Ionizing radiation fills the universe. Daily ionizing particles and rays collide with molecules in ≈1% of the 100 trillion cells that make up the average human. These collisions generate clusters of free radicals known as reactive oxygen species that randomly damage cellular constituents including DNA (1). Certain types of ionizing radiation are more effective at generating reactive oxygen species; one α-particle is at least 10 times more damaging than one γ-ray. To take these differences into account, the Sievert (Sv), a unit that multiplies the absorbed dose in grays (Gy) by the relative effectiveness of the particle or ray to inflict damage, was developed. On this scale, natural background radiation is ≈0.01 mSv/day, although there are areas on earth that have values 5-fold higher (2), and space-station inhabitants may receive ≈1 mSv/day (3). At the other end of the scale, acute exposures of >150 mSv, a range known as high-dose radiation, have measurable and often serious immediate effects on humans (4). Between background and high-dose radiation is the range of exposures known as low-dose radiation. Low-dose radiation has no immediately noticeable effects on humans; nevertheless there is great interest in its long-term biological effects, which may include cancer in exposed individuals and genetic defects in their progeny.
Research into the biological effects of low-dose radiation exposure is hindered by a lack of assays sensitive enough to measure the relevant cellular alterations. More-sensitive assays are being developed, an important one being the ability to detect the cellular presence of the most serious and potentially lethal type of cellular damage, the DNA double-strand break (DSB). This assay is based on the finding that one of the highly conserved histone proteins that package the DNA into chromatin, H2AX, becomes phosphorylated at the sites of nascent DNA DSBs (5–8). The response is highly amplified and rapid, involving the phosphorylation of hundreds to thousands of H2AX molecules within minutes on several megabase equivalents of chromatin flanking the DSB. When visualized with an antibody, the phosphorylated H2AX species, named γ-H2AX, appears as nuclear foci (Fig. 1). In this issue of PNAS, Rothkamm and Löbrich (9) report that the number of DSBs formed, as measured by the number of γ-H2AX foci formed, is linear with dose from 1 mGy to 2 Gy and is also in line with pulse-field gel electrophoresis measurements at higher doses. Greatly extending previous quantitative measurements (10), their findings demonstrate that γ-H2AX focus formation is several orders of magnitude more sensitive than other current methods for detecting DSBs (7).
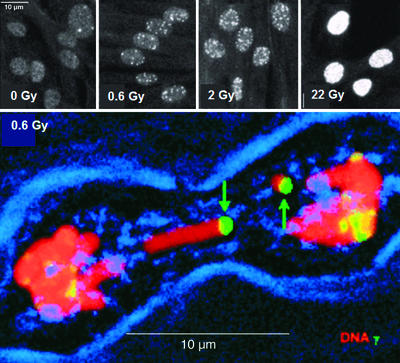
Figure 1.
γ-H2AX foci formation in IMR90 cell cultures and in muntjac mitotic chromosomes. (Upper) IMR90 cell cultures were exposed to the indicated dose from a 137Cs source. After 15 min of recovery at 37°C, the cultures were fixed and processed for immunofluorescence. White dots are γ-H2AX foci. (Lower) Muntjac fibroblast cultures were exposed and permitted to recover for 90 min at 37°C. After processing for immunofluorescence, cultures were scanned through a confocal microscope for mitotic cells. Red, DNA; green, γ-H2AX; blue, phase showing the cell membrane. [Reproduced with permission from ref. 7 (Copyright 1999, The Rockefeller University Press).]
How γ-H2AX foci function in DNA DSB repair and rejoining is poorly understood, but the foci do serve to recruit DNA-repair proteins to DSB sites (11). γ-H2AX foci also form as part of normal cellular processes involving DSBs, including homologous recombination during meiosis and genetic recombination during immune system development (12–15); mice lacking H2AX are viable but deficient in these two areas as well as being sensitive to ionizing radiation. Elucidating the biological roles of γ-H2AX foci will bring greater understanding into the various cellular mechanisms for DNA DSB formation and repair.
