Abstract
The development of protein-based vaccines remains a major challenge in the fields of immunology and drug delivery. Although numerous protein antigens have been identified that can generate immunity to infectious pathogens, the development of vaccines based on protein antigens has had limited success because of delivery issues. In this article, an acid-sensitive microgel material is synthesized for the development of protein-based vaccines. The chemical design of these microgels is such that they degrade under the mildly acidic conditions found in the phagosomes of antigen-presenting cells (APCs). The rapid cleavage of the microgels leads to phagosomal disruption through a colloid osmotic mechanism, releasing protein antigens into the APC cytoplasm for class I antigen presentation. Ovalbumin was encapsulated in microgel particles, 200–500 nm in diameter, prepared by inverse emulsion polymerization with a synthesized acid-degradable crosslinker. Ovalbumin is released from the acid-degradable microgels in a pH-dependent manner; for example, microgels containing ovalbumin release 80% of their encapsulated proteins after 5 h at pH 5.0, but release only 10% at pH 7.4. APCs that phagocytosed the acid-degradable microgels containing ovalbumin were capable of activating ovalbumin-specific cytoxic T lymphocytes. The acid-degradable microgels developed in this article should therefore find applications as delivery vehicles for vaccines targeted against viruses and tumors, where the activation of cytoxic T lymphocytes is required for the development of immunity.
Keywords: polymer‖crosslinker‖encapsulation‖vaccination‖cytotoxic T lymphocyte
There is a great need for the development of vaccines against AIDS, hepatitis C, and cancer (1, 2). Traditional vaccination strategies, based on live attenuated viruses, have been ineffective at generating vaccines against these diseases, largely as a result of their high toxicity, and new vaccination strategies are greatly needed (3). Vaccines based on protein antigens have considerable promise because of their low toxicity and widespread applicability and constitute a new vaccination strategy (2). However, delivery problems have limited the clinical success of protein-based vaccines, and new protein delivery vehicles capable of enhancing the efficacy of protein-based vaccines are needed.
A key limitation of current protein-based vaccines is their inability to activate cytotoxic T lymphocytes (CTLs), a process that is critical for the development of immunity against viruses and tumors. CTLs are activated by antigen-presenting cells (APCs) through the class I antigen presentation pathway. APCs generally only present cytoplasmic proteins as class I antigens, although class I antigen presentation of proteins residing in phagosomes also occurs under certain circumstances (4).
Microparticles, 0.2–5 μm in diameter, have recently gained interest as delivery vehicles for protein-based vaccines because of their ability to enhance the class I antigen presentation of protein antigens (5). Two mechanisms have been proposed to explain the ability of microparticles to enhance the class I antigen presentation of protein antigens. The first involves disruption of phagosomes by microparticles, leading to the release of protein antigens into the cytoplasm of APCs, where they are processed for antigen presentation as endogenous proteins. The second uses microparticles to deliver protein antigens to phagolysosomal compartments that contain MHC I receptors that are being recycled from the plasma membrane. Once delivered these proteins are subsequently degraded by phagolysosomal enzymes into antigenic peptides that complex MHC I receptors and are then trafficked to the cell surface for antigen presentation (5–10).
The microparticles investigated previously as delivery vehicles for protein-based vaccines have been composed of materials such as polycaprolactone, poly(lactic-co-glycolic acid), and polystyrene (5, 9, 10). Some of these materials degrade via base-catalyzed hydrolysis whereas others cannot be degraded. However, for the development of protein-based vaccines, drug delivery vehicles that degrade under mildly acidic conditions are desirable to allow for targeting to the acidic environment of the phagosomes of APCs. At present, the most common strategy for engineering acid sensitivity in microparticles involves incorporating cationic groups that become protonated at acidic pHs, leading to microparticle swelling and drug release (11, 12). However, for protein delivery it would be preferable to develop acid-sensitive materials that remain neutral, thus avoiding the potential toxicity of polycations and their considerable propensity for charge interactions with proteins (13).
