Abstract
Small GTP-binding proteins such as those from the RAC family are cytosolic signal transduction proteins that often are involved in processing of extracellular stimuli. Plant RAC proteins are implicated in regulation of plant cell architecture, secondary wall formation, meristem signaling, and defense against pathogens. We isolated a RacB homolog from barley (Hordeum vulgare) to study its role in resistance to the barley powdery mildew fungus (Blumeria graminis f.sp. hordei). RacB was constitutively expressed in the barley epidermis and its expression level was not strongly influenced by inoculation with B. graminis. However, after biolistic bombardment of barley leaf segments with RacB-double-stranded RNA, sequence-specific RNA interference with RacB function inhibited fungal haustorium establishment in a cell-autonomous and genotype-specific manner. Mutants compromised in function of the Mlo wild-type gene and the Ror1 gene (genotype mlo5 ror1) that are moderately susceptible to B. graminis showed no alteration in powdery mildew resistance upon RacB-specific RNA interference. Thus, the phenotype, induced by RacB-specific RNA interference, was apparently dependent on the same processes as mlo5-mediated broad resistance, which is suppressed by ror1. We conclude that an RAC small GTP-binding protein is required for successful fungal haustorium establishment and that this function may be linked to MLO-associated functions.
Complete resistance of barley (Hordeum vulgare) to the biotrophic, fungal pathogen Blumeria graminis f.sp. hordei (Bgh) is mediated by major resistance genes such as the Mla genes or by loss of MLO function in Mlo-mutant genotypes such as mlo5-barley (Jørgensen, 1994; Schulze-Lefert and Vogel, 2000). The latter is expressed exclusively via penetration resistance, which is characterized by formation of cell wall appositions and accumulation of phytoalexins, pathogenesis-related gene transcripts, and hydrogen peroxide (Stolzenburg et al., 1984; Zeyen et al., 1993; Peterhänsel et al., 1997; von Röpenack et al., 1998; Hückelhoven et al., 1999, 2000b). All of these characteristics are also found in susceptible barley, albeit to a lower extent, meaning that the mlo alleles confer a primed responsiveness for these defense reactions or the functional MLO is a control element of these fundamental resistance mechanisms (Büschges et al., 1997; Peterhänsel et al., 1997).
It is intriguing that Bgh-resistant mlo genotypes show hypersusceptibility to Magnaporthe grisea and to toxic culture filtrates of Cochliobolus sativus (Jarosch et al., 1999; Kumar et al., 2001). Thus, Mlo exerts an ambivalent role in controlling resistance to the biotroph Bgh and susceptibility to the hemibiotroph M. grisea. The MLO protein is a membrane-spanning protein reminiscent of a G-protein coupled receptor (Devoto et al., 1999). In animals, such proteins interact with heterotrimeric G-proteins and/or small GTP-binding proteins via different cytoplasmic domains (Naor et al., 2000). Small GTP-binding proteins such as those of the RAC family are cytosolic signal transduction proteins that often are involved in processing of extracellular stimuli. Plant RAC proteins are involved in regulation of plant cell architecture, secondary wall formation, meristem signaling, and defense against pathogens (Valster et al., 2000). Mammalian RAC1, in its GTP-binding form, is essential for stable assembly of an active NADPH oxidase complex in the plasma membrane of phagocytic and nonphagocytic cells. This complex is responsible for generation of superoxide radical anion (O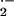 ) that is a signal molecule for cell proliferation in low concentrations, whereas it causes host cell death and pathogen killing in higher concentrations (Irani et al., 1997; Burstein et al., 1998; Irani and Goldschmidt-Clermont, 1998; Subauste et al., 2000).
) that is a signal molecule for cell proliferation in low concentrations, whereas it causes host cell death and pathogen killing in higher concentrations (Irani et al., 1997; Burstein et al., 1998; Irani and Goldschmidt-Clermont, 1998; Subauste et al., 2000).
Interaction of plant RAC homologs with the NADPH oxidase complex appears to regulate activity of NADPH oxidase that produces O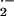 in response to pathogen attack (Hassanain et al., 2000; Ono et al., 2001). Rice (Oryza sativa) Rac1, when overexpressed in rice in its constitutive active form, leads to hypersensitive reaction (HR) at sites of attack by M. grisea and, therefore, to pathogen resistance. Expression of dominant negative forms of Rac1 consistently results in enhanced susceptibility to M. grisea (Kawasaki et al., 1999; Ono et al., 2001).
in response to pathogen attack (Hassanain et al., 2000; Ono et al., 2001). Rice (Oryza sativa) Rac1, when overexpressed in rice in its constitutive active form, leads to hypersensitive reaction (HR) at sites of attack by M. grisea and, therefore, to pathogen resistance. Expression of dominant negative forms of Rac1 consistently results in enhanced susceptibility to M. grisea (Kawasaki et al., 1999; Ono et al., 2001).
Reactive oxygen intermediates (ROI) play multiple roles in plant pathogen interactions. O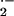 or H2O2 induce defense mechanisms including pathogenesis-related gene expression and the HR. On the other hand, ROI are also signals that restrict cell death and lead to production of antioxidants. Spatial and quantitative differences in the occurrence of ROI are crucial for their mode of action (Levine et al., 1994; Tenhaken et al., 1995; Jabs et al., 1996). In barley, O
or H2O2 induce defense mechanisms including pathogenesis-related gene expression and the HR. On the other hand, ROI are also signals that restrict cell death and lead to production of antioxidants. Spatial and quantitative differences in the occurrence of ROI are crucial for their mode of action (Levine et al., 1994; Tenhaken et al., 1995; Jabs et al., 1996). In barley, O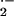 production takes place during attack by Bgh at sites of successful penetration of epidermal cells, but not at sites where fungal penetration is prevented (Hückelhoven and Kogel, 1998). In contrast, H2O2 accumulates subcellularly in barley at sites were penetration by Bgh is successfully prevented as well as in entire cells that undergo HR. Together, accumulation patterns of O
production takes place during attack by Bgh at sites of successful penetration of epidermal cells, but not at sites where fungal penetration is prevented (Hückelhoven and Kogel, 1998). In contrast, H2O2 accumulates subcellularly in barley at sites were penetration by Bgh is successfully prevented as well as in entire cells that undergo HR. Together, accumulation patterns of O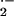 and H2O2 differ temporally and spatially in barley during attack by Bgh (Thordal-Christensen et al., 1997; Hückelhoven and Kogel, 1998; Kogel and Hückelhoven, 1999; Hückelhoven et al., 1999, 2000a, 2000b).
and H2O2 differ temporally and spatially in barley during attack by Bgh (Thordal-Christensen et al., 1997; Hückelhoven and Kogel, 1998; Kogel and Hückelhoven, 1999; Hückelhoven et al., 1999, 2000a, 2000b).
