Abstract
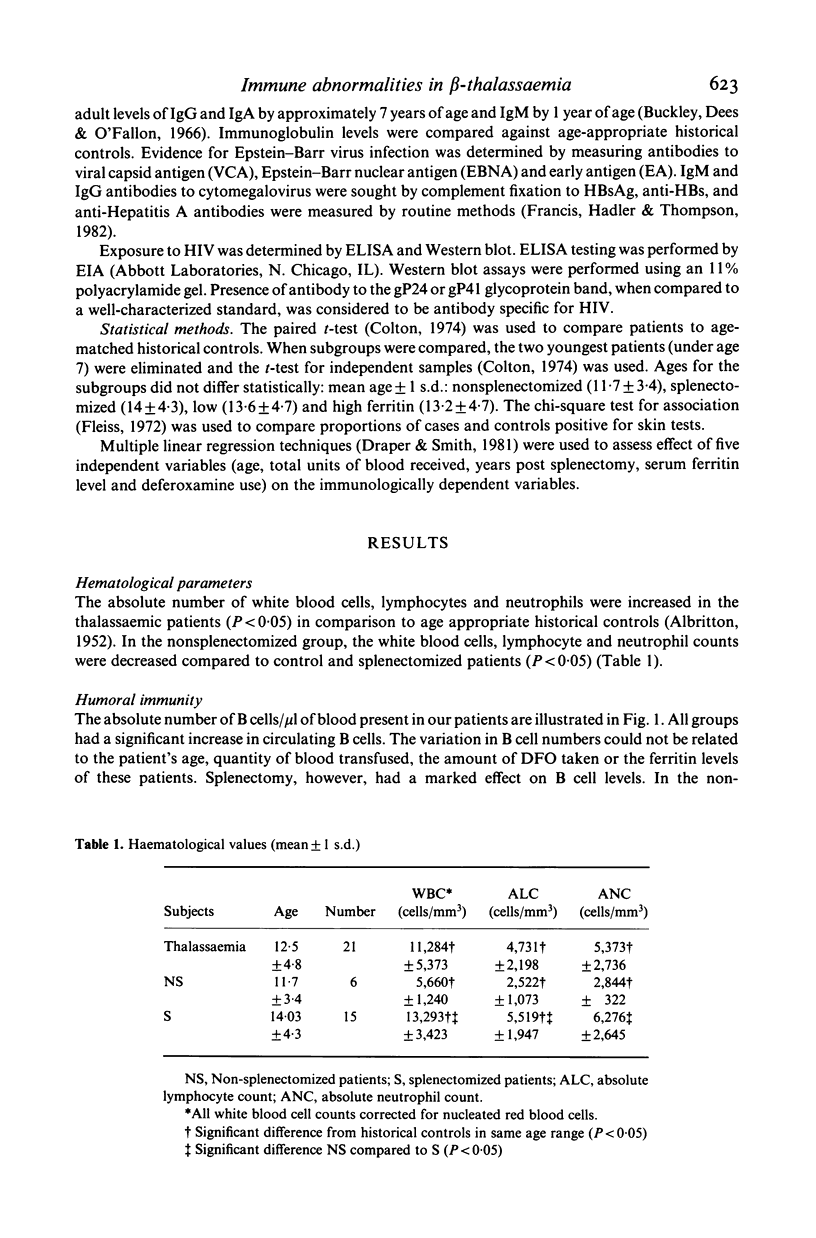
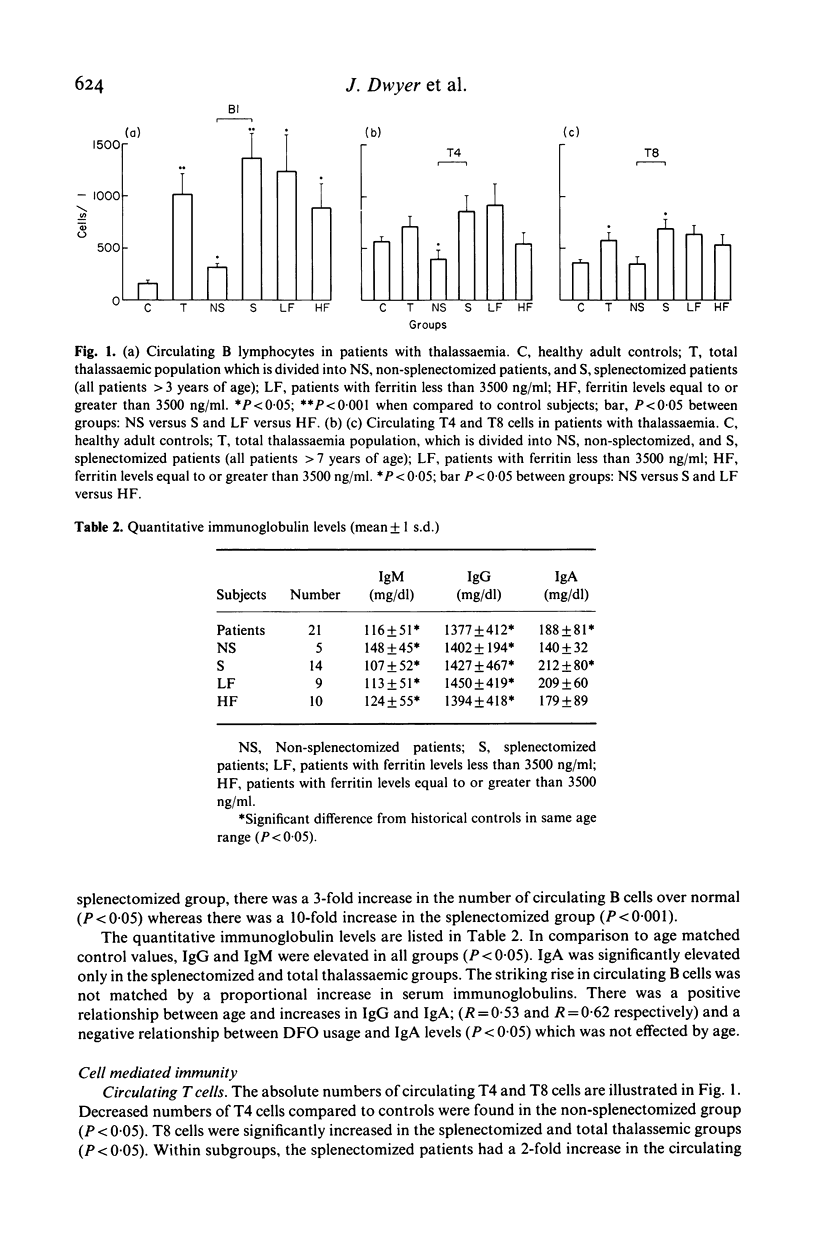
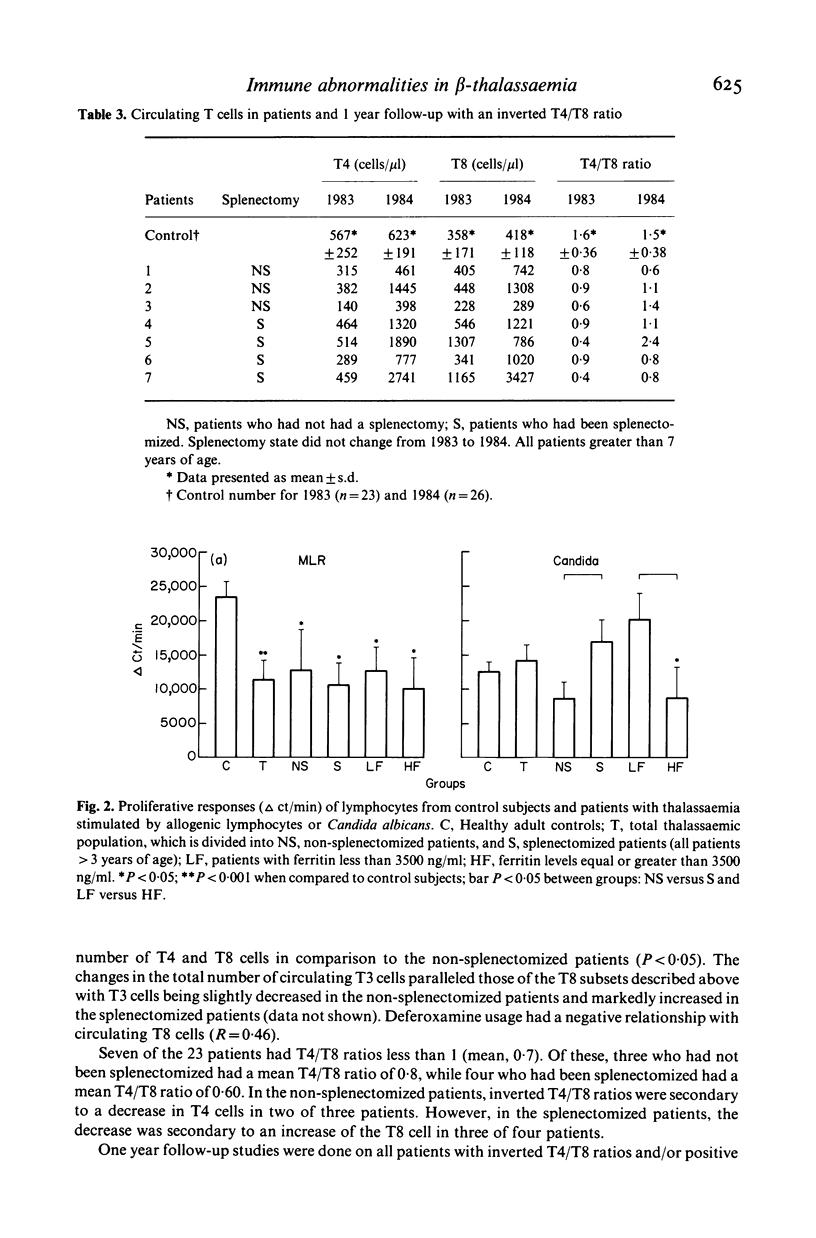
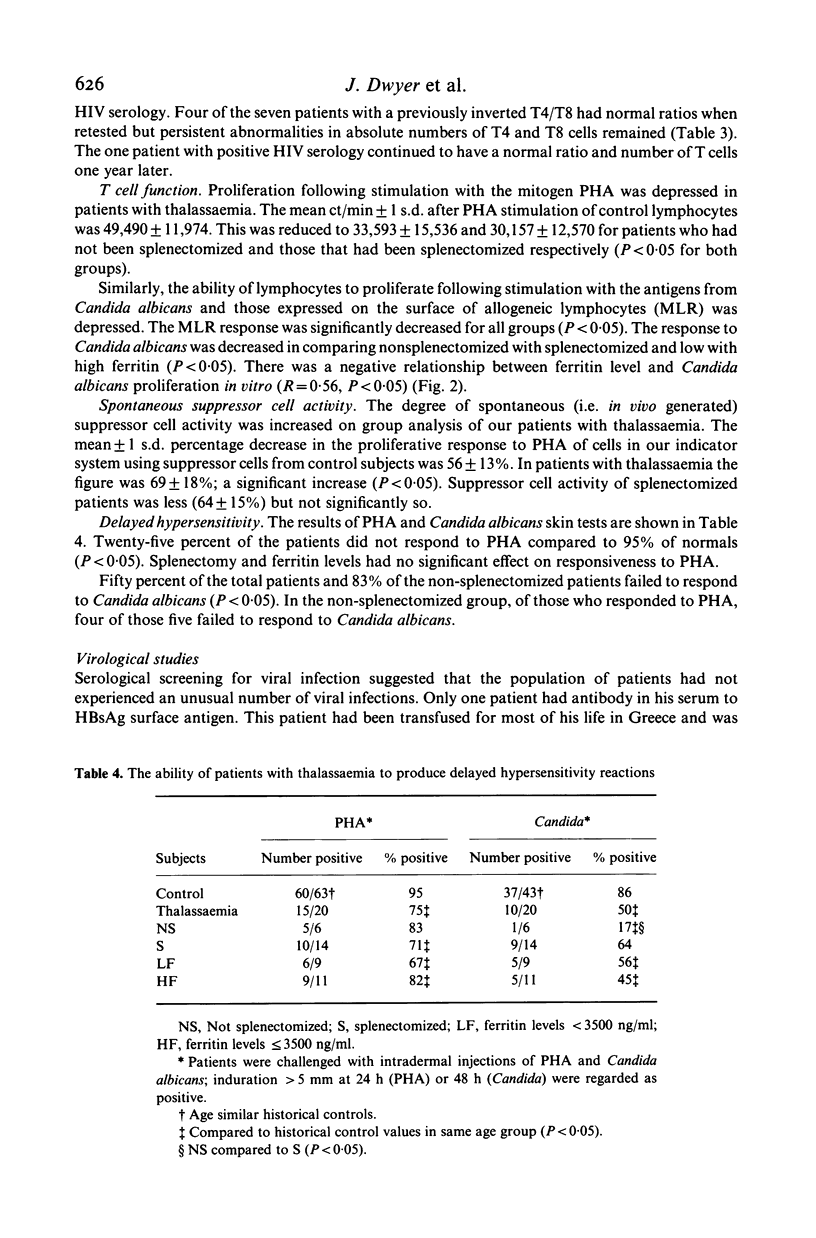
We have studied both the humoral and cell mediated immune systems of 23 children with beta-thalassaemia major. In children who had not been splenectomized, a 3-fold expansion in the number of circulating B cells and a modest polyclonal gammopathy was present. Of these patients 70% had decreased numbers of circulating T4 cells; 83% were unresponsive to skin testing with Candida albicans, and the majority had decreased lymphocyte proliferative responses in vitro. In children who had been splenectomized, there was a 10-fold increase in the number of circulating B lymphocytes and a 2-fold increase in the number of T4 and T8 cells present in peripheral blood. Additionally, these patients as a group were more responsive to both skin testing and lymphocyte stimulation in vitro with Candida albicans. Seven patients had an inverted T4/T8 ratio. One child has positive serology to HIV by ELISA and Western Blot techniques with a normal T4/T8 ratio. Thus, while children with thalassaemia are at risk for exposure to HIV, the immunological abnormalities associated with the disease and/or its treatment necessitates cautious interpretation of any AIDS-related immunological changes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan C. F., Nishiya K., Pollack M. S., Dupont B., de Sousa M. Differential inhibition of the MLR by iron: association with HLA phenotype. Immunogenetics. 