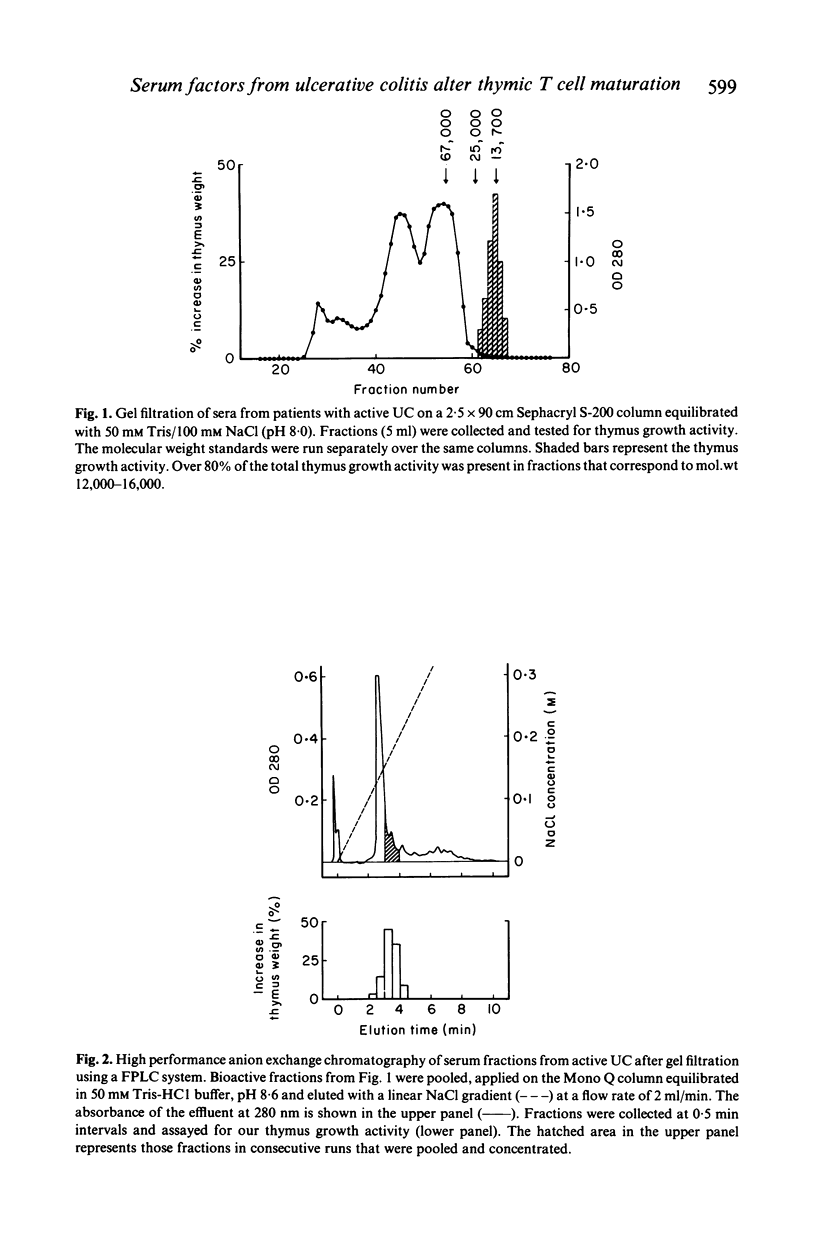
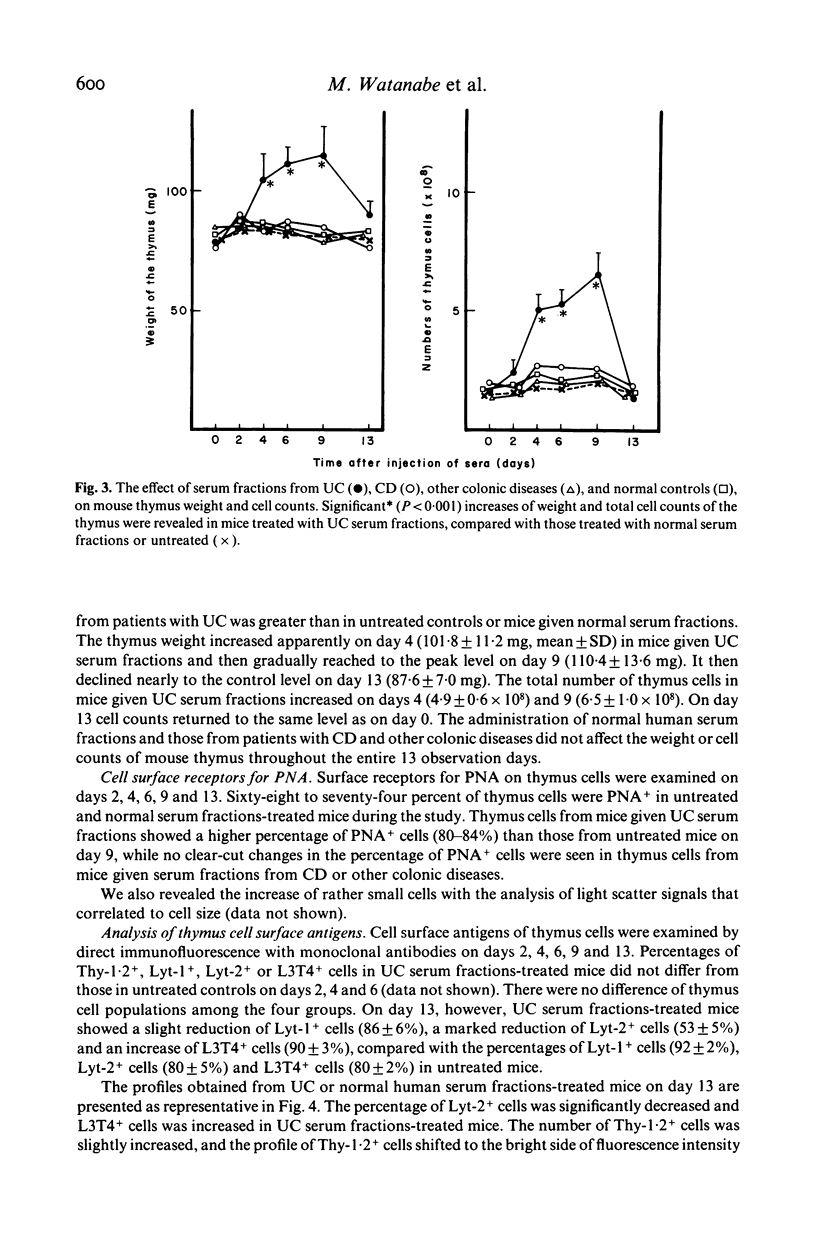
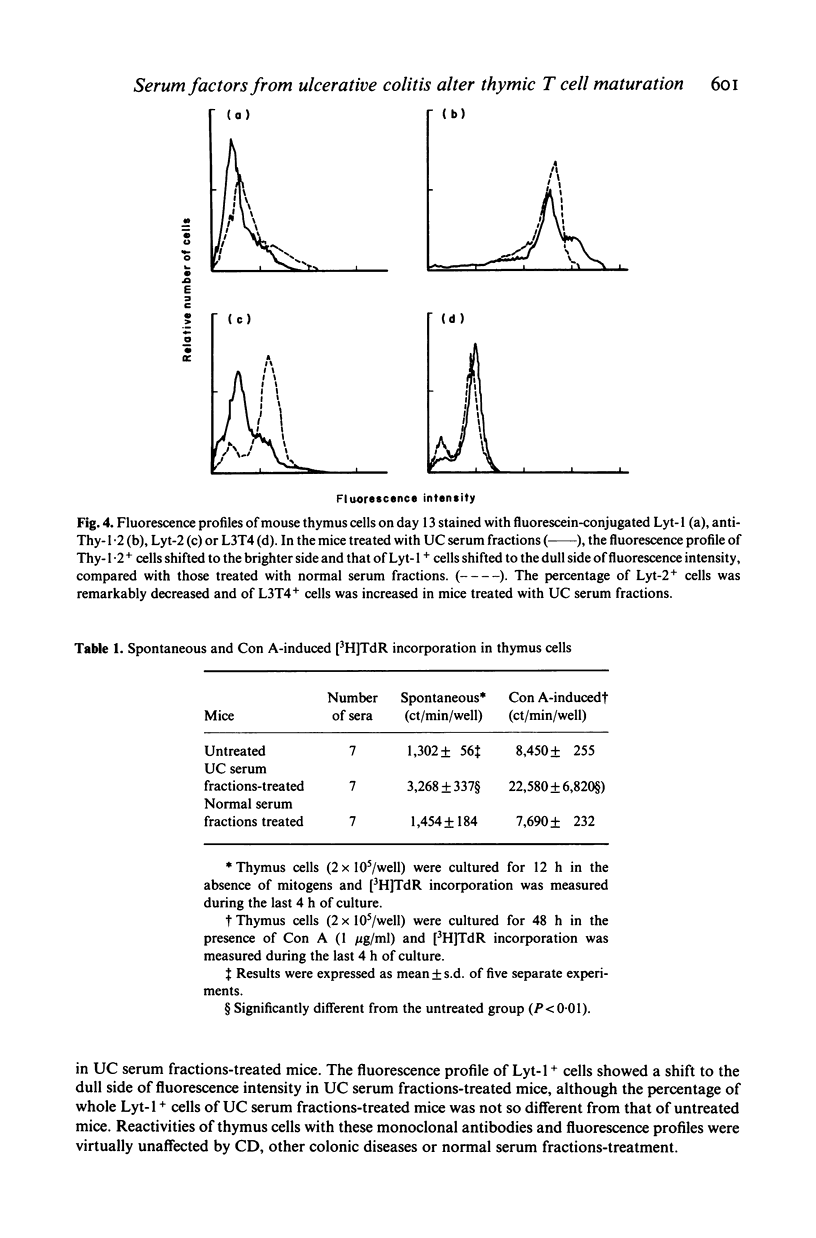
Abstract
Recently it has been reported that patients with ulcerative colitis (UC) often have thymus abnormalities, although the precise mechanisms which induce those abnormalities remain unclear. We have examined the effect of serum fractions from patients with UC and other colonic diseases on mouse thymus to clarify the possible existence of factors which have thymus growth activity. These fractions were separated from sera of patients with UC by gel filtration and anion exchange high performance liquid chromatography. In mice given UC serum fractions; (i) remarkable increases in weight and total cell number of the thymus were observed from day 4 to day 9; (ii) a significant increase in the number of peanut agglutinin (PNA)+ thymus cells was demonstrated using flow cytometry on day 9; (iii) on quantitative analysis of surface antigens the percentage of Lyt-2+ thymus cells decreased and that of L3T4+ thymus cells increased remarkably on day 13; the number of bright Thy-1.2+ cells and of dull Lyt-1+ cells increased. In contrast, the serum fractions from patients with other colonic diseases and from normal persons caused little change in mouse thymus throughout the study. The results suggest that factors fractionated from the serum of patients with