Rothkamm and Löbrich (9) used amounts of γ radiation as low as 1 mGy, a dose that generates on average one track of clustered reactive oxygen species per nucleus and thus is considered the lowest dose that can affect a whole cell culture or animal. With 1 mGy, ≈3% of irradiated cells sustain a DNA DSB. Compared with metabolic DSBs, radiation-induced DSBs are more heterogeneous, some being easy and others impossible to repair. This heterogeneity results from random collateral DNA damage generated near the DSB sites by other free radicals usually from the same cluster of reactive oxygen species. The existence of these sites, termed locally multiply damaged sites (16), has been substantiated by using enzymes specific for different types of DNA damage (17, 18). Of the DNA lesions in locally multiply damaged sites, ≈20% were found to be DSBs, and the rest were composed of single-strand breaks, altered bases, and damaged deoxyribose backbones. DSBs are more likely to be lethal and mutagenic than other DNA lesions for two reasons: both broken DNA strands may have lost the same genetic information, preventing accurate repair, and the linear continuity of mitotic chromosomes necessary for accurate transfer of genetic information to daughter cells is destroyed.
In the absence of direct data, the biological effects of low-dose radiation are currently estimated by extrapolating from the biological effects of high-dose radiation on Japanese atomic-bomb survivors and other groups of exposed workers such as uranium miners, radium painters, or nuclear submarine builders (see † for review). These data are subject to many uncertainties including uncontrolled conditions and inadequate dosimetry. This extrapolation is embodied in the linear nonthreshold model, which postulates that low-dose radiation is just as harmful per gray as high-dose radiation (19); thus any dose no matter how small is potentially harmful. Generating a wide margin of safety but perhaps entailing unnecessarily large expenditures for radiation safety, the linear nonthreshold model is subject to considerable discussion and controversy.
However, the biological effects of low-dose radiation are considerably more complex than predicted by the linear nonthreshold model, and some data seem to support other models. One is a threshold model that postulates that low-dose radiation is harmless below a certain level. Analysis of the epidemiological data, mainly of the life-span study of atomic-bomb survivors, seems to indicate in some cases a linearity between dose and risk at low doses but also cannot exclude a threshold at 60 mSv (20, 21).
Another model is the adaptive-response model, which postulates that certain doses of low-dose radiation may even be beneficial. Typically the adaptive response is induced with 1–100 mGy of γ-rays, doses 100–10,000 times larger than the natural background of ≈0.01 mSv/day. This model was first proposed in 1984 to explain the finding that cultures of human lymphocytes growing in low concentrations of radioactive thymidine developed fewer chromosomal aberrations than cultures of nonradioactive lymphocytes when both were challenged with high-dose radiation (22). Other studies also seem to support this model (23–26, ‡).
Yet another model is the bystander-effect model, which postulates that low-dose radiation may be even more damaging than that predicted by the linear nonthreshold model. For example, in cell cultures irradiated so that only 1% of the cells sustained a collision with an α-particle, sister chromatid exchanges were observed in >30% of the cells (27). Other studies have also supported this model (28–31). These effects suggest that irradiated cells may signal their distress to other cells, perhaps by direct cell-to-cell interaction or by molecules secreted into the medium. The latter form of cellular communication is supported by findings showing that the bystander effect could be induced in nonirradiated cell cultures incubated with conditioned medium from irradiated cultures (32, 33). One possible candidate for the signal is IL-8 (34), a cytokine with important roles as a chemoattractant and activator of polymorphonuclear leukocytes that is up-regulated and secreted in a variety of cell types during oxidative stress.
Quiescent cells signal their distress to their neighbors through secretion of IL-8 and other signaling molecules.
The adaptive response and the bystander effect can occur in the same experimental system. When cultures of C3H10T½ cells were irradiated through a microbeam with known numbers of α-particles, survival was less (65%) than that predicted from the linear nonthreshold model (90%), which is a bystander effect. However, when the cultures were irradiated with 20 mGy of γ-rays 6 h before α-particle exposure, survival was increased (75%), which is an adaptive response (35). This study points to what may be important differences in the two phenomena. The bystander effect is typically induced by the more-damaging α-particles, whereas the adaptive response is typically induced with γ-rays.
