Abstract
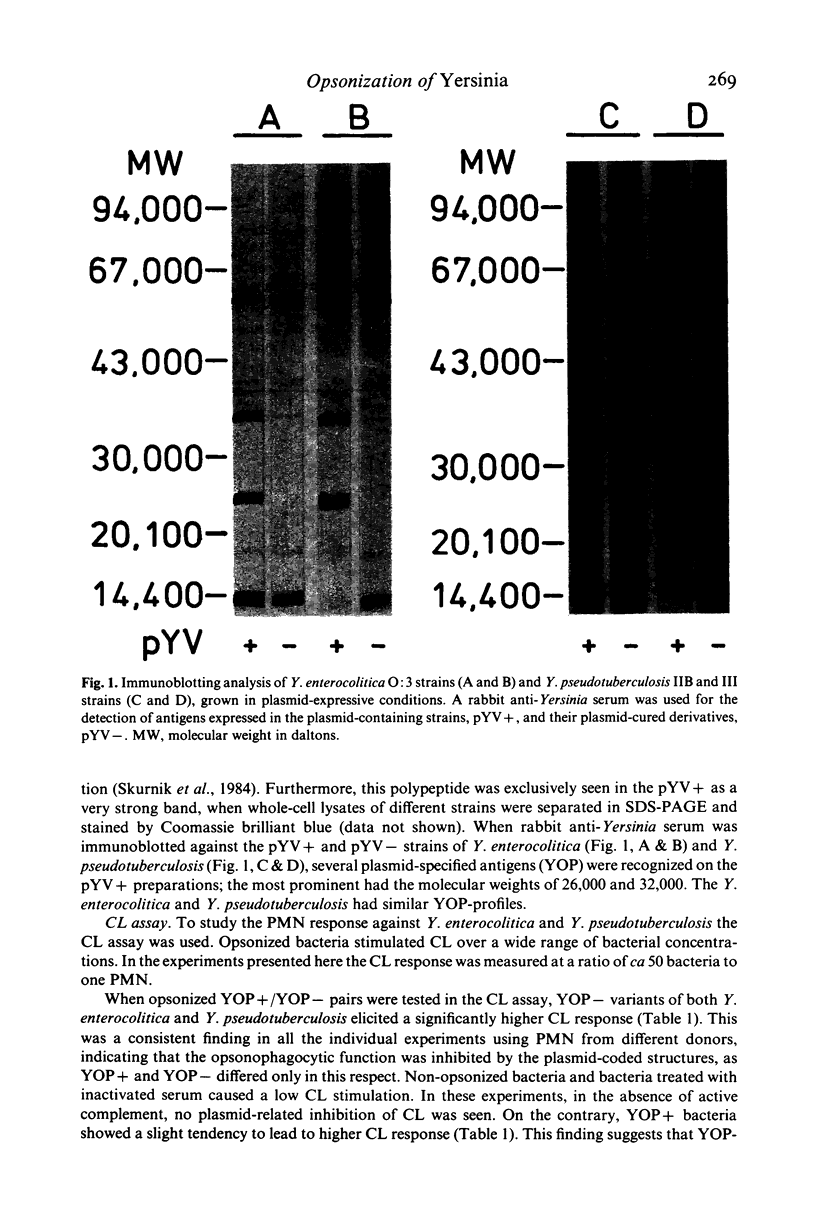
Plasmid-cured variants of virulent strains of Yersinia enterocolitica and Y. pseudotuberculosis were obtained by selection after growth in calcium-deficient medium. To obtain antigen preparations consisting of whole bacteria the original plasmid-containing strains and the plasmid-cured variants were grown in conditions favouring expression of the temperature-inducible outer membrane proteins of Yersinia (YOP) (37 degrees C, calcium-deficient culture medium). The presence or absence of the YOP on the bacteria was verified by immunoblotting. Opsonophagocytosis of YOP-negative Yersinia preparations (YOP-) was compared to that of YOP-containing ones (YOP+) in human polymorphonuclear leukocyte (PMN) chemiluminescence (CL) assay. The attachment of complement C3b on the surface of the bacteria after opsonization with normal human serum was determined by using a fluorescent anti-C3c-antibody and flow cytometry. YOP+ bacteria resisted opsonization in the absence of specific antibodies, as indicated by diminished C3b-fixation on bacteria and weaker CL response. This implies that virulence-plasmid-coded structures provide Y. enterocolitica and Y. pseudotuberculosis with an ability to avoid complement-mediated opsonization and phagocytosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho K., Ahvonen P., Lassus A., Sievers K., Tiilikainen A. HL-A 27 in reactive arthritis. A study of Yersinia arthritis and Reiter's disease. Arthritis Rheum. 1974 Sep-Oct;17(5):521–526. doi: 10.1002/art.1780170505. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. The Vwa+ virulence factor of yersiniae: the molecular basis of the attendant nutritional requirement for Ca++. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S748–S758. doi: 10.1093/clinids/5.supplement_4.s748. [DOI] [PubMed] [Google Scholar]
- Bölin I., Norlander L., Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982 Aug;37(2):506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Engels W., Endert J., Kamps M. A., van Boven C. P. Role of lipopolysaccharide in opsonization and phagocytosis of Pseudomonas aeruginosa. Infect Immun. 1985 Jul;49(1):182–189. doi: 10.1128/iai.49.1.182-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W., Endert J., Van Boven C. P. A quantitative method for assessing the third complement factor (C3) attached to the surface of opsonized Pseudomonas aeruginosa: interrelationship between C3 fixation, phagocytosis and complement consumption. J Immunol Methods. 1985 Jul 16;81(1):43–53. doi: 10.1016/0022-1759(85)90120-6. [DOI] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granfors K., Viljanen M., Tiilikainen A., Toivanen A. Persistence of IgM, IgG, and IgA antibodies to Yersinia in yersinia arthritis. J Infect Dis. 1980 Apr;141(4):424–429. doi: 10.1093/infdis/141.4.424. [DOI] [PubMed] [Google Scholar]
- HIGUCHI K., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961 Apr;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Grossman N., Schmetz M., Leive L. C3 binds preferentially to long-chain lipopolysaccharide during alternative pathway activation by Salmonella montevideo. J Immunol. 1986 Jan;136(2):710–715. [PubMed] [Google Scholar]