We show here that a barley RAC homolog is required for parasitic entry of the biotrophic powdery mildew fungus into epidermal host cells and, therefore, that this protein has a negative function in disease resistance of barley to Bgh.
RESULTS
Isolation of a Barley RACB Open Reading Frame
We recently isolated a partial coding sequence of a barley putative Rac1 homolog (GenBank accession no. AJ290420; Hückelhoven et al., 2001). In this study, we isolated a complete open reading frame of the barley Rac homolog (see “Materials and Methods”) that encodes a protein with more than 98% identity to RACB from rice and maize (Zea mays) and more than 55% identity to human RAC1 or RAC2. Therefore, the cDNA clone now is designated as barley RacB (GenBank accession no. AJ344223). The barley RACB homolog contains several conserved motifs that are essential for RAC function in animal systems. The CXXL motif is conserved at the C terminus. The Cys residue of this motif is the site of post-translational isoprenylation that directs active RAC proteins into the plasma membrane. The so-called effector loop of RAC protein can also be found in barley RACB (amino acids 28–48). This motif is responsible for interaction with target protein of RAC homologs such as NADPH oxidase. Barley RAC residues 127 to 140 resemble a specific effector loop that might be required for induction of O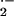 generation via RAC (Hassanain et al., 2000). Motifs typically responsible for GTP binding and GTP hydrolysis, respectively, are also present in barley RACB. Together, the isolated barley cDNA encodes a protein that contains all typical motifs of small RAC GTP-binding proteins.
generation via RAC (Hassanain et al., 2000). Motifs typically responsible for GTP binding and GTP hydrolysis, respectively, are also present in barley RACB. Together, the isolated barley cDNA encodes a protein that contains all typical motifs of small RAC GTP-binding proteins.
RacB Is Expressed in Epidermal Tissue
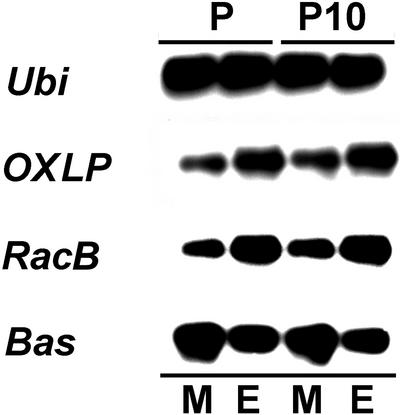
In our previous study, we described constitutive expression of the barley RacB homolog (designated as Rac1) in barley primary leaves. RacB expression was unaffected by inoculation with the powdery mildew fungus (Bgh; Hückelhoven et al., 2001). In this study, we wanted to know whether RacB is expressed in the epidermis of barley that is the only tissue attacked by Bgh. We analyzed tissue-specific expression of RacB in peeled abaxial epidermal strips and the residual part of primary leaves. Susceptible barley cultivars Pallas and resistant P10 were inoculated densely on the abaxial sides with Bgh race A6 by 24 h before sampling. As a positive control for epidermis-specific gene expression, an oxalate-oxidase like-protein gene (OXLP) was selected (Wei et al., 1998). Ubiquitin 1 (Ubi) was used as a marker for tissue-unspecific expression, and chloroplast-directed BAS (thioredoxin-dependent peroxide reductase) was selected as a marker for mesophyll expression. As shown in Figure 1, expression of RacB was stronger in peeled epidermal strips than in the rest of the leaves. Tissue specificity of RacB expression was similar to that of OXLP and different from that of Ubi and BAS.
Figure 1.
RacB is expressed in epidermal tissue. Reverse transcriptase (RT)-PCR with RNA from cv Pallas and cv BCPMla12 (P10) 24 HAI with BghA6. For extraction of total RNA, abaxial epidermal strips (E, inoculated site of the leaves) were separated from the mesophyll and adaxial epidermis (M). Ubi was selected as a marker for tissue-unspecific gene expression. OXLP was selected as a positive control for gene expression in the epidermal layer. Bas was selected as a positive control for gene expression in mesophyll cells. RT-PCR was carried out with 25 cycles under specific conditions. RT-PCR-products were denatured in gel, blotted, and detected by antisense RNA probes under stringent conditions.
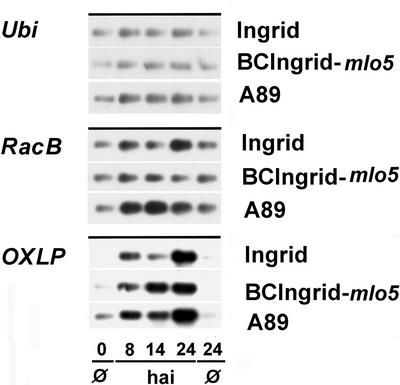
We compared early expression of RacB in a highly resistant barley mlo line BCIngrid-mlo5, the respective susceptible near-isogenic parent Ingrid, and a susceptible mutant A89 (mlo5 ror1) between 0 and 24 h after inoculation (HAI). In Ingrid, about 50% to 60% of fungal penetration attempts lead to haustoria formation between 12 and 24 HAI, whereas penetration rate in BCIngrid-mlo5 was close to 0%. Cultivar A89, a Mlo-Ror1 double-mutant line derived from BCIngrid-mlo5, is penetrated at 20% to 35% of the interaction sites by the Bgh isolate used (Hückelhoven et al., 2000b). RacB gene expression was slightly enhanced in response to Bgh inoculation as compared with Ubi expression that was taken as a constitutive marker. In the same RNA batch, the expression of OXLP as a positive control for Bgh-induced gene expression was enhanced from 8 HAI onward. At 14 HAI, when the first immature haustoria can be found in epidermal cells, OXLP expression was somewhat stronger in cv A89 and resistant BCIngrid-mlo5 than in Ingrid (Fig. 2).