UC disturb intra-thymic T cell maturation and enhance the proliferation of thymus cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiso S., Watanabe M., Hibi T., Yoshida T., Tsuchiya M., Tsuru S. Characterization of immunoregulatory T cells and lymphocytophilic antibodies in ulcerative colitis: analysis with monoclonal antibodies. J Clin Lab Immunol. 1982 Nov;9(2):109–112. [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Lymphocytes as models for the study of mammalian cellular differentiation. Immunol Rev. 1977 Jan;33:105–124. doi: 10.1111/j.1600-065x.1977.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Cantor H., Weissman I. Development and function of subpopulations of thymocytes and T lymphocytes. Prog Allergy. 1976;20:1–64. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Hibi T., Aiso S., Yoshida T., Watanabe M., Asakura H., Tsuru S., Tsuchiya M. Anti-colon antibody and lymphocytophilic antibody in ulcerative colitis. Clin Exp Immunol. 1982 Jul;49(1):75–80. [PMC free article] [PubMed] [Google Scholar]
- Kirsner J. B., Shorter R. G. Recent developments in nonspecific inflammatory bowel disease (second of two parts). N Engl J Med. 1982 Apr 8;306(14):837–848. doi: 10.1056/NEJM198204083061404. [DOI] [PubMed] [Google Scholar]
