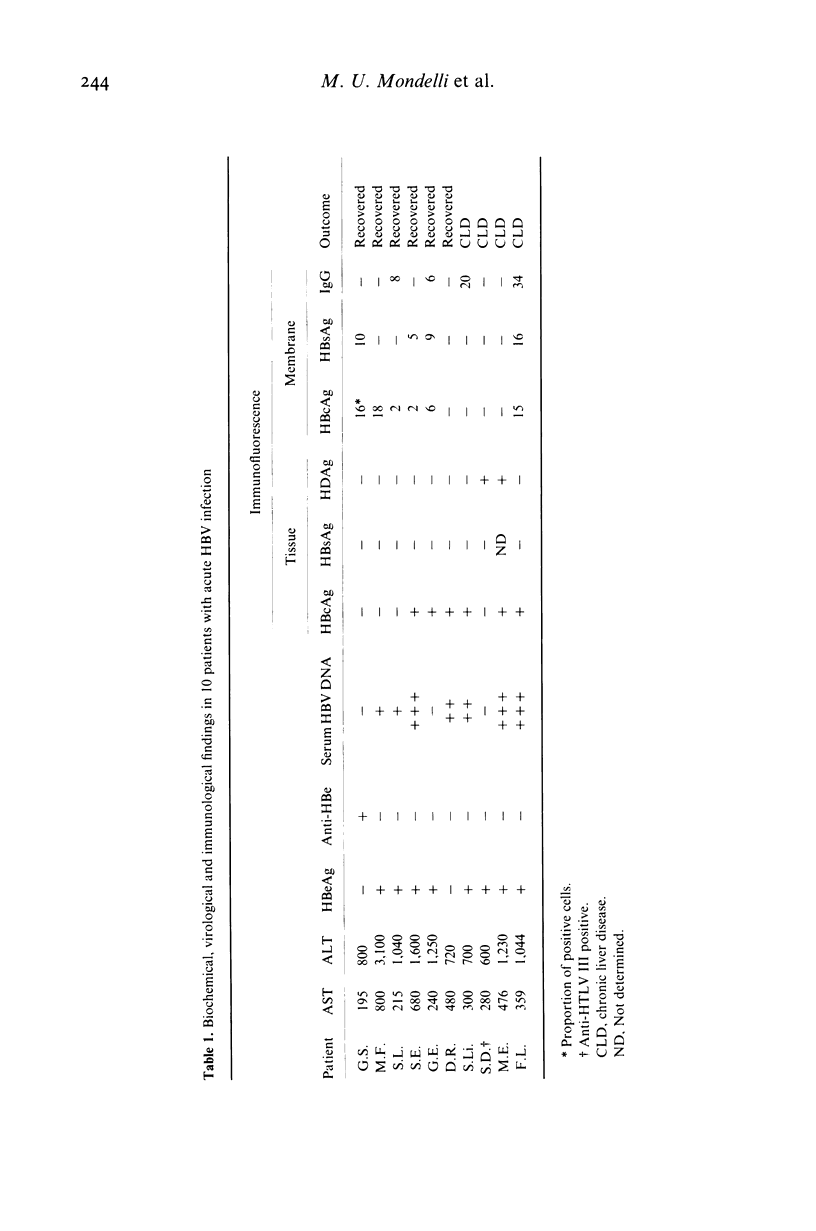
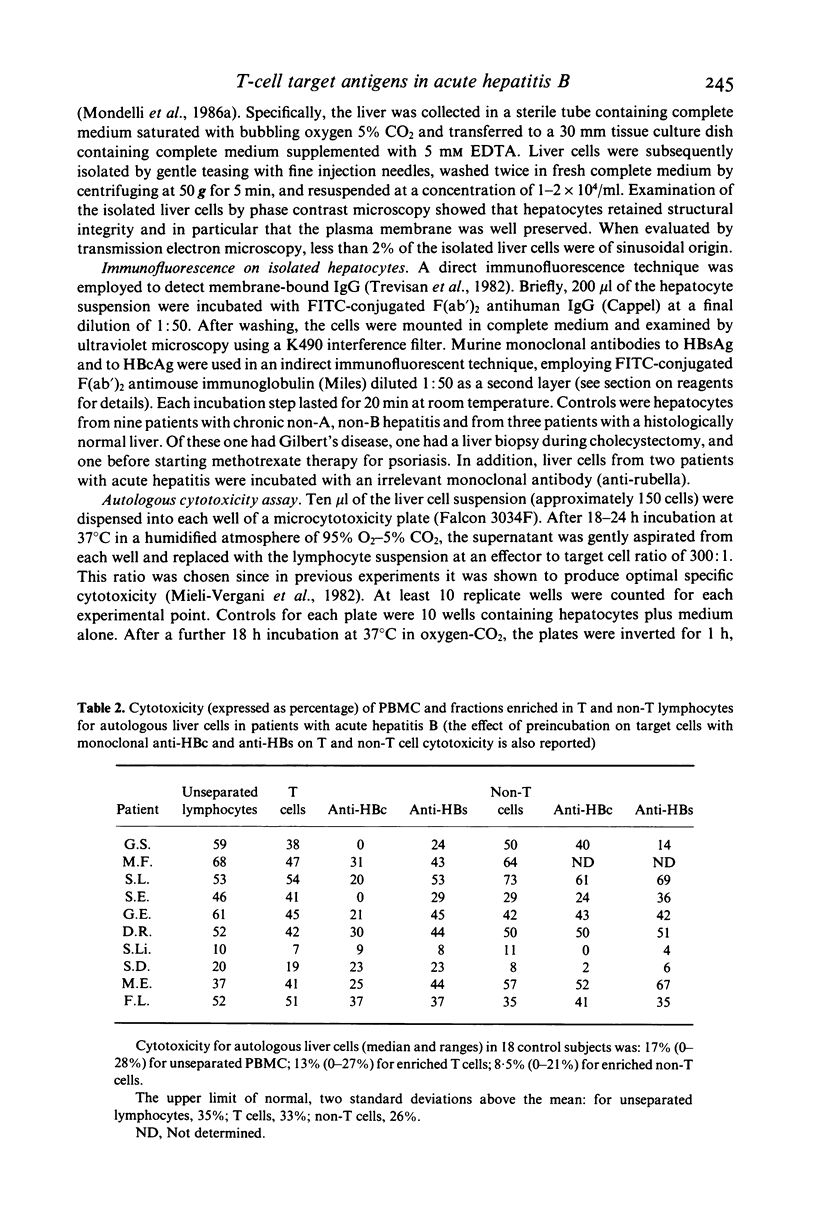
We have investigated the specificity of cell-mediated cytotoxicity for autologous liver cells in 10 patients with acute hepatitis B in relation to the expression of hepatitis B virus (HBV) antigens and IgG on the surface of hepatocytes. Hepatitis B core antigen (HBcAg) was expressed on hepatocytes from six patients and hepatitis B surface antigen (HBcAG) from four, though always in association with HBcAg. Peripheral blood mononuclear cells (PBMC) were significantly cytotoxic in eight of the patients, and fractionation experiments revealed that the cytotoxic effect was equally mediated by T and non-T cells. Monoclonal antibody blocking experiments showed that HBcAg was the major target antigen recognized by T cells, although some inhibition of cytotoxicity was observed after pretreatment of target cells with monoclonal anti-HBs in patients with liver cell surface HBsAg. In contrast, non-T cells were not consistently inhibited by either monoclonal anti-HBc or anti-HBs. These findings further suggest that the HBV nucleoprotein serves as a target for recognition by cytotoxic T cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti A., Pontisso P., Fattovich G., Schiavon E., Chemello L., Bortolotti F., Tremolada F., Realdi G. Changes in serum hepatitis B virus (HBV) DNA positivity in chronic HBV infection: results of a long-term follow-up study of 138 patients. J Infect Dis. 1986 Oct;154(4):562–569. doi: 10.1093/infdis/154.4.562. [DOI] [PubMed] [Google Scholar]
- Alberti A., Realdi G., Bortolotti F., Rigoli A. M. T-lymphocyte cytotoxicity to HBsAg-coated target cells in hepatitis b virus infection. Gut. 1977 Dec;18(12):1004–1009. doi: 10.1136/gut.18.12.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti A., Realdi G., Tremolada F., Spina G. P. Liver cell surface localization of hepatitis B antigen and of immunoglobulins in acute and chronic hepatitis and in liver cirrhosis. Clin Exp Immunol. 1976 Sep;25(3):396–402. [PMC free article] [PubMed] [Google Scholar]