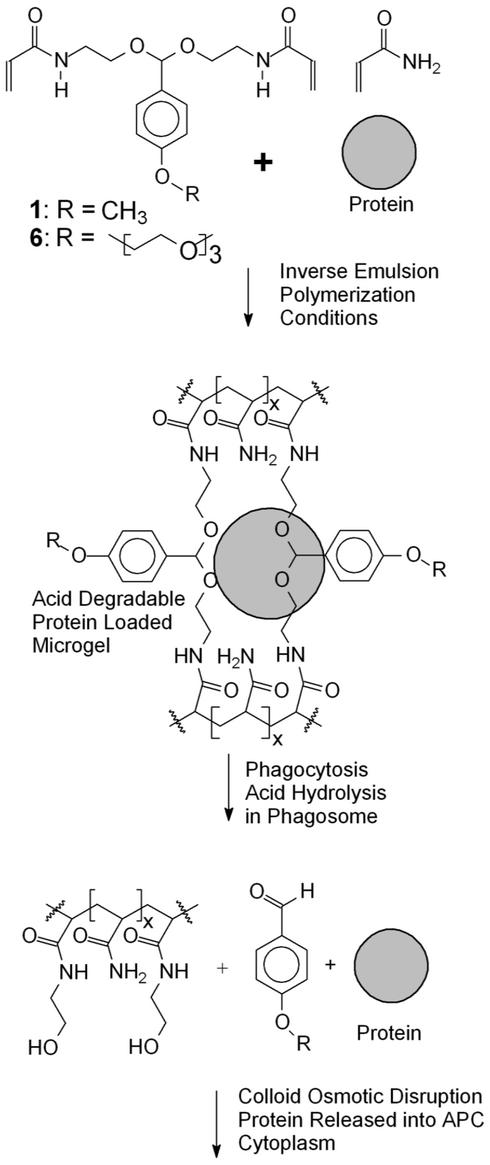
In this article acid-sensitive protein-loaded microgels, 0.2–0.5 μm in diameter, are synthesized for the development of protein-based vaccines that can efficiently activate CTLs. These microgels are designed to disrupt phagosomes and deliver protein antigens into the cytoplasm of APCs for class I antigen presentation. They are chemically stable at pH 7.4 but degrade into linear polymer chains and small molecules under mildly acidic conditions. The molecular design of these microgels should allow them to circulate in the blood, at pH 7.4, but then rapidly hydrolyze after phagocytosis in the acidic environment of the phagosome, to induce a colloid osmotic disruption of the phagosome and deliver phagocytosed proteins into the APC cytoplasm.
These microgels are made with acid-degradable crosslinkers (Scheme S1). We previously reported the synthesis and characterization of an acetal crosslinker (1), in which the acetal linkage has a half-life of 24 h at pH 7.4 and only 5 min at pH 5.0 (14). Microgels synthesized with 1 should degrade rapidly after phagocytosis but be relatively stable at pH 7.4. Compound 1 was used successfully to prepare microgels by inverse microemulsion polymerization with very high concentrations of the ionic surfactant dioctyl sulfosuccinate (14). However, neutral, biocompatible surfactants are preferred for the synthesis of protein-loaded microgels because of their reduced interactions with proteins and lower toxicity. Because crosslinking monomer 1 is relatively hydrophobic, we hypothesized that decreasing its hydrophobicity would improve its performance because inverse emulsion polymerizations are very sensitive to the hydrophobic/hydrophilic balance of the reactants (15).
Scheme 1.
Molecular design of microgels for protein delivery to APCs. These microgels are synthesized with acid-degradable crosslinkers (6 or 1). They are designed to degrade under the acidic conditions of the phagosome, disrupt it by a colloid osmotic mechanism, and release phagocytosed proteins into the APC cytoplasm.
