Abstract
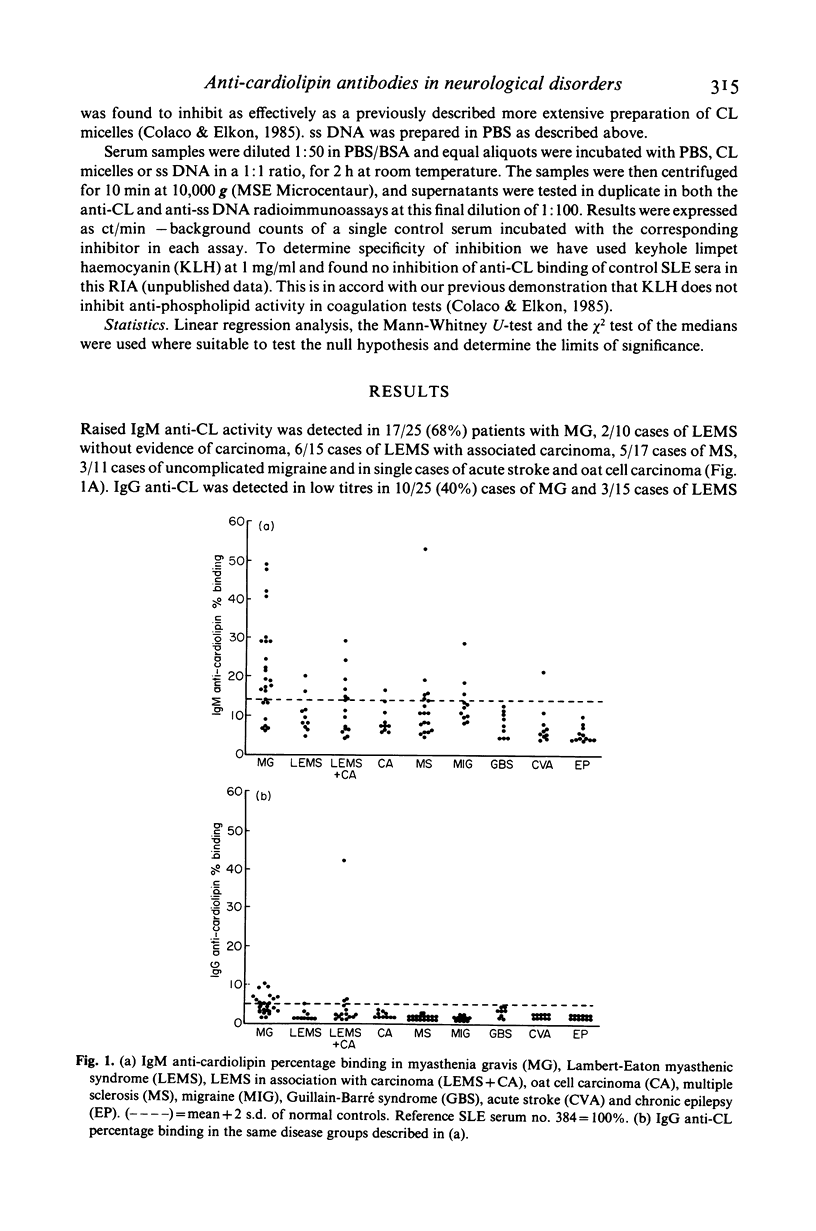
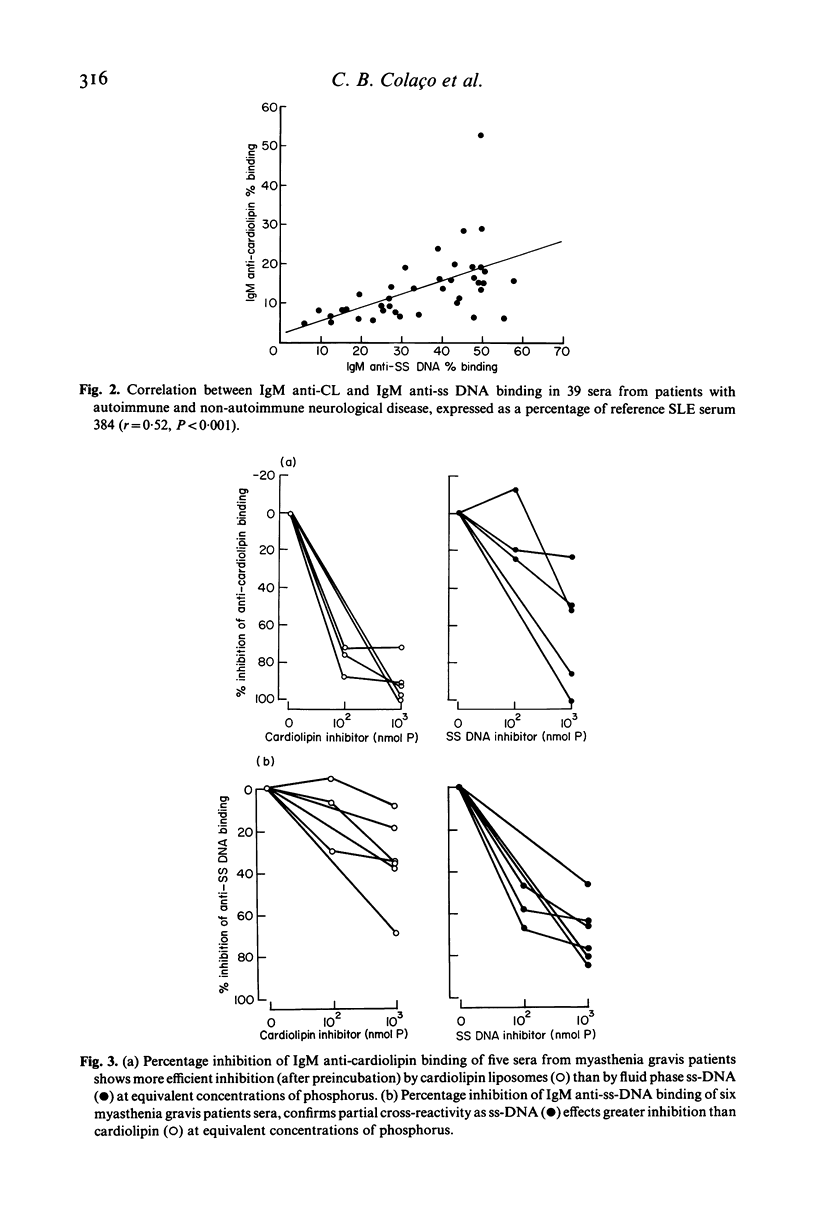
Antiphospholipid (PL) antibodies have been detected in sera from patients with chronic neurological diseases associated with disorders of immunity. In an isotype specific radioimmunoassay for anti-cardiolipin (CL) antibodies, we found IgM anti-CL (greater than 2 s.d. above mean of controls) in 17/25 (68%) patients with myasthenia gravis (MG), 8/25 (32%) with the Lambert-Eaton myasthenic syndrome (LEMS), 5/17 (29%) with multiple sclerosis and 3/11 (27%) cases of migraine. IgG anti-CL was only found in low titres in sera from 10 patients with MG and three with LEMS. Significant anti-CL activity could not be detected in sera from nine patients with acute Guillain-Barré Syndrome (GBS), 12 chronic cases of epilepsy, 8/9 with oat cell carcinoma and 9/10 with acute stroke. Further tests on 39 sera with the highest anti-CL activity, from all of the above disease groups, showed a significant correlation between IgM anti-CL and IgM anti-ss DNA activities. In a series of competitive inhibition assays six sera from patients with MG were shown to have a proportion of both specific and cross-reactive IgM anti-CL and IgM anti-ss DNA antibodies. Anti-phospholipid antibodies occur in certain neurological diseases, at lower titres than seen in SLE, yet their cross-reactive binding to ss DNA suggests similar antibacterial origins as have been proposed for lupus auto-antibodies. In the absence of overt infection they might reflect a breakdown of tolerance for non-organ specific membrane antigens in diseases with predominantly organ specific membrane bound putative autoimmunogens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. M., Lampman G. W., Furie B. C., Naparstek Y., Schwartz R. S., Stollar B. D., Furie B. Homology of the NH2-terminal amino acid sequences of the heavy and light chains of human monoclonal lupus autoantibodies containing the dominant 16/6 idiotype. J Clin Invest. 