Figure 2.
RacB expression in resistant and susceptible barley lines. RNA was isolated from cv Ingrid (Mlo, Ror1, susceptible), cv BCIngrid-mlo5 (mlo5, Ror1, resistant), and cv A89 (mlo5, ror1, moderately susceptible) immediately before (0 Ø) inoculation at 8, 14, and 24 HAI with Bgh and 24 HAI from noninoculated control plants (24 Ø). Ubi was selected as a marker for constitutive gene expression. OXLP was selected as a positive control for Bgh-induced gene expression in the epidermal layer. RT-PCRs were carried out with 20 to 25 cycles under specific conditions. PCR products were denatured in gel, blotted, and detected by antisense RNA probes under stringent conditions.
Sequence-Specific RNA Interference (RNAi) by RacB-double-stranded (ds) RNA Enhances Penetration Resistance
We addressed the question of whether RACB is involved in cellular accessibility or maintenance of basal resistance of barley to powdery mildew fungus. Host cell wall penetration and haustorium formation are the key steps in establishing host-pathogen compatibility. However, even susceptible barley cultivars such as Pallas or Ingrid prevent penetration at up to 50% of interaction sites, indicating a significant level of basal resistance. We used sequence-specific RNAi to induce gene silencing of RacB. RNAi produces phenotypes in plants that are very similar to those of knockout mutants (Waterhouse et al., 1998). It recently was shown that RNAi also functions transiently in barley if dsRNA is delivered into epidermal cells by biolistic bombardment (Schweizer et al., 2000). To test the efficiency of RNAi in induction of post-transcriptional gene silencing of RACB, we bombarded barley epidermal cells with p-green fluorescent protein (GFP):RACB that had been constructed for expression of a GFP:RACB fusion protein under control of the cauliflower mosaic virus 35 S promoter, together with RacB-dsRNA or heterologous control dsRNA (human thyroid hormone receptor dsRNA, TR), respectively. In four independent experiments, sequence-specific silencing of GFP:RACB led to a significant reduction of green fluorescing cells by 75% (Table I). This shows that dsRNA of RacB is suitable for inducing silencing of RACB in bombarded cells.
Table I.
Effect of RacB-dsRNA on transient expression of a RACB:GFP fusion protein
| No. of Green Fluorescing Cells per Leafa | |
|---|---|
| Control-dsRNA | 11.3 ± 2.0 |
| RacB-dsRNA | 2.9 ± 1.8 |
Mean ± se of four independent experiments.
To elucidate the role of small GTP-binding proteins in basal resistance or cellular accessibility, we bombarded Pallas leaf segments with RacB-dsRNA together with a GFP expression vector (pGFP; Schweizer et al., 1999). Leaves were subsequently inoculated with Bgh, and the outcome of the interaction was evaluated 48 h later by in vivo light and fluorescence microscopy (Nielsen et al., 1999). Penetration into GFP-expressing cells was confirmed by detection of haustoria in living cells and by judgment of fungal development on these cells by fluorescence and light microscopy (see “Materials and Methods”).
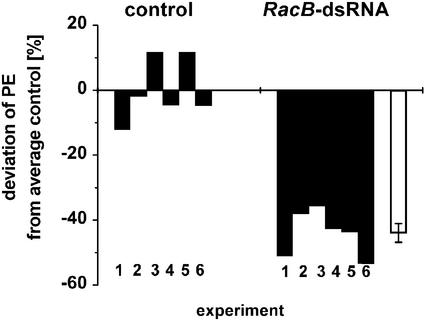
In each of six independent experiments, bombardment of cv Pallas with RacB-dsRNA led to a reduced number of cells that were successfully invaded by Bgh as compared with leaf segments bombarded with heterologous TR-dsRNA. The resistance-inducing effect of RacB-dsRNA resulted in an average reduction of penetration efficiency (PE) of Bgh by 44% (Fig. 3).
Figure 3.
RacB-dsRNA interferes with the PE of Bgh in barley. Relative PE was evaluated in six independent experiments with Bgh on barley cv Pallas. PE of Bgh was reduced in cells that were bombarded with RacB-dsRNA compared with cells that were bombarded with control dsRNA (TR, human thyroid receptor-dsRNA). Negative and positive deviation of PE indicate reduced or enhanced PE, respectively, compared with average penetration frequency in six control experiments (adjusted to zero). Black columns, Relative PE at minimum 100 interaction sites in an independent experiment. White column, Average of the independent experiments with RacB-dsRNA. Error bar shows se (relative PE of control and RacB-dsRNA are significantly different at P = 0.000001 level, Student's t test).
