Abstract
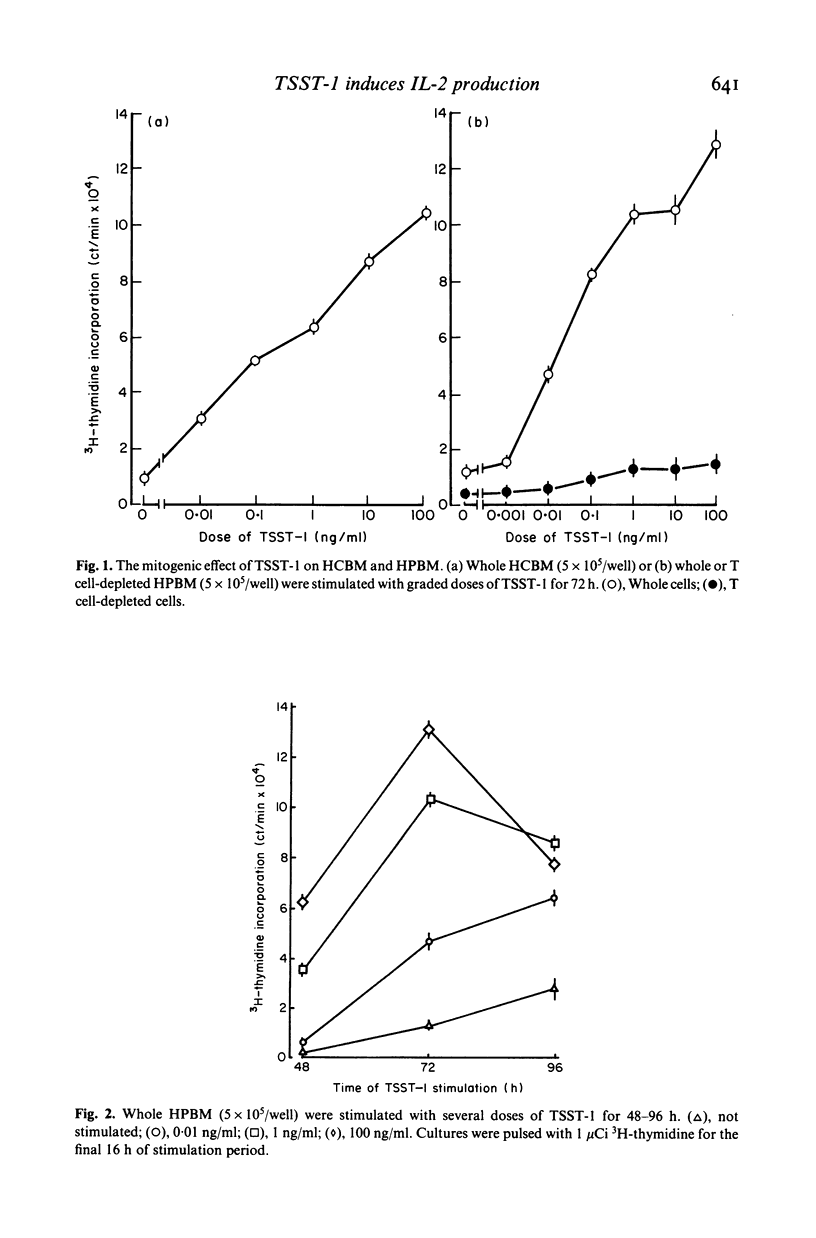
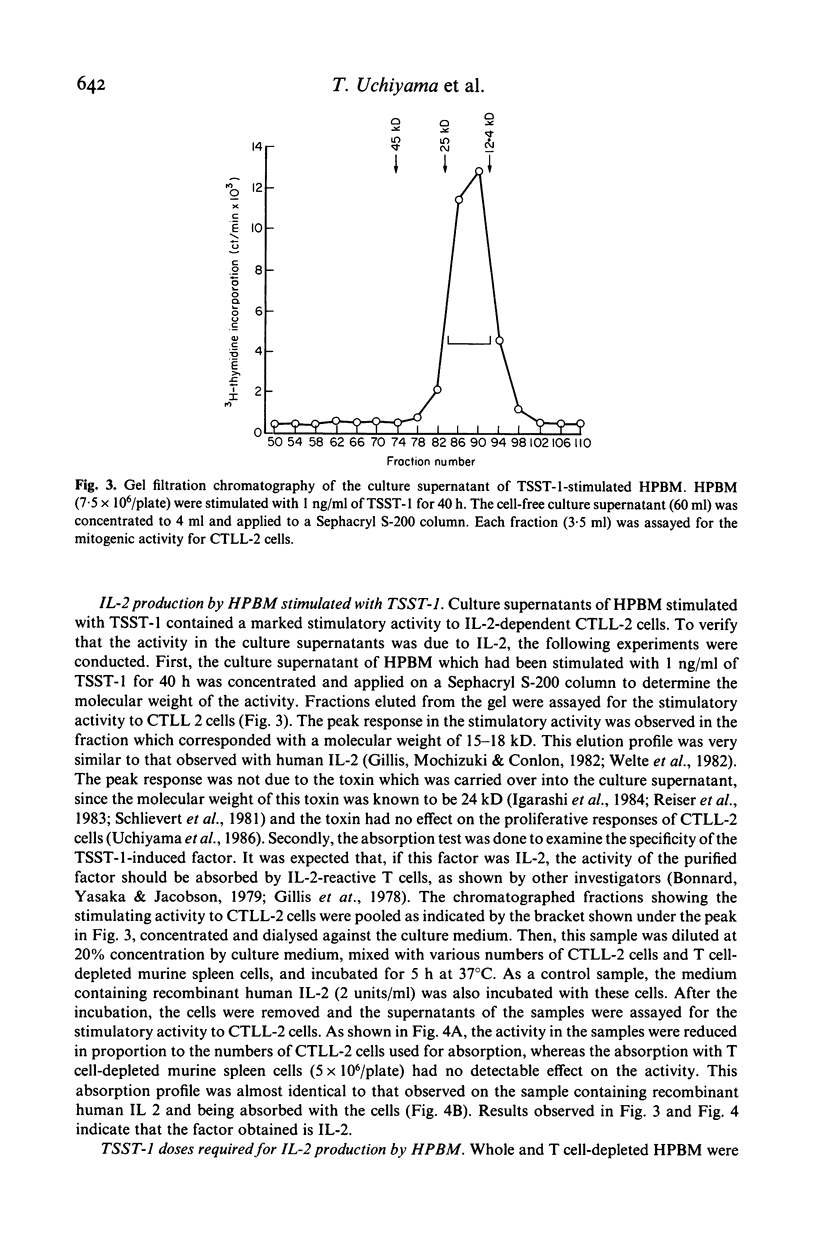
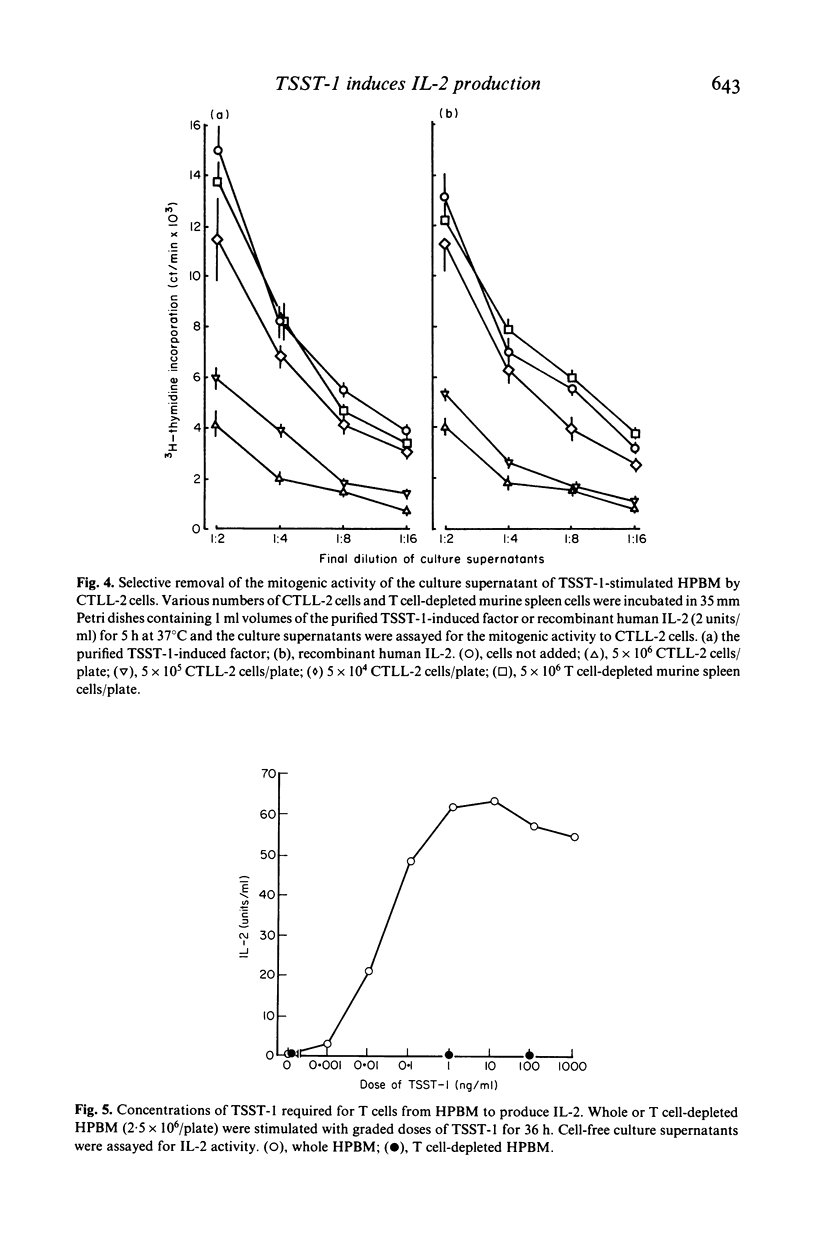
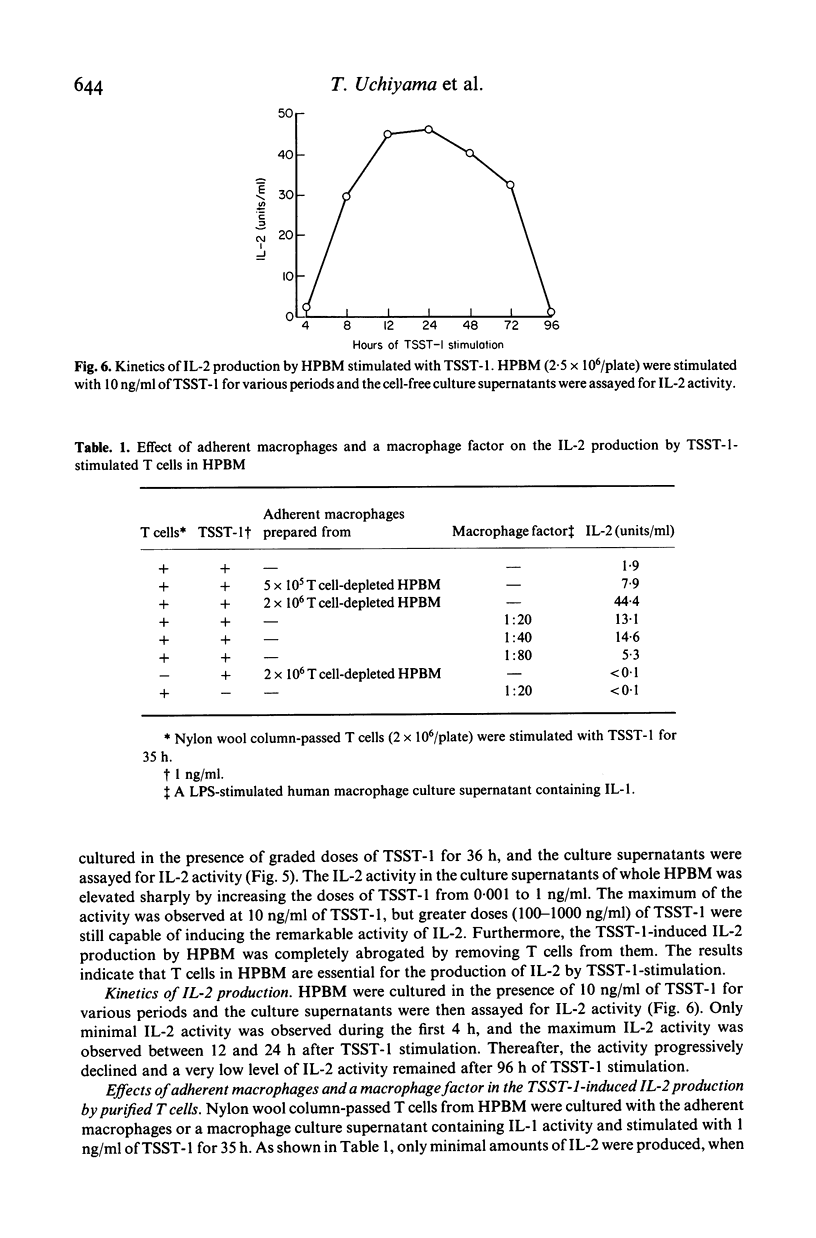
Toxic shock syndrome toxin-1 (TSST-1) is an exotoxin produced by Staphylococcus aureus isolated from patients with toxic shock syndrome. We investigated the proliferative response of human lymphocytes and their interleukin 2 (IL-2) production after stimulation with TSST-1 in vitro. Human cord blood mononuclear cells (HCBM) and human peripheral blood mononuclear cells (HPBM) could proliferate with TSST-1 stimulation. T cell-depleted HPBM showed only a marginal response to this toxin. A IL-2-like factor with a molecular weight of 15-18 kD was obtained from the supernatants of TSST-1-stimulated HPBM cultures. The factor was absorbed by CTLL-2 cells but not by T cell-depleted murine spleen cells, indicating that it is IL-2. HPBM are very sensitive to TSST-1: a low concentration of TSST-1 (0·01 ng/ml in 36 h stimulation) and a short period of stimulation (8 h at 10 ng/ml of the toxin) were fully effective for HPBM to produce substantial amounts of IL-2. Removal of T cells abrogated the TSST-1-induced IL-2 production by HPBM. Reconstituted cell cultures of nylon wool column-passed T cells and macrophages produced IL-2 by TSST-1 stimulation and, furthermore, the accessory activity of the macrophages could be partially replaced by a macrophage-derived factor containing interleukin 1. These findings indicate that T cells require macrophages or IL-1 for TSST-1-induced production of IL-2. The roles of lymphokines, including IL-2, in the development of this illness are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergdoll M. S., Crass B. A., Reiser R. F., Robbins R. N., Davis J. P. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981 May 9;1(8228):1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- Bonnard G. D., Yasaka K., Jacobson D. Ligand-activated T cell growth factor-induced proliferation: absorption of T cell growth factor by activated T cells. J Immunol. 1979 Dec;123(6):2704–2708. [PubMed] [Google Scholar]
- Bonventre P. F., Weckbach L., Staneck J., Schlievert P. M., Thompson M. Production of staphylococcal enterotoxin F and pyrogenic exotoxin C by Staphylococcus aureus isolates from toxic shock syndrome-associated sources. Infect Immun. 1983 Jun;40(3):1023–1029. doi: 10.1128/iai.40.3.1023-1029.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvano S. E., Quimby F. W., Antonacci A. C., Reiser R. F., Bergdoll M. S., Dineen P. Analysis of the mitogenic effects of toxic shock toxin on human peripheral blood mononuclear cells in vitro. Clin Immunol Immunopathol. 1984 Oct;33(1):99–110. doi: 10.1016/0090-1229(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Falkoff R. M., Peters M., Fauci A. S. T cell enrichment and depletion of human peripheral blood mononuclear cell preparations. Unexpected findings in the study of the functional activities of the separated populations. J Immunol Methods. 1982;50(1):39–49. doi: 10.1016/0022-1759(82)90302-7. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Mochizuki D. Y., Conlon P. J., Hefeneider S. H., Ramthun C. A., Gillis A. E., Frank M. B., Henney C. S., Watson J. D. Molecular characterization of interleukin 2. Immunol Rev. 1982;63:167–209. doi: 10.1111/j.1600-065x.1982.