Abstract
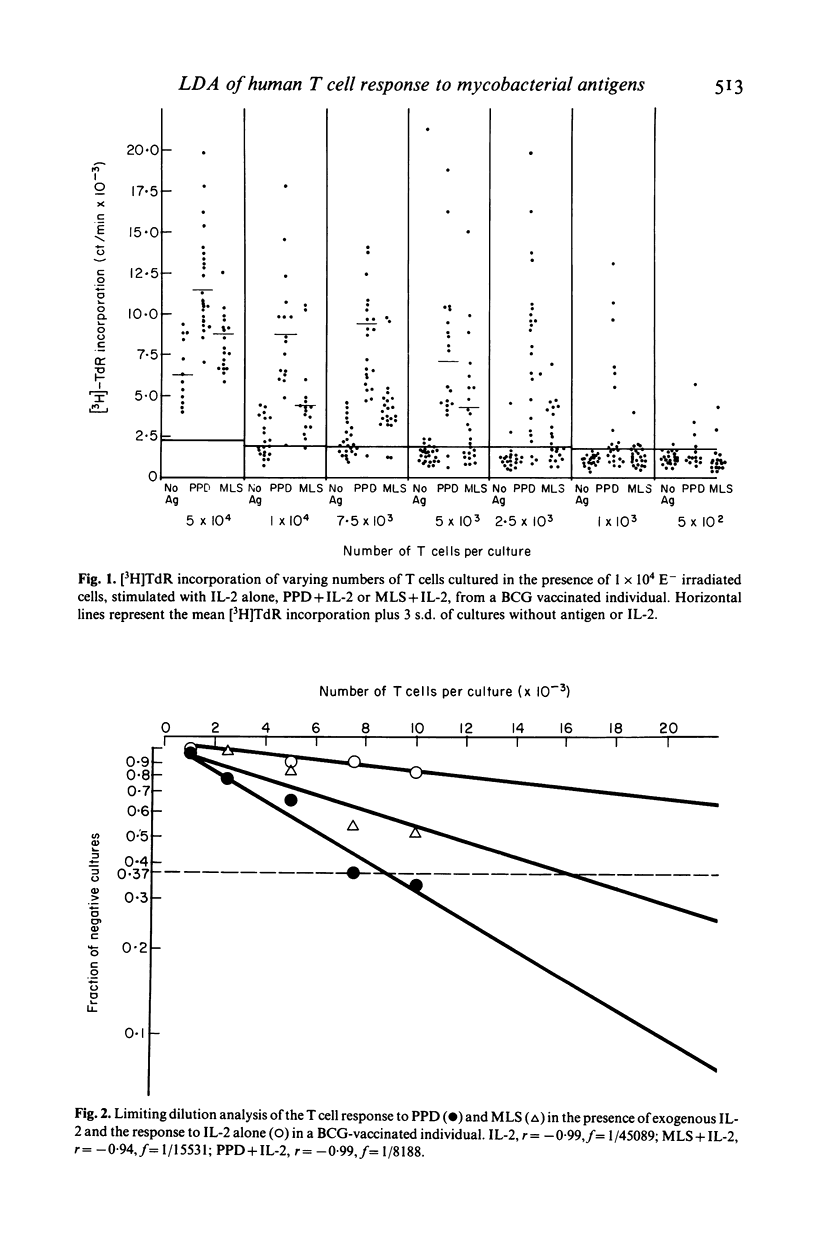
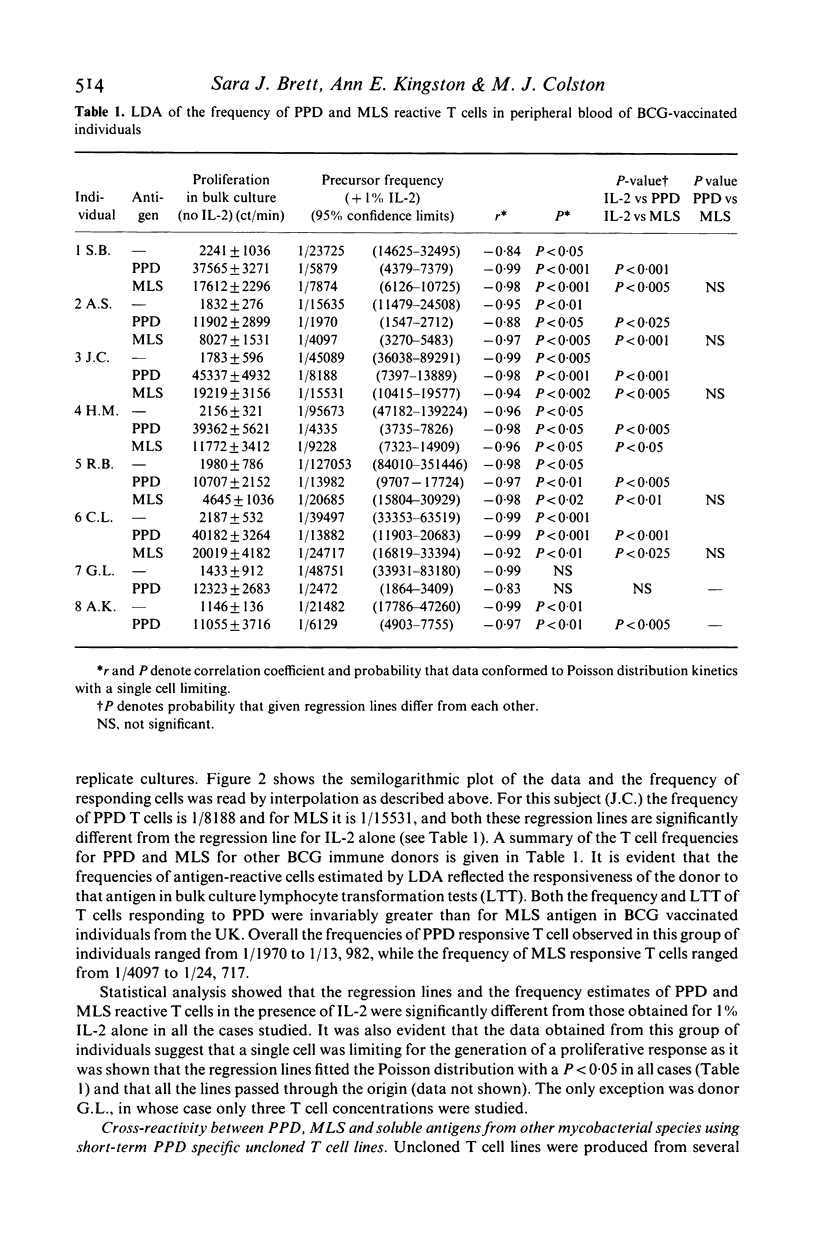
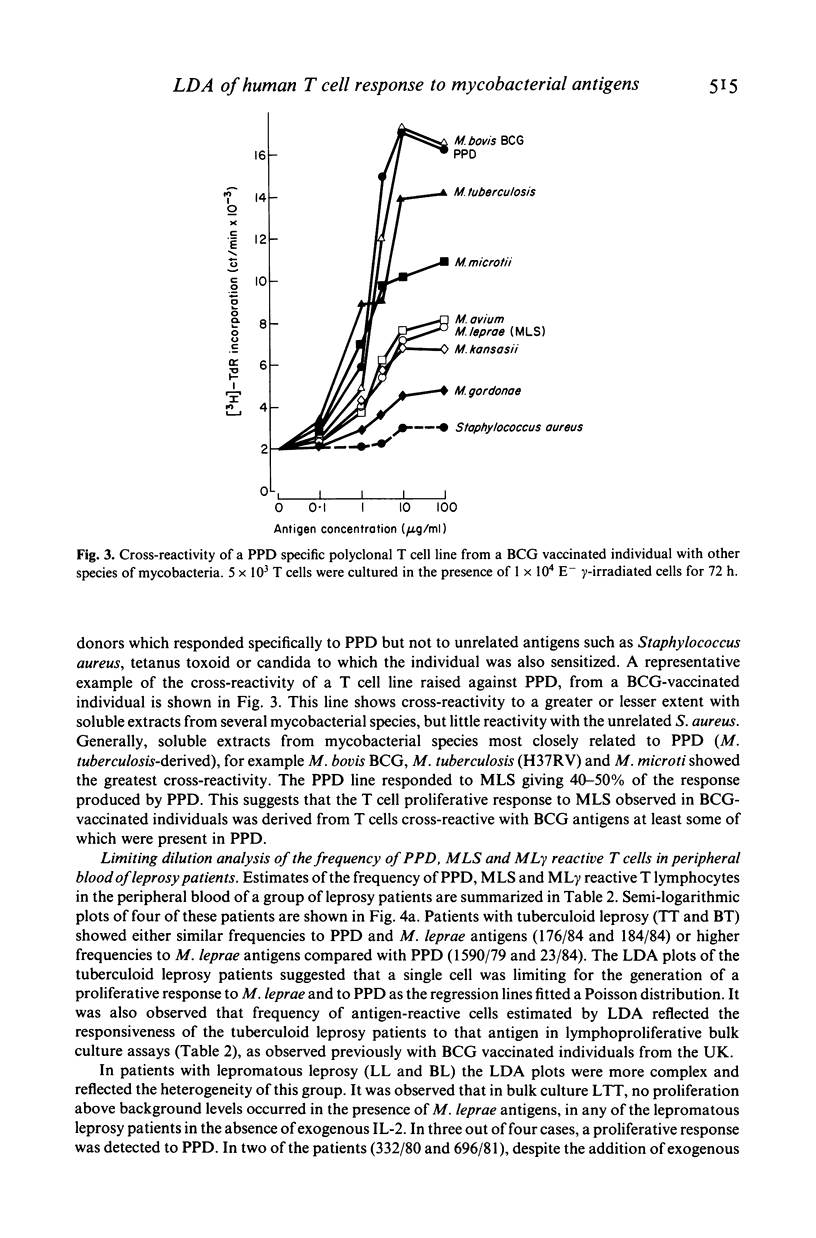
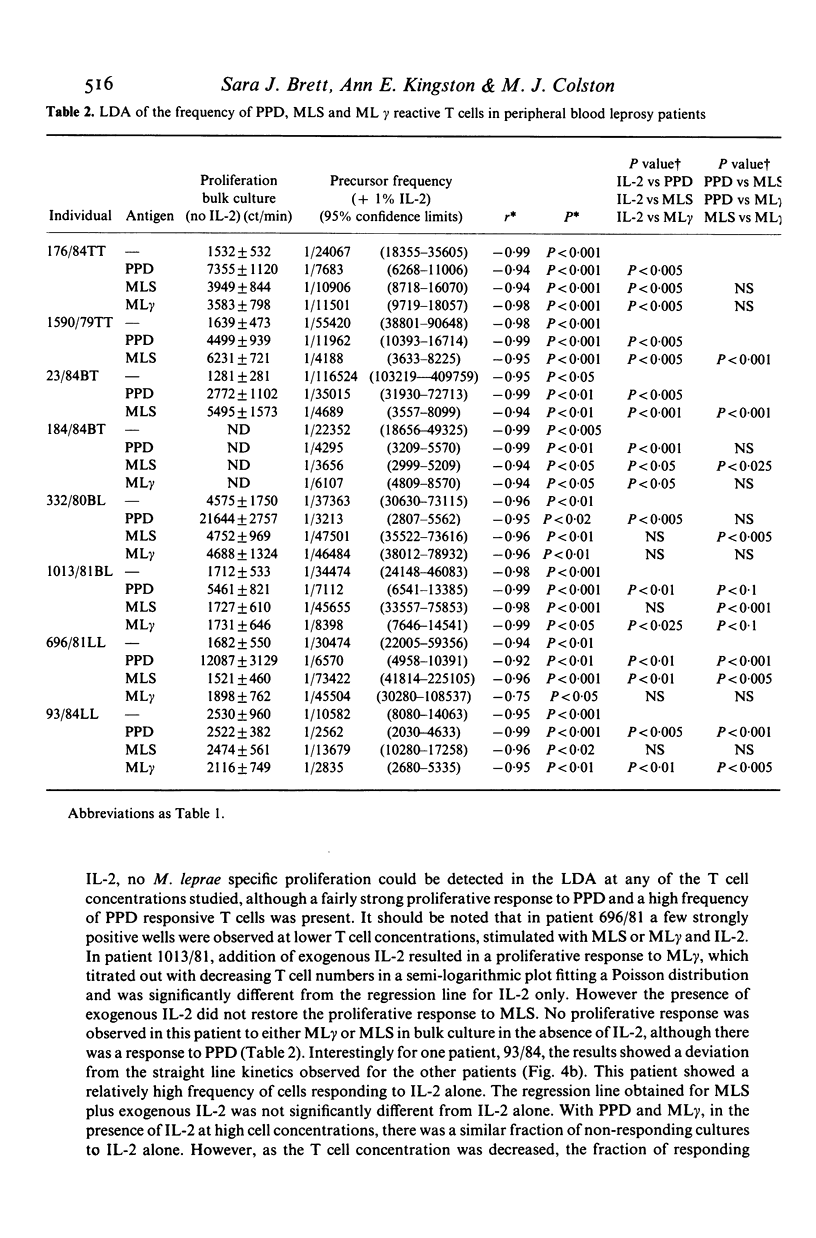
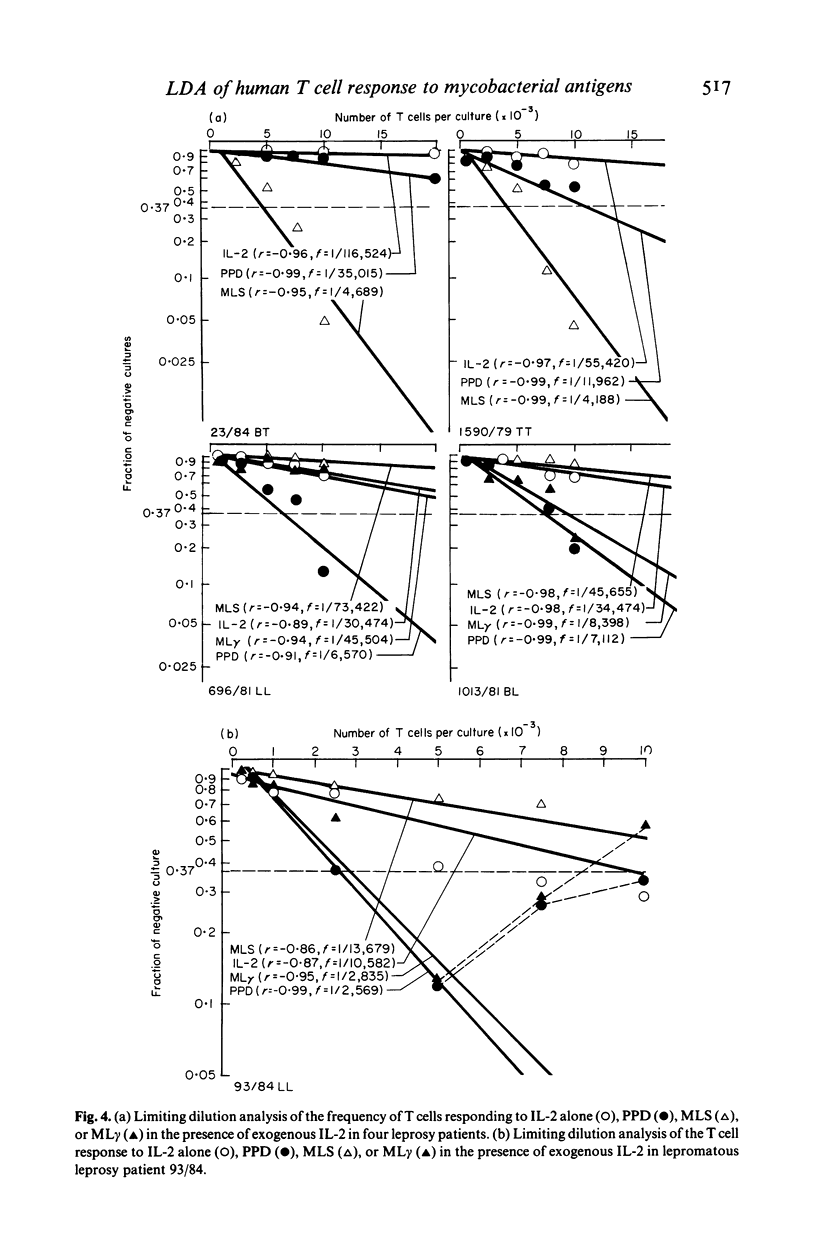
The number of peripheral blood T lymphocytes responding to soluble mycobacterial antigens from Mycobacterium tuberculosis purified protein derivative (PPD) and M. leprae (MLS) was estimated by limiting dilution analysis. Antigen-induced lymphocyte activation was measured by means of [3H]TdR incorporation on day 10 of culture in the presence of suboptimal concentrations of interleukin 2 (IL-2). In the peripheral blood of BCG-vaccinated individuals from the UK, the frequency of T lymphocytes responding to PPD was 1.5 to 4 times greater than to MLS. Frequencies between 1/1970 and 1/13, 982 were observed in response to PPD and between 1/4097 and 1/24, 717 in response to MLS. A proportion of cells in the peripheral blood were also observed to respond to IL-2 only. The frequency of cells observed in limiting dilution analysis for PPD and MLS