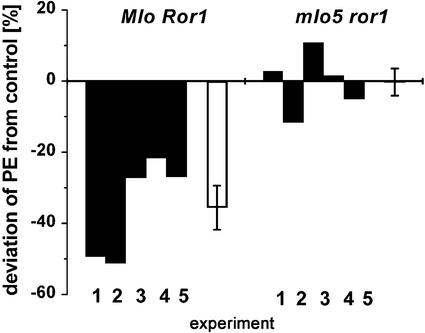
Broad prehaustorial resistance in barley against Bgh is controlled negatively by the wild-type MLO protein. Barley mlo5 genotypes without a functional MLO protein are race nonspecifically resistant to penetration by Bgh (Büschges et al., 1997; Jørgensen, 1994). Because RacB-dsRNA inhibited haustorium formation in cv Pallas that bears no functional resistance gene against BghA6, we speculated that RacB and Mlo might be functionally linked. To test this hypothesis, we selected a mlo5 genotype (cv A89, mlo5 ror1, background Ingrid) that is moderately susceptible to Bgh due to the mutation in Ror1 (Freialdenhoven et al., 1996). In this double-mutant genotype, we tested the impact of RacB-dsRNA in comparison with wild-type Mlo genotypes. In five independent experiments, RacB-dsRNA did not prevent haustoria establishment in cv A89, whereas in the same experiments, PE was reduced by RacB-dsRNA in cv Pallas and cv Ingrid (Mlo Ror1 genotypes; Fig. 4). Thus, resistance induced by RacB-dsRNA such as mlo-mediated resistance does not work in cv A89. It is remarkable that the RacB-dsRNA effect was stronger in cv Pallas than in cv Ingrid (Fig. 4, experiments 1 and 2 or 3–5, respectively). Absolute PEs are shown in Table II.
Figure 4.
The influence of RacB-dsRNA on the PE of Bgh is dependent on the barley genotype. Relative PE was evaluated in five independent experiments with Bgh on barley lines Pallas, Ingrid, or A89. The PE of Bgh is reduced in cv Pallas (Mlo Ror1, experiments 1 and 2) or cv Ingrid (Mlo Ror1, experiments 3–5) cells that were bombarded with RacB-dsRNA compared with cells bombarded with control dsRNA (not shown). Penetration of susceptible double-mutant A89 (mlo5 ror1, experiments 1–5) was not affected by RacB-dsRNA. Black columns, Relative PE in an independent experiment. White columns, Average of five independent experiments with RacB-dsRNA. Error bars show ses (influence of RacB-dsRNA on PE in Mlo Ror1 and mlo5 ror1 genotypes, respectively, is significantly different at P < 0.002, Student's t test).
Table II.
Penetration frequencies of Bgh on barley leaves bombarded with dsRNA
| Line | Penetration Frequencya
|
|
|---|---|---|
| Control-dsRNA | RacB-dsRNA | |
| % | ||
| Pallas (Mlo Ror1) | 57.0 ± 2.3 | 31.8 ± 1.6 |
| Ingrid (Mlo Ror1) | 53.8 ± 6.5 | 39.0 ± 4.0 |
| A89 (mlo5 ror1) | 27.4 ± 0.6 | 27.5 ± 1.6 |
No. of penetrated cells divided by no. of attacked cells multiplied by 100 (mean ± se).
To rule out the possibility that RacB-dsRNA influences the transformation rate or the survival rate of attacked cells, we compared the number of GFP-expressing cells on control and RacB-dsRNA bombarded leaves (Table III). Microscopic evaluation showed that RacB-dsRNA did not influence the number of total or attacked GFP-expressing cells in any genotypes used. This demonstrates that RNAi by RacB-dsRNA strongly affects processes linked to successful establishment of the fungus but not cell death of host cells.
Table III.
Transformation rates on barley leaves bombarded with dsRNA
| Line | No. of GFP-Expressing Cells per Shotab
|
nc | |||
|---|---|---|---|---|---|
| Control-dsRNA
|
RacB-dsRNA
|
||||
| Total | Attacked | Total | Attacked | ||
| Pallas (Mlo Ror1) | 34.3 ± 4.6 | 16.0 ± 2.2 | 33.9 ± 4.8 | 15.5 ± 1.4 | 6 (21) |
| Ingrid (Mlo Ror1) | 51.0 ± 8.9 | 27.6 ± 8.7 | 49.9 ± 5.6 | 31.5 ± 7.8 | 3 (11) |
| A89 (mlo5 ror1) | 34.4 ± 5.4 | 18.1 ± 4.0 | 34.1 ± 5.5 | 16.7 ± 3.8 | 5 (22) |
Four leaves were bombarded per shot.
Mean ± se.
No. of independent experiments (shots in n experiments each for control and RacB-dsRNA).
DISCUSSION
We have shown that RacB-dsRNA specifically interferes in barley epidermal cells with haustorium establishment by the plant parasitic, biotrophic powdery mildew fungus. Delivery of RacB-dsRNA into epidermal cells induced resistance with a similar efficiency as Mlo-dsRNA (Schweizer et al., 2000). Therefore, our results tag an RAC small GTP-binding protein as a host element that is required for successful invasion by Bgh.
Several lines of evidence could exclude nonspecific effects of RacB-dsRNA. First, in all experiments, the effect of RacB-dsRNA was compared with that of nonspecific TR-dsRNA, which has no plant homologs. An effect of TR-dsRNA was excluded in several experiments (data not shown). Second, the effect of RacB-dsRNA was genotype specific (Fig. 4). Third, RacB-dsRNA did not influence the number of nonattacked or attacked GFP-expressing cells (Table III). Fourth, when we bombarded barley with pGFP:RACB for expression of a GFP:RACB fusion protein together with RacB-dsRNA, the number of cells showing GFP fluorescence was reduced by 75% compared with experiments with heterologous TR-dsRNA. This shows that RacB-dsRNA induced gene silencing of the RacB:GFP-transgene. Thus, the biological effects of RacB-dsRNA are most likely a result of post-transcriptional gene silencing of endogenous RacB. In barley, high sequence identities of dsRNA and target genes are necessary for RNAi (Schweizer et al., 2000). However, because RacB is probably very similar to other barley Rac genes, we cannot exclude that we might have affected the expression of RAC proteins other than RACB by RacB-dsRNA.