- Laird W. J., Cavanaugh D. C. Correlation of autoagglutination and virulence of yersiniae. J Clin Microbiol. 1980 Apr;11(4):430–432. doi: 10.1128/jcm.11.4.430-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Pai C. H. Inhibition of human neutrophil chemiluminescence by plasmid-mediated outer membrane proteins of Yersinia enterocolitica. Infect Immun. 1985 Jul;49(1):145–151. doi: 10.1128/iai.49.1.145-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila L., Granfors K., Toivanen A. Acute anterior uveitis after yersinia infection. Br J Ophthalmol. 1982 Mar;66(3):209–212. doi: 10.1136/bjo.66.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A. M., Weir D. M. The effect of Pseudomonas alginate on rat alveolar macrophage phagocytosis and bacterial opsonization. Clin Exp Immunol. 1985 Jan;59(1):190–196. [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Martinez R. J. Role of a plasmid in the pathogenicity of Yersinia species. Curr Top Microbiol Immunol. 1985;118:29–51. doi: 10.1007/978-3-642-70586-1_3. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L., Granberg I., Granfors K., Toivanen A. Restriction map of virulence plasmid in Yersinia enterocolitica O:3. Plasmid. 1986 Nov;16(3):225–227. doi: 10.1016/0147-619x(86)90062-4. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Lee N., Bowden C. Stimulation of human leukocytes by protein II+ gonococci is mediated by lectin-like gonococcal components. Infect Immun. 1985 Oct;50(1):116–122. doi: 10.1128/iai.50.1.116-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenberg-Arska M., Salters M. E., van Strijp J. A., Geuze J. J., Verhoef J. Electron microscopic study of phagocytosis of Escherichia coli by human polymorphonuclear leukocytes. Infect Immun. 1985 Dec;50(3):852–859. doi: 10.1128/iai.50.3.852-859.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M., Bölin I., Heikkinen H., Piha S., Wolf-Watz H. Virulence plasmid-associated autoagglutination in Yersinia spp. J Bacteriol. 1984 Jun;158(3):1033–1036. doi: 10.1128/jb.158.3.1033-1036.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertti R., Granfors K., Lehtonen O. P., Mertsola J., Mäkelä A. L., Välimäki I., Hänninen P., Toivanen A. An outbreak of Yersinia pseudotuberculosis infection. J Infect Dis. 1984 Feb;149(2):245–250. doi: 10.1093/infdis/149.2.245. [DOI] [PubMed] [Google Scholar]
- Toivanen A., Granfors K., Lahesmaa-Rantala R., Leino R., Ståhlberg T., Vuento R. Pathogenesis of Yersinia-triggered reactive arthritis: immunological, microbiological and clinical aspects. Immunol Rev. 1985 Aug;86:47–70. doi: 10.1111/j.1600-065x.1985.tb01137.x. [DOI] [PubMed] [Google Scholar]
- Vuento R., Leino R., Viander M., Toivanen A. In vitro lymphoproliferative response to Yersinia: depressed response in arthritic patients years after Yersinia infection. Clin Exp Rheumatol. 1983 Jul-Sep;1(3):219–224. [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Inhibition of complement-mediated opsonization and phagocytosis of Streptococcus pyogenes by D fragments of fibrinogen and fibrin bound to cell surface M protein. J Exp Med. 1985 Dec 1;162(6):1983–1997. doi: 10.1084/jem.162.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink D. L., Feeley J. C., Wells J. G., Vanderzant C., Vickery J. C., Roof W. D., O'Donovan G. A. Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature. 1980 Jan 10;283(5743):224–226. doi: 10.1038/283224a0. [DOI] [PubMed] [Google Scholar]