We now report a more hydrophilic acid-degradable crosslinker, 6, containing a hydrophilic triglyme moiety (Scheme S2). This modification dramatically increased the hydrophilicity of the crosslinker, making it compatible with a variety of inverse emulsion polymerization procedures. Protein-loaded microgel particles were synthesized by using the Food and Drug Administration-approved neutral biocompatible surfactants Tween 80/Span 80, at low surfactant concentrations (3% with respect to the organic phase). The protein release rates from these particles were investigated at both pH 5.0 and 7.4. The ability of these particles to deliver antigens to APCs and induce class I antigen presentation was also determined by using the LacZ class I antigen presentation assay (16).
Scheme 2.
Synthesis of acid-degradable crosslinker 6.
Materials and Methods
The synthesis and characterization of compound 6, the procedures used to determine protein encapsulation and release, and the toxicity of the acid-degradable microgels are described in detail in the Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Inverse Microemulsion Polymerization with 6.
Acid-degradable microgels were prepared via inverse emulsion polymerization. The organic phase of the polymerization consisted of 5 ml of hexane containing 150 mg of a 3:1 weight ratio of Span 80 (sorbitan monooleate) and Tween 80 (polyethyleneglycol-sorbitan monooleate). The aqueous phase of the polymerization consisted of 0.5 ml of 300 mM sodium phosphate at pH 8.0 containing different ratios of crosslinker 6 and acrylamide (250 mg combined) and 12 mg of the free radical initiator potassium peroxodisulfate and ovalbumin (see Table 1 for exact amounts). The aqueous and organic phases were deoxygenated with nitrogen. An inverse emulsion between the organic and aqueous phases was formed by mixing them and then sonicating for 30 s. Polymerization of the inverse emulsion was then initiated while stirring with a magnetic bar by adding 10 μl of N,N,N′,N′-tetramethylethylene diamine. The stirred polymerization was allowed to proceed for 10 min at room temperature.
Table 1.
Encapsulation of ovalbumin in microgels made with 6
| Sample | Mole percent crosslinking | Crosslinker, mg | Acrylamide, mg | Cascade blue-labeled albumin, mg | Encapsulated albumin/ particle weight, μg/mg | Weight % yield of microgels |
|---|---|---|---|---|---|---|
| A | 1.6 | 25 | 225 | 5.4 | 10.4 | 38.0 |
| B | 3.5 | 50 | 200 | 5.7 | 11.1 | 61.0 |
| C | 12.8 | 125 | 125 | 5.4 | 9.5 | 43.0 |
After polymerization, the mixture was centrifuged at 2,800 rpm for 10 min and the solvent was decanted off. The microgels were carefully washed with hexane (2 × 20 ml) and acetone (4 × 20 ml) and isolated by centrifugation at 2,800 rpm for 10 min. The recovered microgels were vacuum-dried overnight and analyzed by scanning electron microscopy (WDX ISI-ds130C, Microspec, Concord, MA) at 15 kV.
MHC Class I Antigen Presentation Assay.
RAW 309.1 CR cells were plated at 5 × 104 cells per well in a 96-well plate and allowed to grow overnight. Ovalbumin-loaded microgels were dispersed in high DMEM by sonication for 5 min. The microgel samples were centrifuged for 5 min and the supernatant was pipetted off to remove any unbound protein. The washed microgels were then incubated with RAW 309.1 CR cells for 6 h in 5% FBS, and the microgels were removed from the RAW cells by washing several times with DMEM. B3Z cells (1 × 105) were added to the antigen-fed RAW cells, and the two cell lines were incubated together for 16 h in RPMI media and then washed with PBS. The β-galactosidase activity of the B3Z cells was assayed by incubating them with a 100 μl of Z buffer (100 μM mercaptoethanol/9 mM MgCl2/0.15 mM chlorophenol red β-galactoside) for 2 h. The UV absorbance of the liberated chlorophenol red was measured at 595 nm with a plate reader.
Results and Discussion
Design and Synthesis of 6.