1981;12(1-2):129–140. doi: 10.1007/BF01561656. [DOI] [PubMed] [Google Scholar]
- Buckley R. H., Dees S. C., O'Fallon W. M. Serum immunoglobulins. I. Levels in normal children and in uncomplicated childhood allergy. Pediatrics. 1968 Mar;41(3):600–611. [PubMed] [Google Scholar]
- Buffe D., Rimbaut C. Immunosuppresive effect of a human hepatic glycoferroprotein, alpha2H globulin. A study on the transformation of normal human lymphocytes. Immunology. 1975 Jul;29(1):175–184. [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M., Johnson C., Desaules M. Behaviour of human immunoregulatory cells in culture. I. Variables requiring consideration for clinical studies. Clin Exp Immunol. 1979 Dec;38(3):499–513. [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M., Johnson C. The regulation of T cell responses by spontaneously active suppressor cells. Clin Exp Immunol. 1982 Nov;50(2):406–415. [PMC free article] [PubMed] [Google Scholar]
- Falcão R. P. Human blood lymphocyte subpopulations from birth to eight years. Clin Exp Immunol. 1980 Jan;39(1):203–207. [PMC free article] [PubMed] [Google Scholar]
- Gascón P., Zoumbos N. C., Young N. S. Immunologic abnormalities in patients receiving multiple blood transfusions. Ann Intern Med. 1984 Feb;100(2):173–177. doi: 10.7326/0003-4819-100-2-173. [DOI] [PubMed] [Google Scholar]