1985 Apr;75(4):1138–1143. doi: 10.1172/JCI111808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaco C. B., Elkon K. B. The lupus anticoagulant. A disease marker in antinuclear antibody negative lupus that is cross-reactive with autoantibodies to double-stranded DNA. Arthritis Rheum. 1985 Jan;28(1):67–74. doi: 10.1002/art.1780280111. [DOI] [PubMed] [Google Scholar]
- Colaco C. B., Scadding G., Newsom-Davis J. Anti-cardiolipin antibodies in neurological diseases. Lancet. 1984 Jan 21;1(8369):164–164. doi: 10.1016/s0140-6736(84)90093-x. [DOI] [PubMed] [Google Scholar]
- Colaço C. B., Male D. K. Anti-phospholipid antibodies in syphilis and a thrombotic subset of SLE: distinct profiles of epitope specificity. Clin Exp Immunol. 1985 Feb;59(2):449–456. [PMC free article] [PubMed] [Google Scholar]
- Compston D. A., Vincent A., Newsom-Davis J., Batchelor J. R. Clinical, pathological, HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain. 1980 Sep;103(3):579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- Cooke A., Lydyard P. M., Roitt I. M. Autoimmunity and idiotypes. Lancet. 1984 Sep 29;2(8405):723–725. doi: 10.1016/s0140-6736(84)92628-x. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Daube J., O'Brien P., Pineda A., Low P. A., Windebank A. J., Swanson C. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. N Engl J Med. 1986 Feb 20;314(8):461–465. doi: 10.1056/NEJM198602203140801. [DOI] [PubMed] [Google Scholar]
- Jacob L., Tron F., Bach J. F., Louvard D. A monoclonal anti-DNA antibody also binds to cell-surface protein(s). Proc Natl Acad Sci U S A. 1984 Jun;81(12):3843–3845. doi: 10.1073/pnas.81.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E. A., Lassus A. The occurrence of circulating anticoagulants in patients with syphilitic and biologically false positive antilipoidal antibodies. Ann Clin Res. 1974 Apr;6(2):105–108. [PubMed] [Google Scholar]
- Koike T., Sueishi M., Funaki H., Tomioka H., Yoshida S. Anti-phospholipid antibodies and biological false positive serological test for syphilis in patients with systemic lupus erythematosus. Clin Exp Immunol. 1984 Apr;56(1):193–199. [PMC free article] [PubMed] [Google Scholar]