The resistance inducing effect of RacB-dsRNA effect was somewhat stronger in cv Pallas than in cv Ingrid. Because RACB apparently plays a negative role in broad resistance to Bgh, different levels of broad resistance in cv Pallas and cv Ingrid might influence RACB activity. In the barley double-mutant A89 (mlo5-ror1), RacB-dsRNA did not interfere with resistance. Therefore, it appears that the function of a RAC protein is linked to elements of the MLO/ROR network. Because MLO and ROR1 are involved in broad resistance against Bgh, this finding suggests that RacB-dsRNA interferes with race-unspecific penetration resistance of barley against Bgh, and that the same processes underlying mlo-mediated resistance limit this effect. Because RACB and MLO are required for fungal entry in barley epidermal cells, we speculate that they might be linked functionally. It is interesting that functional RACB and functional MLO play negative roles in resistance to Bgh, whereas losses of RAC1 or MLO function lead to hypersusceptibility to the fungal parasite M. grisea (Jarosch et al., 1999; Ono et al., 2001). Thus, MLO and RAC G-proteins are signal transduction elements that play ambivalent roles in resistance to biotrophic Bgh and hemibiotrophic M. grisea.
The mechanism by which RAC interferes with penetration resistance needs to be elucidated. One possibility might be that RAC interacts with the cytoskeleton. In mammals, RAC activation is triggered by bacterial pathogens that invade nonphagocytic cells and in phagocytes during phagocytosis. Thereby, RAC is involved in actin reorganization processes during plasma membrane ruffling or bacterial engulfment (Knodler et al., 2001). Both processes appear to resemble the process of plasma membrane invagination during establishment of a fungal haustorium in a plant cell. If barley RAC is needed for plasma membrane invagination, loss of RAC function should lead to inhibition of haustorium formation, as shown here. We speculate that the Bgh triggers a RAC small GTP-binding protein and that this process depends on MLO allowing plasma membrane invagination as a prerequisite for establishment of compatibility. Also, active RAC could be involved in cytoskeleton organization processes that antagonize formation of cell wall appositions. Cytoskeleton reorganization appears to be required for penetration resistance of barley coleoptiles to nonhost pathogens such as Erysiphe pisi (Kobayashi et al., 1997).
RAC proteins are involved in activation of the O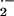 generating NADPH oxidase complex (Bokoch, 1995; Hassanain et al., 2000). In previous studies, we have shown that enhanced O
generating NADPH oxidase complex (Bokoch, 1995; Hassanain et al., 2000). In previous studies, we have shown that enhanced O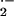 generation in barley cells attacked by Bgh temporally and spatially coincided with successful penetration and haustorium formation, but not with processes resulting in penetration resistance. Resistant mlo5 genotypes did not produce O
generation in barley cells attacked by Bgh temporally and spatially coincided with successful penetration and haustorium formation, but not with processes resulting in penetration resistance. Resistant mlo5 genotypes did not produce O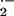 during the period of attempted penetration (Hückelhoven and Kogel, 1998; Kogel and Hückelhoven, 1999). Thus, it is tempting to speculate that barley RACB functions via activation of NADPH oxidase and that O
during the period of attempted penetration (Hückelhoven and Kogel, 1998; Kogel and Hückelhoven, 1999). Thus, it is tempting to speculate that barley RACB functions via activation of NADPH oxidase and that O generation influences penetration resistance to Bgh negatively. In contrast to O
generation influences penetration resistance to Bgh negatively. In contrast to O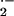 , H2O2 accumulates at sites of formation of cell wall appositions in which Bgh sticks (Thordal-Christensen et al., 1997; Hückelhoven et al., 1999, 2000b). Thus, H2O2 is strictly associated with barley defense reactions. Together, the balance of O
, H2O2 accumulates at sites of formation of cell wall appositions in which Bgh sticks (Thordal-Christensen et al., 1997; Hückelhoven et al., 1999, 2000b). Thus, H2O2 is strictly associated with barley defense reactions. Together, the balance of O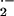 and H2O2 might be crucial for accessibility of epidermal cells.
and H2O2 might be crucial for accessibility of epidermal cells.
MATERIALS AND METHODS
Plant Materials, Pathogen, and Inoculation
The barley (Hordeum vulgare) lines Ingrid, Pallas, and the backcross line BCIngrid-mlo5 were obtained from Lisa Munk (Royal Veterinary and Agricultural University, Copenhagen). Their generation was described previously (Kølster et al., 1986). The mutant A89 was obtained from Paul Schulze-Lefert (Max-Plank-Institute for Plant Breeding Research, Köln, Germany). Plants were grown in a growth chamber at 18°C with 60% relative humidity and a photoperiod of 16 h (60 μmol m−2 s−1 photon flux density). The barley powdery mildew fungus, Blumeria graminis (DC) Speer f.sp. hordei Em. Marchal, race A6 (Wiberg, 1974) was inoculated onto barley primary leaves to give a density of 50 conidia mm−2. Bgh was maintained on barley cv Siri under the same conditions.
Isolation of epidermal tissue for expression analysis was performed by scribing adaxial sides of leaf tips with a scalpel without harming the abaxial epidermis. Leaf tips were folded back and taken as a handle to peel off epidermal strips that were cut off the leaf tips and frozen in liquid nitrogen immediately.