- Grady R. W., Akbar A. N., Giardina P. J., Hilgartner M. W., de Sousa M. Disproportionate lymphoid cell subsets in thalassaemia major: the relative contributions of transfusion and splenectomy. Br J Haematol. 1985 Apr;59(4):713–724. doi: 10.1111/j.1365-2141.1985.tb07367.x. [DOI] [PubMed] [Google Scholar]
- Guglielmo P., Cunsolo F., Lombardo T., Sortino G., Giustolisi R., Cacciola E., Cacciola E. T-subset abnormalities in thalassaemia intermedia: possible evidence for a thymus functional deficiency. Acta Haematol. 1984;72(6):361–367. doi: 10.1159/000206421. [DOI] [PubMed] [Google Scholar]
- Kapadia A., de Sousa M., Markenson A. L., Miller D. R., Good R. A., Gupta S. Lymphoid cell sets and serum immunoglobulins in patients with thalassaemia intermedia: relationship to serum iron and splenectomy. Br J Haematol. 1980 Jul;45(3):405–416. doi: 10.1111/j.1365-2141.1980.tb07161.x. [DOI] [PubMed] [Google Scholar]
- Khalifa A. S., Fattah S. A., Maged Z., Sabry F., Mohamed H. A. Immunoglobulin levels, opsonic activity and phagocytic power in Egyptian thalassemic children. Acta Haematol. 1983;69(2):136–139. doi: 10.1159/000206875. [DOI] [PubMed] [Google Scholar]
- Lederman H. M., Cohen A., Lee J. W., Freedman M. H., Gelfand E. W. Deferoxamine: a reversible S-phase inhibitor of human lymphocyte proliferation. Blood. 1984 Sep;64(3):748–753. [PubMed] [Google Scholar]