- Lafer E. M., Rauch J., Andrzejewski C., Jr, Mudd D., Furie B., Furie B., Schwartz R. S., Stollar B. D. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981 Apr 1;153(4):897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang B., Newsom-Davis J., Wray D., Vincent A., Murray N. Autoimmune aetiology for myasthenic (Eaton-Lambert) syndrome. Lancet. 1981 Aug 1;2(8240):224–226. doi: 10.1016/s0140-6736(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Lennon V. A., Lambert E. H., Whittingham S., Fairbanks V. Autoimmunity in the Lambert-Eaton myasthenic syndrome. Muscle Nerve. 1982;5(9S):S21–S25. [PubMed] [Google Scholar]
- Lindstrom J. M., Seybold M. E., Lennon V. A., Whittingham S., Duane D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976 Nov;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Duggan D., Schattner A., Madaio M. P., Goni F., Frangione B., Stollar B. D., Kabat E. A., Schwartz R. S. Immunochemical similarities between monoclonal antibacterial Waldenstrom's macroglobulins and monoclonal anti-DNA lupus autoantibodies. J Exp Med. 1985 Jun 1;161(6):1525–1538. doi: 10.1084/jem.161.6.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe J., Gahan S., Cuzner M. L. Serum antibodies against central nervous system proteins in human demyelinating disease. Clin Exp Immunol. 1985 Feb;59(2):383–390. [PMC free article] [PubMed] [Google Scholar]
- Oosterhuis H. J., de Haas W. H. Rheumatic diseases in patients with myasthenia gravis. An epidemiological and clinical investigation. Acta Neurol Scand. 1968;44(2):219–227. doi: 10.1111/j.1600-0404.1968.tb05568.x. [DOI] [PubMed] [Google Scholar]
- Palosuo T., Vaarala O., Kinnunen E. Anticardiolipin antibodies in Guillain-Barré syndrome. Lancet. 1985 Oct 12;2(8459):839–839. doi: 10.1016/s0140-6736(85)90831-1. [DOI] [PubMed] [Google Scholar]
- Plotz P. H. Autoantibodies are anti-idiotype antibodies to antiviral antibodies. Lancet. 1983 Oct 8;2(8354):824–826. doi: 10.1016/s0140-6736(83)90740-7. [DOI] [PubMed] [Google Scholar]