Compound 6, N,N′-bisacryloyl-di-(2-aminoethoxy)-4-(1,4,7,10-tetraoxaundecyl)phenyl]methane, was designed as the key acid-cleavable crosslinking monomer used to prepare acid-degradable protein-loaded microgels by inverse emulsion polymerization. Compound 6 contains two acrylamide units connected by an acid-degradable acetal linkage derived from a benzaldehyde with a solubilizing triglyme para substituent. The acetal linkage of 6 has the appropriate half-life (5 min at pH 5.0 and 24 h at pH 7.4) and pH dependency needed for the delivery of proteins to APCs. The triethylene glycol pendant group incorporated in 6 increases its water solubility, making it much more hydrophilic and therefore more suitable for use in inverse emulsion polymerization than its para-methoxy-substituted analog, 1. Even monomers such as acrylic acid are difficult to polymerize in inverse emulsion because they partition into the organic phase of the polymerization, causing termination (15). The triethylene glycol chain of 6 is also expected to contribute to the stabilization of the final particles while also enhancing their dispersability.
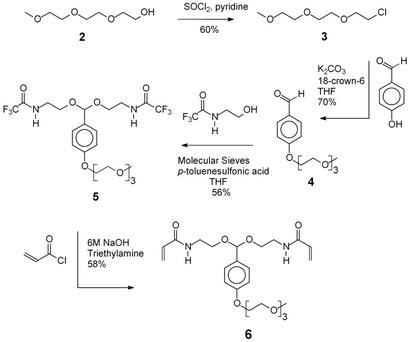
Compound 6 was synthesized in four steps on a multigram scale, as shown in Scheme S2. The first step involved the preparation of 1-chloro-3,6,9-trioxadecane (3) by using the procedure of Loth and Ulrich (17). Compound 3 was then used to alkylate hydroxybenzaldehyde and afford p-(1,4,7,10-tetraoxaundecyl)benzaldehyde (4). Compound 3 was chosen as the alkylating agent since it can be easily synthesized on a large scale (100 g), enabling the preparation of 4 on a 20-g scale (70% yield), using potassium carbonate as the base and 18-crown-6 as the phase transfer catalyst. Hydroxybenzaldehyde could also be alkylated by using 1-tosyl-3,6,9-trioxadecane and 1-bromo-3,6,9-trioxadecane but these approaches were discontinued because significantly lower yields of the desired product were obtained.
Compound 4 was converted to an acetal (5) by reaction with N-(2-hydroxyethyl)-2,2,2-trifluoroacetamide. A potential problem with acetal formations is the separation of the product from residual alcohol. The alcohol is generally used in 4- to 6-fold molar excess over the aldehyde, and even high yielding reactions leave two to four molar equivalents of the alcohol to be removed. Initial attempts at purifying the acetal product 5 from unreacted N-(2-hydroxyethyl)-2,2,2-trifluoroacetamide by using flash chromatography were unsuccessful. However, 5 could be purified by crystallization from ethyl acetate/hexane, allowing for its synthesis on a multigram scale. The final bisacrylamide crosslinker 6 was obtained by cleaving the trifluoroacetyl groups on 5 in 6 M NaOH/dioxane followed by reaction of the resulting diamine with an excess of acryloyl chloride. Final purification of compound 6 was achieved by crystallization from ethyl acetate/hexane.
Preparation of a Microgel.
The inverse emulsion polymerization procedure developed by Kriwet et al. (18) was used for the preparation of microgels by using monomer 6. The surfactants selected were Tween 80 and Span 80. Since both are Food and Drug Administration-approved for human use, they are ideally suited for the preparation of microgels targeted for therapeutic applications. Tween 80 and Span 80 are also neutral lipids, which should not denature encapsulated proteins as would be the case for ionic surfactants such as SDS.