- Matzner Y., Hershko C., Polliack A., Konijn A. M., Izak G. Suppressive effect of ferritin on in vitro lymphocyte function. Br J Haematol. 1979 Jul;42(3):345–353. doi: 10.1111/j.1365-2141.1979.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Munn C. G., Markenson A. L., Kapadia A., de Sousa M. Impaired T-cell mitogen responses in some patients with thalassemia intermedia. Thymus. 1981 Aug;3(2):119–128. [PubMed] [Google Scholar]
- Musumeci S., Schiliro G., Romeo M. A., Sciotto A., Rosalba A., Pizzarelli G. Lymphocyte changes in beta-thalassaemia major. Arch Dis Child. 1979 Dec;54(12):954–957. doi: 10.1136/adc.54.12.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri-Aria K. T., Lobo-Yeo A., Vergani D., Mieli-Vergani G., Mowat A. P., Eddleston A. L. Immunoregulation of immunoglobulin production in normal infants and children. Clin Exp Immunol. 1985 Mar;59(3):679–686. [PMC free article] [PubMed] [Google Scholar]
- Petricciani J. C., Seto B., Wells M., Quinnan G., McDougal J. S., Bodner A. J. An analysis of serum samples positive for HTLV-III antibodies. N Engl J Med. 1985 Jul 4;313(1):47–48. doi: 10.1056/NEJM198507043130111. [DOI] [PubMed] [Google Scholar]