- Le Brigand H., Leuasseur P., Merlier M., Rojas-Miranda A., Gaud C., Noviant Y. Cent thymectomies chez des myasthéniques. Résultats lointains. Rev Neurol (Paris) 1972 Apr;126(4):267–274. [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Berrih S., Bach J. F. Peanut agglutinin. I. A new tool for studying T lymphocyte subpopulations. J Immunol. 1978 Aug;121(2):438–443. [PubMed] [Google Scholar]
- Mathieson B. J., Sharrow S. O., Campbell P. S., Asofsky R. An Lyt differentiated thymocyte subpopulation detected by flow microfluorometry. Nature. 1979 Feb 8;277(5696):478–480. doi: 10.1038/277478a0. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Shimabukuro K., Kurita K., Tsuchiya M. Thymus abnormalities in ulcerative colitis- comparative study with other autoimmune diseases. Gastroenterol Jpn. 1976;11(3):208–214. doi: 10.1007/BF02777706. [DOI] [PubMed] [Google Scholar]
- Papatestas A. E., Alpert L. I., Osserman K. E., Osserman R. S., Kark A. E. Studies in myasthenia gravis: effects of thymectomy. Results on 185 patients with nonthymomatous and thymomatous myasthenia gravis, 1941-1969. Am J Med. 1971 Apr;50(4):465–474. doi: 10.1016/0002-9343(71)90336-6. [DOI] [PubMed] [Google Scholar]
- Penit C. In vivo thymocyte maturation. BUdR labeling of cycling thymocytes and phenotypic analysis of their progeny support the single lineage model. J Immunol. 1986 Oct 1;137(7):2115–2121. [PubMed] [Google Scholar]