Microgels with crosslinking ratios ranging from 1.6% to 12.8% were prepared by using this inverse emulsion polymerization procedure with crosslinker 6 and acrylamide. A representative scanning electron microscopy image of the particles is shown in Fig. 1; the particle size varies between 200 and 500 nm in the dry state. This size distribution is suitable for protein delivery to APCs, which internalize particles between 200 nm and 5 μm by phagocytosis (19). Thus, the ability of these particles to encapsulate proteins was investigated.
Figure 1.

Scanning electron microscopy image of microgels made with acrylamide and 6 at 1.6% crosslinking.
Synthesis of Ovalbumin-Loaded Microgels.
Inverse emulsion polymerization is an attractive method for the synthesis of protein-loaded particles because it does not chemically modify or denature the encapsulated protein (20–22). In this procedure, the protein is physically entrapped in the microgel by polymerizing the monomer and crosslinker in the presence of the protein. A key parameter in the synthesis of protein-loaded microgels is their “pore size,” which needs to be smaller than the radius of the protein for efficient encapsulation. Ovalbumin was chosen as the model protein for the encapsulation studies because numerous immunological assays have been developed for this protein. Ovalbumin labeled with Cascade blue was encapsulated in microgels containing 1.6, 3.5, and 12.8 mol percent of 6. The results of the protein encapsulation experiment are listed in Table 1, with protein loadings varying from 9 to 11 μg of protein per mg of microgel. Based on the starting protein/monomers ratio, this finding represents a 50% encapsulation efficiency. The encapsulation efficiency obtained with 6 was similar to that observed by O'Hagan et al. (23), for the encapsulation of ovalbumin in nondegradable microgels composed of 2.5 mol percent methylene bisacrylamide and 97.5 mol percent acrylamide.
The encapsulation efficiency of microgels made with 6 did not change appreciably with the crosslinking ratio. For example, the 1.6% crosslinked microgel has a protein loading of 10.4 μg of protein per mg of microgel and the 12.8% crosslinked microgel has a protein loading of 9.5 μg of protein per mg of microgel. This lack of correlation between crosslinking ratio and protein encapsulation has been observed by Ekman et al. (20) for the encapsulation of human serum albumin in nondegradable microgels composed of methylene bisacrylamide and acrylamide. A representative scanning electron microscopy image of the protein-loaded microgels is shown in Fig. 2; the microgels have a size between 200 and 500 nm in diameter, comparable to the results obtained in the absence of protein. Therefore, these protein-loaded microgels were deemed suitable for protein delivery to APCs.