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Gutterman J. U., Blumenschein G. R., Alexanian R., Yap H. Y., Buzdar A. U., Cabanillas F., Hortobagyi G. N., Hersh E. M., Rasmussen S. L., Harmon M. Leukocyte interferon-induced tumor regression in human metastatic breast cancer, multiple myeloma, and malignant lymphoma. Ann Intern Med. 1980 Sep;93(3):399–406. doi: 10.7326/0003-4819-93-3-399. [DOI] [PubMed] [Google Scholar]
- Igarashi H., Fujikawa H., Usami H., Kawabata S., Morita T. Purification and characterization of Staphylococcus aureus FRI 1169 and 587 toxic shock syndrome exotoxins. Infect Immun. 1984 Apr;44(1):175–181. doi: 10.1128/iai.44.1.175-181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima T., Dinarello C. A., Gill D. M., Wolff S. M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984 May;73(5):1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Larkin S. M., Williams D. N., Osterholm M. T., Tofte R. W., Posalaky Z. Toxic shock syndrome: clinical, laboratory, and pathologic findings in nine fatal cases. Ann Intern Med. 1982 Jun;96(6 Pt 2):858–864. doi: 10.7326/0003-4819-96-6-858. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Ettinghausen S. E., Rayner A. A., Sharrow S. O., Seipp C. A., Custer M. C., Rosenberg S. A. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985 Oct;135(4):2865–2875. [PubMed] [Google Scholar]
- Micusan V. V., Mercier G., Bhatti A. R., Reiser R. F., Bergdoll M. S., Oth D. Production of human and murine interleukin-2 by toxic shock syndrome toxin-1. Immunology. 1986 Jun;58(2):203–208. [PMC free article] [PubMed] [Google Scholar]