- Askonas B. A., Webster R. G. Monoclonal antibodies to hemagglutinin and to H-2 inhibit the cross-reactive cytotoxic T cell populations induced by influenza. Eur J Immunol. 1980 Feb;10(2):151–156. doi: 10.1002/eji.1830100215. [DOI] [PubMed] [Google Scholar]
- Charpentier B., Michelson S., Martin B. Definition of human cytomegalovirus-specific target antigens recognized by cytotoxic T cells generated in vitro by using an autologous lymphocyte system. J Immunol. 1986 Jul 1;137(1):330–336. [PubMed] [Google Scholar]
- Chemello L., Mondelli M., Bortolotti F., Schiavon E., Pontisso P., Alberti A., Rondanelli E. G., Realdi G. Natural killer activity in patients with acute viral hepatitis. Clin Exp Immunol. 1986 Apr;64(1):59–64. [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Bieber M. S., Josepho C. A., Xavier C., Anderson D. S. Functional properties of lymphocyte subpopulations in hepatitis B virus infection. II. Cytotoxic effector cell killing of targets that naturally express hepatitis B surface antigen and liver-specific lipoprotein. J Immunol. 1981 Jan;126(1):45–49. [PubMed] [Google Scholar]
- Dienstag J. L., Bhan A. K. Enhanced in vitro cell-mediated cytotoxicity in chronic hepatitis B virus infection: absence of specificity for virus-expressed antigen on target cell membranes. J Immunol. 1980 Nov;125(5):2269–2276. [PubMed] [Google Scholar]
- Effros R. B., Frankel M. E., Gerhard W., Doherty P. C. Inhibition of influenza-immune T cell effector function by virus-specific hybridoma antibody. J Immunol. 1979 Sep;123(3):1343–1346. [PubMed] [Google Scholar]
- Finberg R., Spriggs D. R., Fields B. N. Host immune response to reovirus: CTL recognize the major neutralization domain of the viral hemagglutinin. J Immunol. 1982 Nov;129(5):2235–2238. [PubMed] [Google Scholar]