Isolation of Barley RacB, Cloning, Sequencing, and Probe Generation
We isolated cDNA fragments by the use of one-step RT-PCR kits (Invitrogen, Carlsbad, CA or Qiagen, Hilden, Germany). A complex RNA pool out of barley seedlings was used as a template. RNA was isolated from cv Pallas at 3, 5, and 7 d after germination. In addition, RNA was isolated from cv Pallas and backcross lines bearing mlo5, Mlg, or Mla12 at 1, 2, and 5 d after inoculation with BghA6 at the 7th d after germination. All isolated RNAs were diluted to a concentration of 1 μg μL−1 and they were pooled. Primers were designed using GenBank or expressed sequence tag database information for specific barley sequences or rice (Oryza sativa) sequences. To amplify a putative barley RacB cDNA, we designed primers from rice and barley sequences. Primers 5′-GGATCCGATGAGCGCGTCCAGGTT-3′ (from GenBank accession no. AF250327) and 5′-GTCGACCTTCGCCCTTGTTCTTTGTC-3′ (from GenBank accession no. BF260616) were suitable to generate a 642-bp RT-PCR product including 618-bp barley-specific sequence (GenBank accession no. AJ344223). We isolated cDNAs from gels and cloned them into pGEM-T-Vektor (Promega, Mannheim, Germany). cDNAs were sequenced from plasmids by use of the Thermo Sequenase Fluorescent Labeled Primer Cycle Sequencing kit (Amersham Biosciences, Freiburg, Germany) and were analyzed for similarities in the GenBank database using the BLAST algorithm (Altschul et al., 1997). Because the 5′ end of the isolated complete RacB open reading frame contained primer-derived sequences, we carried out RACE. First-strand cDNA synthesis and RACE were carried out as suggested by the manufacturer (GeneRacer; Invitrogen, Karlsruhe, Germany). First strand cDNA synthesis started from mRNA that was isolated from total RNA using the Dynabeads mRNA Purification kit (Dynal, Hamburg, Germany) according to the manufacturer's instructions. Hot-start touch-down RACE-PCR included the GeneRacer 5′primer and the RacB-specific primer 5′-GGATCCGATGAGCGCGTCCAGGTT-3′. Touch-down PCR was carried out with initial denaturation (5 min at 94°C), five cycles at a 70°C annealing temperature, five cycles at 68°C, and 28 cycles at 66°C. Each annealing was followed by a 1-min primer extension at 72°C and a 30-s denaturation at 94°C. The final extension time at 72°C was 10 min. The resulting RACE product of approximately 400 bp was reamplified with the gene-specific primer and the 5′GeneRacer nested primer, and was then isolated, cloned, and sequenced as already described.
For probe generation, plasmids were amplified in Escherichia coli, isolated, and used for in vitro transcription using T7 or SP6 RNA polymerases and digoxygenin- or fluorescein-labeled nucleotides (DIG-Luminescence Detection kit; Roche Molecular Biochemicals, Mannheim, Germany).
RNA Extraction and RT-PCR
Total RNA was extracted from eight to 10 primary leaf segments (5 cm long) or from 20 epidermal strips (mentioned before) using RNA extraction buffer (Applied Genetechnology Systems, Heidelberg) according to the manufacturer's instructions. RNA contents of the extracts were measured by UV photometry and were adjusted after checking in ethidium bromide-stained gels taking rRNA bands as a measure.
The OneStep RT-PCR kit (Qiagen) was used for semiquantitative RT-PCR following the manufacturer's instructions. To estimate template amounts, the RT-PCR reaction was stopped during the exponential phase of amplification, maintaining initial differences in target transcript amounts. PCR products were separated in agarose gels, denatured, blotted on nylon membranes, and detected with specific nonradioactively labeled RNA probes according to the DIG System user's guide (Roche Molecular Biochemicals). Prior to immunodetection of DNA-RNA hybrids, blots were washed stringently two times for 20 min in 0.1% (w/v) SDS and 0.1× SSC (15 mm sodium chloride and 1.5 mm sodium citrate, pH 7.0) at 68°C.
The primers were: 5′-GTTCATCAAGTGCGTCACC-GTG-3′ (5′ primer) and 5′-TTAGCTTCCTCAGTTCTTC-CCTG-3′ (3′ primer) for a 387-bp RacB cDNA fragment; 5′-CGCGCCGCAGCCGAGTACGAC-3′ (5′ primer) and 5′-GTCACAAAAACA-CATGTAACC-3′ (3′ primer) for a 674-bp barley BAS cDNA fragment (GenBank accession no. Z34917); 5′-GGC-CGACATGCATTCACCAG-3′ (5′ primer) and 5′-CATCT-GATATTGCTGGGTCTG-3′ (3 ′ primer) for a 506-bp OXLP cDNA fragment (GenBank accession no. X93171); and 5′-CCAAGATGCAGATCTTCGTGA-3′ (5′ primer) and 5′-TTCGCGATAGGTAAAAGAGCA-3′ (3′ primer) for a 513-bp Ubi cDNA fragment (GenBank accession no. M60175).
Construction of pGFP:RACB
For expression of a GFP:RACB fusion protein, cDNAs of GFP (GFPemd-b in pGFP; Schweizer et al., 1999) and RacB were amplified from plasmids by PCR using primers with attached restriction sites. PCR products were cloned into pGEM-T, amplified in E. coli, digested using primer-specific restriction enzymes, isolated from gels, and cloned one after another in pGY1 (Schweizer et al., 1999). Primers were designed in a way that allowed cloning of GFP upstream of the RacB 5′ end under elimination of the GFP stop codon. The primers used were 5′-GGATCCATGGTGAG-CAAGGGCGAG-3′ and 5′-GGATCCTTGTACAGCTCGT-CCAT-3′ for GFP and the RacB primers already mentioned. Orientation of the inserts was checked by PCR.
Transient Transformation, RNAi, and Evaluation of Fungal Development
A transient transformation protocol originally developed for wheat (Triticum aestivum) to assess gene function in the interaction with powdery mildew was used to induce RNAi via biolistic delivery of dsRNA into epidermal cells of barley leaf segments as described by Schweizer et al. (1999) and Schweizer et al. (2000; compare also Nielsen et al., 1999). For the transient transformation assay, plants were grown in a growth chamber at 24°C (20°C in the dark) with 60% relative humidity and a photoperiod of 16 h (240 μmol m−2 s−1 photon flux density). In principle, 312 μg of 1.1-μm tungsten particles was coated with dsRNA (2 μg) together with pGFP (1 μg; GFP under control of cauliflower mosaic virus 35S promoter) as a transformation marker for each shot. dsRacB RNA was obtained by annealing of sense and antisense RNA synthesized in vitro (Schweizer et al., 2000). Leaf segments were bombarded with coated particles 4 h before inoculation with Bgh, race A6. Inoculation with 100 conidia mm−2 led to an attack rate of approximately 50% on transformed cells. Interaction outcome was judged subsequently by fluorescence and light microscopy. For each individual experiment, at least 100 interaction sites were evaluated. Transformed GFP-expressing cells were identified under blue light excitation. Three different categories of transformed cells were distinguished: (a) penetrated cells, which contained an easily visible haustorium; (b) cells that were attacked by a fungal appressorium but did not contain a haustorium; (c) and cells that did not contain a haustorium and were not attacked by Bgh. Cells that contained more than one haustorium were scored as one penetrated cell independent of the number of fungal penetration attempts. Cells with multiple attacks from Bgh without a haustorium were scored as one nonpenetrated cell. Stomata cells and stomata guard cells were excluded from the evaluation. Surface structures of Bgh were detected by light microscopy or by fluorescence staining of the fungus with 0.1% calcofluor (w/v in water) for 30 s.