Rothkamm and Löbrich (9) report findings that also support a cellular communication model. In studies examining the incidence of DSBs in quiescent cultures of normal human fibroblasts after low-dose radiation, the expected numbers of DSBs, proportional to dose, were found at 3 min. Over 24 h the numbers decreased; however, they did not return to the preexposure average of 0.05 DSB per cell but stabilized at 0.1 DSB per cell for at least 14 days independent of dose. Thus 5% of the cells in the unirradiated cultures contained DSBs compared with 10% in the exposed cultures. However, when the quiescent cultures were exposed daily to 1.2 or 5 mGy for 10 days, the percentage of cells sustaining DSBs returned in each case to 10% within 24 h of the last irradiation. Thus only the first exposure increased the percentage of cells with DSBs after 24 h; subsequent exposures had no effect. When irradiated quiescent cultures were subcultured to induce growth, the percentage of cells with DSBs returned to the basal level of 5% at 7 days. However, apoptotic cells and cells with micronuclei were more abundant in the growing cultures derived from irradiated quiescent cultures than in those derived from the controls. In addition, cell survival after 200 and 1.2 mGy (80% and 90%, respectively) was significantly less than that predicted from the linear nonthreshold model (93% and 99+%).
To explain their findings, the authors suggest that quiescent cells signal their distress to their neighbors, perhaps as previously discussed, through secretion of IL-8 and other signaling molecules. Concerning the increased apoptosis and decreased survival in cell populations derived from the irradiated cultures, the authors suggest that these responses may be beneficial by eliminating injured or less-healthy cells from organisms subjected to low-dose irradiation. If cultures of quiescent normal human fibroblasts are a valid model system, then similar biological effects may occur in organs of animals subjected to low-dose radiation.
Understanding the biological consequences of exposures to low-dose radiation is becoming increasingly important for humans and other organisms as greater exposures to ionizing radiation occur from new man-made sources and space travel. If doses 100 times background are harmless (threshold model) or even beneficial (adaptive-response model), exposure standards could be relaxed, resulting in substantial savings. In addition, the adaptive response and bystander effect could prove useful in cancer therapy if normal and tumor cells are found to respond differently. However, until the mechanisms of these two phenomena are understood and their effects become predictable, few would propose a relaxation of standards. This contribution of Rothkamm and Löbrich (9) is a significant advance toward the elucidation of the immediate as well as long-term biological consequences of low-dose radiation and their effects on human health and safety.
Footnotes
See companion article on page 5057.
Giussani, A., Ballarini, F. & Ottolenghi, A. (2002) Sixth European ALARA Network Workshop: Occupational Exposure-Optimisation in the Medical Field and Radiopharmaceutical Industry, October 23–25, 2002, Madrid.
Mitchel, R. E. J. & Boreham, D. R. (2000) Tenth International Conference of the International Radiation Protection Association IRPA-10, May 15–19, 2000, Hiroshima, Japan.
References
- 1.Riley P A. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 2.Wei L, Zha Y, Tao Z, He W, Chen D, Yuan Y, Zhao R. High Background Radiation Research in Yangjiang, China. Beijing: Atomic Energy Press; 1996. [Google Scholar]
- 3. Lyndon B. Johnson Space Center (2002) NASA Factsheet: Understanding Space Radiation, FS-2002-10-080-JSC, www.jsc.nasa.gov/news/factsheets/radiation.pdf.