- Holt C. A., Osorio K., Lilly F. Friend virus-specific cytotoxic T lymphocytes recognize both gag and env gene-encoded specificities. J Exp Med. 1986 Jul 1;164(1):211–226. doi: 10.1084/jem.164.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancki D. W., Lorber M. I., Loken M. R., Fitch F. W. A clone-specific monoclonal antibody that inhibits cytolysis of a cytolytic T cell clone. J Exp Med. 1983 Mar 1;157(3):921–935. doi: 10.1084/jem.157.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. Cytotoxic T lymphocytes reactive with vesicular stomatitis virus: analysis of specificity with monoclonal antibodies directed to the viral glycoprotein. J Immunol. 1983 Mar;130(3):1408–1412. [PubMed] [Google Scholar]
- McMichael A. J., Michie C. A., Gotch F. M., Smith G. L., Moss B. Recognition of influenza A virus nucleoprotein by human cytotoxic T lymphocytes. J Gen Virol. 1986 Apr;67(Pt 4):719–726. doi: 10.1099/0022-1317-67-4-719. [DOI] [PubMed] [Google Scholar]
- Mieli-Vergani G., Vergani D., Portmann B., White Y., Murray-Lyon I., Marigold J. H., Woolf I., Eddleston A. L., Williams R. Lymphocyte cytotoxicity to autologous hepatocytes in HBsAg positive chronic liver disease. Gut. 1982 Dec;23(12):1029–1036. doi: 10.1136/gut.23.12.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Mondelli M., Eddleston A. L. Mechanisms of liver cell injury in acute and chronic hepatitis B. Semin Liver Dis. 1984 Feb;4(1):47–58. doi: 10.1055/s-2008-1040645. [DOI] [PubMed] [Google Scholar]
- Mondelli M., Mieli-Vergani G., Bortolotti F., Cadrobbi P., Portmann B., Alberti A., Realdi G., Eddleston A. L., Mowat A. P. Different mechanisms responsible for in vitro cell-mediated cytotoxicity to autologous hepatocytes in children with autoimmune and HBsAg-positive chronic liver disease. J Pediatr. 1985 Jun;106(6):899–906. doi: 10.1016/s0022-3476(85)80234-1. [DOI] [PubMed] [Google Scholar]
- Mondelli M., Tedder R. S., Ferns B., Pontisso P., Realdi G., Alberti A. Differential distribution of hepatitis B core and E antigens in hepatocytes: analysis by monoclonal antibodies. Hepatology. 1986 Mar-Apr;6(2):199–204. doi: 10.1002/hep.1840060208. [DOI] [PubMed] [Google Scholar]