- Reisner Y., Itzicovitch L., Meshorer A., Sharon N. Hemopoietic stem cell transplantation using mouse bone marrow and spleen cells fractionated by lectins. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2933–2936. doi: 10.1073/pnas.75.6.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Scollay R., Shortman K. Identification of early stages of T lymphocyte development in the thymus cortex and medulla. J Immunol. 1985 Jun;134(6):3632–3642. [PubMed] [Google Scholar]
- Tomono T., Ikeda H., Tokunaga E. High-performance ion-exchange chromatography of plasma proteins. J Chromatogr. 1983 Aug 26;266:39–47. doi: 10.1016/s0021-9673(01)90877-5. [DOI] [PubMed] [Google Scholar]
- Toyka K. V., Drachman D. B., Griffin D. E., Pestronk A., Winkelstein J. A., Fishbeck K. H., Kao I. Myasthenia gravis. Study of humoral immune mechanisms by passive transfer to mice. N Engl J Med. 1977 Jan 20;296(3):125–131. doi: 10.1056/NEJM197701202960301. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M. Immunological abnormalities involving the thymus in ulcerative colitis and therapeutic effects of thymectomy. Gastroenterol Jpn. 1984 Jun;19(3):232–246. doi: 10.1007/BF02779175. [DOI] [PubMed] [Google Scholar]
- van Ewijk W., van Soest P. L., van den Engh G. J. Fluorescence analysis and anatomic distribution of mouse T lymphocyte subsets defined by monoclonal antibodies to the antigens Thy-1, Lyt-1, Lyt-2, and T-200. J Immunol. 1981 Dec;127(6):2594–2604. [PubMed] [Google Scholar]