- Propper R. D., Cooper B., Rufo R. R., Nienhuis A. W., Anderson W. F., Bunn H. F., Rosenthal A., Nathan D. G. Continuous subcutaenous administration of deferoxamine in patients with iron overload. N Engl J Med. 1977 Aug 25;297(8):418–423. doi: 10.1056/NEJM197708252970804. [DOI] [PubMed] [Google Scholar]
- Sinniah D., Yadav M. Elevated IgG and decreased complement component C3 and factor B in B-thalassaemia major. Acta Paediatr Scand. 1981 Jul;70(4):547–550. doi: 10.1111/j.1651-2227.1981.tb05738.x. [DOI] [PubMed] [Google Scholar]
- Tovo P. A., Miniero R., Barbera C., Sacchetti L., Saitta M. Serum immunoglobulins in homozygous beta-thalassemia. Acta Haematol. 1981;65(1):21–25. doi: 10.1159/000207144. [DOI] [PubMed] [Google Scholar]
- Vierucci A., de Martino M., Rossi M. E., Vullo C., Borgatti L., London W. T., Blumberg B. S. Raised IgE levels in beta-thalassaemia: correlation with splenectomy and hepatitis B virus infection. Clin Exp Immunol. 1984 Oct;58(1):199–205. [PMC free article] [PubMed] [Google Scholar]
- WOLMAN I. J. TRANSFUSION THERAPY IN COOLEY'S ANEMIA: GROWTH AND HEALTH AS RELATED TO LONG-RANGE HEMOGLOBIN LEVELS. A PROGRESS REPORT. Ann N Y Acad Sci. 1964 Oct 7;119:736–747. doi: 10.1111/j.1749-6632.1965.tb54075.x. [DOI] [PubMed] [Google Scholar]
- de Sousa M., Smithyman A., Tan C. Suggested models of ecotaxopathy in lymphoreticular malignancy. A role for iron-binding proteins in the control of lymphoid cell migration. Am J Pathol. 1978 Feb;90(2):497–520. [PMC free article] [PubMed] [Google Scholar]