- Paris A. L., Herwaldt L. A., Blum D., Schmid G. P., Shands K. N., Broome C. V. Pathologic findings in twelve fatal cases of toxic shock syndrome. Ann Intern Med. 1982 Jun;96(6 Pt 2):852–857. doi: 10.7326/0003-4819-96-6-852. [DOI] [PubMed] [Google Scholar]
- Poindexter N. J., Schlievert P. M. Toxic-shock-syndrome toxin 1-induced proliferation of lymphocytes: comparison of the mitogenic response of human, murine, and rabbit lymphocytes. J Infect Dis. 1985 Jan;151(1):65–72. doi: 10.1093/infdis/151.1.65. [DOI] [PubMed] [Google Scholar]
- Rasheed J. K., Arko R. J., Feeley J. C., Chandler F. W., Thornsberry C., Gibson R. J., Cohen M. L., Jeffries C. D., Broome C. V. Acquired ability of Staphylococcus aureus to produce toxic shock-associated protein and resulting illness in a rabbit model. Infect Immun. 1985 Mar;47(3):598–604. doi: 10.1128/iai.47.3.598-604.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser R. F., Robbins R. N., Khoe G. P., Bergdoll M. S. Purification and some physicochemical properties of toxic-shock toxin. Biochemistry. 1983 Aug 2;22(16):3907–3912. doi: 10.1021/bi00285a028. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M. Alteration of immune function by staphylococcal pyrogenic exotoxin type C: possible role in toxic-shock syndrome. J Infect Dis. 1983 Mar;147(3):391–398. doi: 10.1093/infdis/147.3.391. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Shands K. N., Dan B. B., Schmid G. P., Nishimura R. D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981 Apr;143(4):509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Shands K. N., Schmid G. P., Dan B. B., Blum D., Guidotti R. J., Hargrett N. T., Anderson R. L., Hill D. L., Broome C. V., Band J. D. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980 Dec 18;303(25):1436–1442. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- Todd J., Fishaut M., Kapral F., Welch T. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet. 1978 Nov 25;2(8100):1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- Tofte R. W., Williams D. N. Clinical and laboratory manifestations of toxic shock syndrome. Ann Intern Med. 1982 Jun;96(6 Pt 2):843–847. doi: 10.7326/0003-4819-96-6-843. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Kamagata Y., Wakai M., Yoshioka M., Fujikawa H., Igarashi H. Study of the biological activities of toxic shock syndrome toxin-1. I. Proliferative response and interleukin 2 production by T cells stimulated with the toxin. Microbiol Immunol. 1986;30(5):469–483. doi: 10.1111/j.1348-0421.1986.tb02973.x. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Kamagata Y., Yoshioka M. Mechanism of lipopolysaccharide-induced immunosuppression: immunological activity of B cell subsets responding to T-dependent or T-independent antigens in lipopolysaccharide-preinjected mice. Infect Immun. 1984 Aug;45(2):367–371. doi: 10.1128/iai.45.2.367-371.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Wang C. Y., Mertelsmann R., Venuta S., Feldman S. P., Moore M. A. Purification of human interleukin 2 to apparent homogeneity and its molecular heterogeneity. J Exp Med. 1982 Aug 1;156(2):454–464. doi: 10.1084/jem.156.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]