reflected the relative amounts of proliferation to these two antigens in bulk culture lymphocyte transformation tests. Use of PPD-specific T cell lines suggested that the responsiveness observed to M. leprae antigens in BCG-vaccinated individuals was due to cross-reactivity with antigens shared with M. bovis BCG. In tuberculoid leprosy, the frequency of peripheral blood T lymphocytes responding to M. leprae antigens was either greater than or similar to the frequency of T cells responding to PPD. In contrast, limiting dilution analysis of T lymphocytes from the peripheral blood of lepromatous leprosy patients revealed the complex regulatory heterogeneity of this group. In some patients M. leprae responsive T cells were detected in the presence of exogenous IL-2.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. A., Wallach D., Flageul B., Cottenot F. In vitro proliferative response to M. leprae and PPD of isolated T cell subsets from leprosy patients. Clin Exp Immunol. 1983 Apr;52(1):107–114. [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Ford D., Burger D. Precursor frequency of antigen-specific T cells: effects of sensitization in vivo and in vitro. Cell Immunol. 1983 Jul 15;79(2):334–344. doi: 10.1016/0008-8749(83)90075-8. [DOI] [PubMed] [Google Scholar]
- Gebel H. M., Scott J. R., Parvin C. A., Rodey G. E. In vitro immunization to KLH. II. Limiting dilution analysis of antigen-reactive cells in primary and secondary culture. J Immunol. 1983 Jan;130(1):29–32. [PubMed] [Google Scholar]
- Geha R. S. Dynamics of human circulating antigen reactive cells following secondary immunization with tetanus toxoid. Clin Immunol Immunopathol. 1981 May;19(2):196–205. doi: 10.1016/0090-1229(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Kettman J. R., Soederberg A., Lefkovits I. Activation/suppression of the immune response in vitro by antigen and dextran sulfate. I. Analysis by limiting dilution methods. Eur J Immunol. 1985 Jul;15(7):708–712. doi: 10.1002/eji.1830150713. [DOI] [PubMed] [Google Scholar]