Deviation of PE referring to average control PE was used as a measure for susceptibility of cells that were bombarded with RacB-dsRNA compared with those bombarded with control TR-dsRNA (human thyroid receptor-dsRNA; Fig. 3). In five independent experiments, TR-dsRNA did not change the PE of Bgh compared with water. Deviation of PE was calculated for each experiment as the number of penetrated cells divided by the total number of attacked cells (PE) minus average PE in the controls divided by average PE of the controls multiplied by 100.
Deviation of PE referring to individual control PE was used to compare the impact of RNAi in different genotypes (Fig. 4). Therefore, PE in each experiment with RacB-dsRNA was divided by PE of individual controls, normalized by subtraction of one and multiplication by 100.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owner of all or parts of the material. Obtaining any permission will be the responsibility of the requestor. No restrictions or conditions will be placed on the use of any novel materials described in this paper that would limit their use in noncommercial research purposes.
ACKNOWLEDGMENTS
We thank Dr. Patrick Schweizer (Institut für Pflanzengenetik und Kulturpflanzenforschung, Gatersleben, Germany) for being a great teacher in the transient transformation assay and for providing pGFP. We also thank Dr. Gregor Langen (Justus-Liebig-University, Giessen, Germany) for providing cDNA for RACE.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. DFG Ko1208/8 to R.H. and K.-H.K.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010805.
LITERATURE CITED
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM. Regulation of the phagocyte respiratory burst by small GTP-binding proteins. Trends Cell Biol. 1995;5:109–113. doi: 10.1016/s0962-8924(00)88960-6. [DOI] [PubMed] [Google Scholar]
- Burstein ES, Hesterberg DJ, Gutkind JS, Brann MR, Currier EA, Messier TL. The ras-related GTPase rac1 regulates a proliferative pathway selectively utilized by G-protein coupled receptors. Oncogene. 1998;17:1617–1623. doi: 10.1038/sj.onc.1202067. [DOI] [PubMed] [Google Scholar]
- Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J et al. The barley Mlo gene: a novel control element of plant pathogen resistance. Cell. 1997;88:695–705. doi: 10.1016/s0092-8674(00)81912-1. [DOI] [PubMed] [Google Scholar]
- Devoto A, Piffanelli P, Nilsson I, Wallin E, Panstruga R, von Heijne G, Schulze-Lefert P. Topology, subcellular localization, and sequence diversity of the Mlo family in plants. J Biol Chem. 1999;274:34993–35004. doi: 10.1074/jbc.274.49.34993. [DOI] [PubMed] [Google Scholar]
- Freialdenhoven A, Peterhänsel C, Kurth J, Kreuzaler F, Schulze-Lefert P. Identification of genes required for the function of non-race-specific mlo resistance to powdery mildew in barley. Plant Cell. 1996;8:5–14. doi: 10.1105/tpc.8.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanain HH, Sharma YK, Moldovan L, Khramtsov V, Berliner LJ, Duvick JP, Goldschmidt-Clermont PJ. Plant rac proteins induce superoxide production in mammalian cells. Biochem Biophys Res Commun. 2000;272:783–788. doi: 10.1006/bbrc.2000.2791. [DOI] [PubMed] [Google Scholar]
- Hückelhoven R, Dechert C, Trujillo M, Kogel K-H. Differential expression of putative cell death regulator genes in near-isogenic, resistant and susceptible barley lines inoculated with the powdery mildew fungus. Plant Mol Biol. 2001;47:739–748. doi: 10.1023/a:1013635427949. [DOI] [PubMed] [Google Scholar]
- Hückelhoven R, Fodor J, Preis C, Kogel K-H. Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with H2O2 but not with salicylic acid accumulation. Plant Physiol. 1999;119:1251–1260. doi: 10.1104/pp.119.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hückelhoven R, Fodor J, Trujillo M, Kogel K-H. Barley Mla- and Rar-mutants compromised in the hypersensitive cell death response against Blumeria graminis f.sp. hordei are modified in their ability to accumulate reactive oxygen intermediates at sites of fungal invasion. Planta. 2000a;212:16–24. doi: 10.1007/s004250000385. [DOI] [PubMed] [Google Scholar]
- Hückelhoven R, Kogel K-H. Tissue-specific superoxide generation at interaction sites in resistant and susceptible near-isogenic barley lines attacked by the powdery mildew fungus (Erysiphe graminis f.sp. hordei) Mol Plant-Microbe Interact. 1998;11:292–300. [Google Scholar]
- Hückelhoven R, Trujillo M, Kogel KH. Mutations in Ror1 and Ror2 genes cause modification of hydrogen peroxide accumulation in mlo-barley under attack from the powdery mildew fungus. Mol Plant Pathol. 2000b;1:287–292. doi: 10.1046/j.1364-3703.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- Irani K, Goldschmidt-Clermont PJ. Ras, superoxide and signal transduction. Biochem Pharmacol. 1998;55:1339–1346. doi: 10.1016/s0006-2952(97)00616-3. [DOI] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearch ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science. 1996;273:1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- Jarosch B, Kogel K-H, Schaffrath U. The ambivalence of the barley Mlo locus: Mutations conferring resistance against powdery mildew (Blumeria graminis f.sp. hordei) enhance susceptibility to the rice blast fungus Magnaporte grisea. Mol Plant-Microbe Interact. 1999;12:508–514. [Google Scholar]