- Mondelli M., Vergani G. M., Alberti A., Vergani D., Portmann B., Eddleston A. L., Williams R. Specificity of T lymphocyte cytotoxicity to autologous hepatocytes in chronic hepatitis B virus infection: evidence that T cells are directed against HBV core antigen expressed on hepatocytes. J Immunol. 1982 Dec;129(6):2773–2778. [PubMed] [Google Scholar]
- Naumov N. V., Mondelli M., Alexander G. J., Tedder R. S., Eddleston A. L., Williams R. Relationship between expression of hepatitis B virus antigens in isolated hepatocytes and autologous lymphocyte cytotoxicity in patients with chronic hepatitis B virus infection. Hepatology. 1984 Jan-Feb;4(1):63–68. doi: 10.1002/hep.1840040111. [DOI] [PubMed] [Google Scholar]
- Plata F., Kalil J., Zilber M. T., Fellous M., Lévy D. Identification of a viral antigen recognized by H-2-restricted cytolytic T lymphocytes on a murine leukemia virus-induced tumor. J Immunol. 1983 Nov;131(5):2551–2556. [PubMed] [Google Scholar]
- Tedder R. S., Guarascio P., Yao J. L., Lord R. B., Eddleston A. L. Production of monoclonal antibodies to hepatitis B surface and core antigens, and use in the detection of viral antigens in liver biopsies. J Hyg (Lond) 1983 Feb;90(1):135–142. doi: 10.1017/s0022172400063932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Trevisan A., Realdi G., Alberti A., Ongaro G., Pornaro E., Meliconi R. Core antigen-specific immunoglobulin G bound to the liver cell membrane in chronic hepatitis B. Gastroenterology. 1982 Feb;82(2):218–222. [PubMed] [Google Scholar]
- Wands J. R., Perrotto J. L., Alpert E., Isselbacher K. J. Cell-mediated immunity in acute and chronic hepatitis. J Clin Invest. 1975 May;55(5):921–929. doi: 10.1172/JCI108021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Mackett M., Lefrancois L., Lyles D. S., Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986 Jun 1;163(6):1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Rosenthal K. L. Experiments and speculation on antiviral specificity of T and B cells. Immunol Rev. 1981;58:131–155. doi: 10.1111/j.1600-065x.1981.tb00352.x. [DOI] [PubMed] [Google Scholar]