- Kingston A. E., Stagg A. J., Colston M. J. Investigation of antigen cross-reactivity of Mycobacterium leprae-reactive murine T-cell lines and clones. Immunology. 1986 Jun;58(2):217–223. [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Johnson A. H., Hartzman R. J., Woody J. N. Antigen-specific human T lymphocyte clones: induction, antigen specificity, and MHC restriction of influenza virus-immune clones. J Immunol. 1982 Jan;128(1):233–238. [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Miller R. A. Clonal endurance and clonal burst size: novel indices of helper T cell heterogeneity. Eur J Immunol. 1984 Nov;14(11):992–997. doi: 10.1002/eji.1830141106. [DOI] [PubMed] [Google Scholar]
- Mohagheghpour N., Gelber R. H., Larrick J. W., Sasaki D. T., Brennan P. J., Engleman E. G. Defective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with defective expression of interleukin 2 receptors and is not reconstituted by interleukin 2. J Immunol. 1985 Aug;135(2):1443–1449. [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Nath I., Sathish M., Jayaraman T., Bhutani L. K., Sharma A. K. Evidence for the presence of M. leprae reactive T lymphocytes in patients with lepromatous leprosy. Clin Exp Immunol. 1984 Dec;58(3):522–530. [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Kaplan G., Levy E., Sarno E. N., Kushner P., Granelli-Piperno A., Vieira L., Colomer Gould V., Levis W., Steinman R. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J Exp Med. 1983 Dec 1;158(6):2165–2170. doi: 10.1084/jem.158.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Smelt A. H., Rees R. J., Liew F. Y. Induction of delayed-type hypersensitivity to Mycobacterium leprae in healthy individuals. Clin Exp Immunol. 1981 Jun;44(3):501–506. [PMC free article] [PubMed] [Google Scholar]
- Sohnle P. G., Collins-Lech C. Lymphocyte transformation and the number of antigen-responsive cells in humans. J Immunol. 1981 Aug;127(2):612–615. [PubMed] [Google Scholar]
- van Oers M. H., Pinkster J., Zeijlemaker W. P. Quantification of antigen-reactive cells among human T lymphocytes. Eur J Immunol. 1978 Jul;8(7):477–484. doi: 10.1002/eji.1830080706. [DOI] [PubMed] [Google Scholar]