- Jørgensen JH. Genetics of powdery mildew resistance in barley. Crit Rev Plant Sci. 1994;13:97–119. [Google Scholar]
- Kawasaki T, Henmi K, Ono E, Hatakeyama S, Iwano M, Satoh H, Shimamoto K. The small GTP-binding protein rac is a regulator of cell death in plants. Proc Natl Acad Sci USA. 1999;96:10922–10926. doi: 10.1073/pnas.96.19.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler LA, Celli J, Finlay BB. Pathogenic trickery: deception of host cell processes. Nat Rev Mol Cell Biol. 2001;2:578–588. doi: 10.1038/35085062. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kobayashi I, Funaki Y, Fujimoto S, Takemoto T, Kunoh H. Dynamic reorganization of microfilaments and microtubules is necessary for the expression of non-host resistance in barley coleoptile cells. Plant J. 1997;11:525–537. [Google Scholar]
- Kogel K-H, Hückelhoven R. Superoxide generation in chemically activated resistance of barley in response to powdery mildew inoculation. J Phytopathol. 1999;147:1–4. [Google Scholar]
- Kølster P, Munk L, Stølen O, Løhde J. Near-isogenic barley lines with genes for resistance to powdery mildew. Crop Sci. 1986;26:903–907. [Google Scholar]
- Kumar J, Hückelhoven R, Beckhove U, Nagarajan S, Kogel K-H. A compromised Mlo pathway affects the response of barley to the necrotrophic fungus Bipolaris sorokiniana (teleomorph: Cochliobolus sativus) and its toxins. Phytopathology. 2001;91:127–133. doi: 10.1094/PHYTO.2001.91.2.127. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Naor Z, Benard O, Seger R. Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor. Trends Endocrinol Metab. 2000;11:91–99. doi: 10.1016/s1043-2760(99)00232-5. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Olsen O, Oliver R. A transient expression system to assay putative antifungal genes on powdery mildew infected barley leaves. Physiol Mol Plant Pathol. 1999;54:1–12. [Google Scholar]
- Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K. Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA. 2001;98:759–764. doi: 10.1073/pnas.021273498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhänsel C, Freialdenhoven A, Kurth J, Kolsch R, Schulze-Lefert P. Interaction analyses of genes required for resistance responses to powdery mildew in barley reveal distinct pathways leading to leaf cell death. Plant Cell. 1997;9:1397–1409. doi: 10.1105/tpc.9.8.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lefert P, Vogel J. Closing the ranks to attack by powdery mildew. Trends Plant Sci. 2000;5:343–348. doi: 10.1016/s1360-1385(00)01683-6. [DOI] [PubMed] [Google Scholar]
- Schweizer P, Pokorny J, Abderhalden O, Dudler R. A transient assay system for the functional assessment of defense-related genes in wheat. Mol Plant-Microbe Interact. 1999;12:647–654. [Google Scholar]
- Schweizer P, Pokorny J, Schulze-Lefert P, Dudler R. Double-stranded RNA interferes with gene function at the single-cell level in cereals. Plant J. 2000;24:895–903. doi: 10.1046/j.1365-313x.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- Stolzenburg MC, Aist JR, Israel HW. The role of papillae in resistance to powdery mildew conditioned by the ml-o gene in barley: correlative evidence. Physiol Plant Pathol. 1984;25:337–346. [Google Scholar]
- Subauste MC, Von Herrath M, Benard V, Chamberlain CE, Chuang TH, Chu K, Bokoch GM, Hahn KM. Rho family proteins modulate rapid apoptosis induced by cytotoxic T lymphocytes and Fas. Biol Chem. 2000;275:9725–9733. doi: 10.1074/jbc.275.13.9725. [DOI] [PubMed] [Google Scholar]
- Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb C. Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA. 1995;92:4158–4163. doi: 10.1073/pnas.92.10.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Valster AH, Hepler PK, Chernoff J. Plant GTPases: the Rhos in bloom. Trends Cell Biol. 2000;10:141–146. doi: 10.1016/s0962-8924(00)01728-1. [DOI] [PubMed] [Google Scholar]
- von Röpenack E, Parr A, Schulze-Lefert P. Structural analyses and dynamics of soluble and cell wall-bound phenolics in a broad spectrum resistance to the powdery mildew fungus in barley. J Biol Chem. 1998;273:9013–9022. doi: 10.1074/jbc.273.15.9013. [DOI] [PubMed] [Google Scholar]
- Waterhouse PM, Graham MW, Wang MB. Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc Natl Acad Sci USA. 1998;95:13959–13964. doi: 10.1073/pnas.95.23.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zhang Z, Andersen CH, Schmelzer E, Gregersen PL, Collinge DB, Smedegaard-Petersen V, Thordal-Christensen H. An epidermis/papilla-specific oxalate oxidase-like protein in the defense response of barley attacked by the powdery mildew fungus. Plant Mol Biol. 1998;36:101–112. doi: 10.1023/a:1005955119326. [DOI] [PubMed] [Google Scholar]
- Wiberg A. Genetical studies of spontaneous sources of resistance to powdery mildew in barley. Hereditas. 1974;77:89–148. doi: 10.1111/j.1601-5223.1974.tb01357.x. [DOI] [PubMed] [Google Scholar]
- Zeyen RJ, Ahlstrand GG, Carver TLW. X-ray microanalysis of frozen-hydrated, freeze-dried, and critical point dried leaf specimens: determination of soluble and insoluble chemical elements at Erysiphe graminis epidermal cell papilla sites in barley isolines containing Ml-o and ml-o alleles. Can J Bot. 1993;71:284–296. [Google Scholar]