- Rauch J., Tannenbaum H., Stollar B. D., Schwartz R. S. Monoclonal anti-cardiolipin antibodies bind to DNA. Eur J Immunol. 1984 Jun;14(6):529–534. doi: 10.1002/eji.1830140609. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Smolarsky M. A simple radioimmunoassay to determine binding of antibodies to lipid antigens. J Immunol Methods. 1980;38(1-2):85–93. doi: 10.1016/0022-1759(80)90333-6. [DOI] [PubMed] [Google Scholar]
- Stefansson K., Dieperink M. E., Richman D. P., Gomez C. M., Marton L. S. Sharing of antigenic determinants between the nicotinic acetylcholine receptor and proteins in Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae. Possible role in the pathogenesis of myasthenia gravis. N Engl J Med. 1985 Jan 24;312(4):221–225. doi: 10.1056/NEJM198501243120407. [DOI] [PubMed] [Google Scholar]
- Strauss A. J., Smith C. W., Cage G. W., van der Geld H. W., McFarlin D. E., Barlow M. Further studies on the specificity of presumed immune associations of myasthenia gravis and consideration of possible pathogenic implications. Ann N Y Acad Sci. 1966 Jan 26;135(1):557–579. doi: 10.1111/j.1749-6632.1966.tb45504.x. [DOI] [PubMed] [Google Scholar]
- Thiagarajan P., Shapiro S. S., De Marco L. Monoclonal immunoglobulin M lambda coagulation inhibitor with phospholipid specificity. Mechanism of a lupus anticoagulant. J Clin Invest. 1980 Sep;66(3):397–405. doi: 10.1172/JCI109869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull A. K., Ebringer R., Panayi G. S., Colthorpe D., James D. C., Ebringer A. IgA antibodies to Klebsiella pneumoniae in ankylosing spondylitis. Scand J Rheumatol. 1983;12(3):249–253. doi: 10.3109/03009748309098543. [DOI] [PubMed] [Google Scholar]
- el-Roiey A., Sela O., Isenberg D. A., Feldman R., Colaco B. C., Kennedy R. C., Shoenfeld Y. The sera of patients with Klebsiella infections contain a common anti-DNA idiotype (16/6) Id and anti-polynucleotide activity. Clin Exp Immunol. 1987 Mar;67(3):507–515. [PMC free article] [PubMed] [Google Scholar]