- 4.National Council on Radiation Protection. Guidance on Radiation Received in Space Activities: Report 98. Bethesda, MD: Natl. Council Radiat. Prot.; 1991. [Google Scholar]
- 5.Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 6.Rogakou E P, Pilch D R, Orr A H, Ivanova V S, Bonner W M. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 7.Rogakou E P, Boon C, Redon C, Bonner W M. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannironi C, Bonner W M, Hatch C L. Nucleic Acids Res. 1989;17:9113–9126. doi: 10.1093/nar/17.22.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothkamm K, Löbrich M. Proc Natl Acad Sci USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sedelnikova O A, Panuytin I G, Bonner W M. Radiat Res. 2002;158:486–492. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Paull T T, Rogakou E P, Yamazaki V, Kirchgessner C U, Gellert M, Bonner W M. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 12.Chen H T, Bhandoola A, Difilippantonio M J, Zhu J, Brown M J, Tia X, Rogakou E P, Brotz T, Bonner W M, Ried T, Nussenzweig A. Science. 2000;290:1962–1964. doi: 10.1126/science.290.5498.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahadevaian S K, Turner J M A, Rogakou E P, Baudat F, Blanco-Rodríguez J, Jasin M, Bonner W M, Burgoyne P S. Nat Genet. 2001;27:271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- 14.Petersen S, Casellas R, Reina-San-Martin B, Chen H T, Difilippantonio M J, Wilson P C, Hanitsch L, Celeste A, Muramatsu M, Pilch D R, et al. Nature. 2001;414:660–665. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celeste A, Petersen S, Romanienko P J, Fernandez-Capetillo O, Chen H T, Reina- San-Martin B, Meffre E, Difilippantonio M J, Sedelnikova O A, Redon C, et al. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward J F. Int J Radiat Biol. 1994;66:427–432. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland B M, Bennett P V, Sidorkina O, Laval J. Biochemistry. 2000;39:8026–8031. doi: 10.1021/bi9927989. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland B M, Bennett P V, Sidorkina O, Laval J. Proc Natl Acad Sci USA. 2000;97:103–108. doi: 10.1073/pnas.97.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Council on Radiation Protection. Limitation of Exposure to Ionizing Radiation: Report 116. Bethesda, MD: Natl. Council Radiat. Prot.; 1993. [Google Scholar]
- 20.Pierce D A, Preston D L. Radiat Res. 2000;154:176–186. doi: 10.1667/0033-7587(2000)154[0178:rrcral]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Little M P, Muirhead C R. Int J Radiat Biol. 2000;76:939–953. doi: 10.1080/09553000050050954. [DOI] [PubMed] [Google Scholar]
- 22.Olivieri G, Bodycote J, Wolff S. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 23.Azzam E I, Raaphorst G P, Mitchel R E J. Radiat Res. 1994;138:S28–S31. [PubMed] [Google Scholar]
- 24.Azzam E I, de Toledo S M, Raaphorst G P, Mitchel R E J. Radiat Res. 1996;146:369–373. [PubMed] [Google Scholar]
- 25.Mitchel R E J, Gragtmans N J, Morrison D P. Radiat Res. 1990;121:180–186. [PubMed] [Google Scholar]
- 26.Mitchel R E J, Jackson J S, McCann R A, Boreham D R. Radiat Res. 1999;152:273–279. [PubMed] [Google Scholar]
- 27.Nagasawa H, Little J B. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 28.Deshpande A, Goodwin E H, Bailey S M, Marrone B L, Lehnert B E. Radiat Res. 1996;145:260–267. [PubMed] [Google Scholar]
- 29.Azzam E I, de Toledo S M, Little J B. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawant S G, Randers-Pehrson G, Geard C R, Brenner D J, Hall E J. Radiat Res. 2001;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Chen G, Trosko J E, Waldren C A, Hei T K. Proc Natl Acad Sci USA. 2001;98:14410–14415. doi: 10.1073/pnas.251524798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mothersill C, Seymour C. Radiat Res. 1998;149:256–262. [PubMed] [Google Scholar]
- 33.Mothersill C, Seymour C. Radiat Res. 2001;155:759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan P, LaRue K E A, Goodwin E H, Lehnert B E. Radiat Res. 1999;152:57–63. [PubMed] [Google Scholar]
- 35.Sawant S G, Randers-Pehrson G, Metting N F, Hall E J. Radiat Res. 2001;156:177–180. doi: 10.1667/0033-7587(2001)156[0177:aratbe]2.0.co;2. [DOI] [PubMed] [Google Scholar]