Figure 2.

Scanning electron microscopy image of ovalbumin-loaded microgels made of acrylamide and 6, at 1.6% crosslinking. See Table 1 for ovalbumin content.
Protein Release from Ovalbumin Microgels.
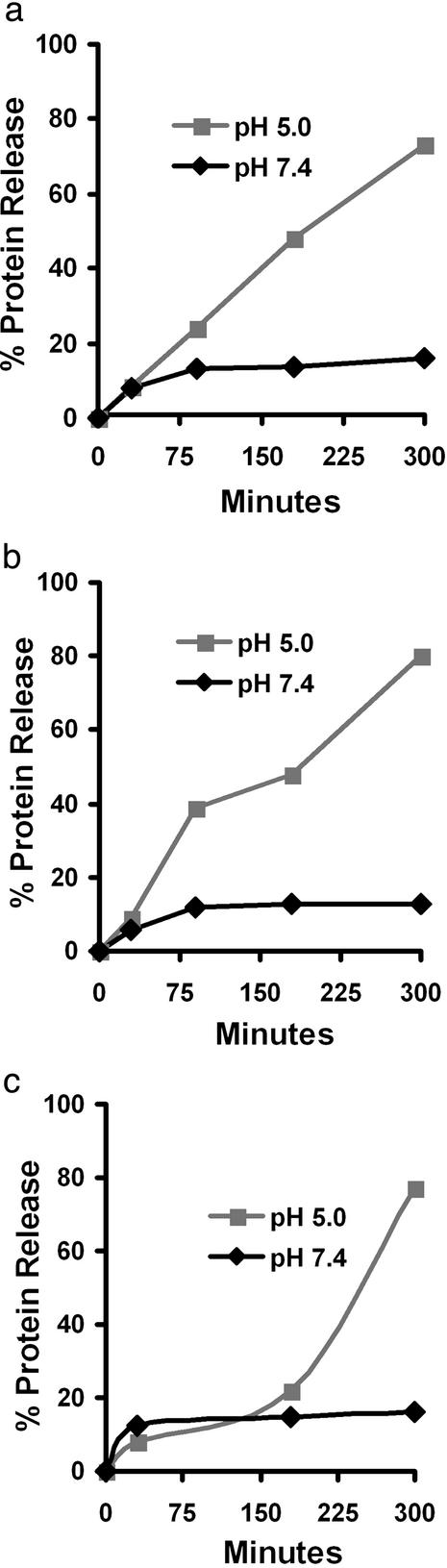
The protein-loaded microgels synthesized in this article are designed to retain encapsulated proteins while circulating in the body, but then degrade and release their contents after phagocytosis by APCs. The release of protein from the microgels was measured at pH 5.0 and 7.4 to understand their behavior in the environments of the phagosome and blood, respectively. Fig. 3 demonstrates that microgels made with 6 release their contents much faster at pH 5.0 than at pH 7.4. For example, at pH 5.0, after 5 h, the 1.6% crosslinked microgels released 80% of encapsulated ovalbumin, whereas at pH 7.4, only 10% was released. This pH dependency is caused by the acid sensitivity of 6, which hydrolyzes rapidly at pH 5.0, thus increasing the effective pore size of the microgels and the diffusion rate of proteins out of the microgels. In contrast, at pH 7.4, 6 remains intact and the majority of encapsulated proteins are retained. A small percentage of encapsulated proteins are initially released at pH 7.4. We speculate that this is caused by the vigorous vortexing and sonication procedures used to disperse the microgels, which could dislodge proteins loosely entrapped within the microgels. Fig. 3 illustrates that the pH sensitivity of microgels made with 6 is suitable for protein delivery to APCs.
Figure 3.
pH-dependent protein release from acid-degradable microgels: 1.6% crosslinked microgels (a), 3.5% crosslinked microgels (b), and 12.8% crosslinked microgels (c). Data points represent the average of three experiments.
MHC Class I Antigen Presentation of Ovalbumin-Loaded Microgels.
The microgels synthesized in this article are designed to disrupt phagosomes and deliver protein antigens into the APC cytoplasm for class I antigen presentation. The LacZ MHC class I antigen presentation assay was performed with these microgels to test their ability to deliver proteins into APCs for class I antigen presentation. This experiment uses the LacZ B3Z hybridoma, which is a CTL that recognizes the peptide SIINFEKL, from ovalbumin, complexed with the MHC class I molecule H-2Kb. This hybridoma produces β-galactosidase after encountering APCs that present SIINFEKL as a class I antigen, thus allowing class I antigen presentation to be quantified by measuring β-galactosidase activity.
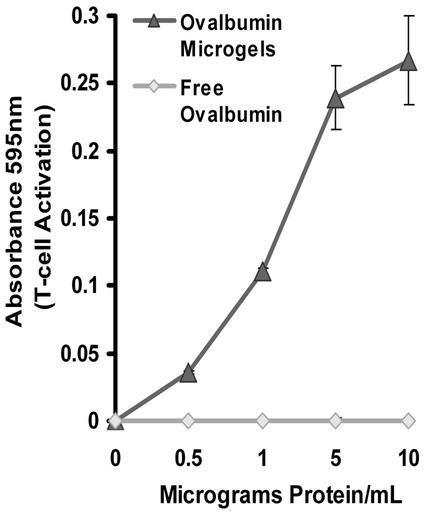
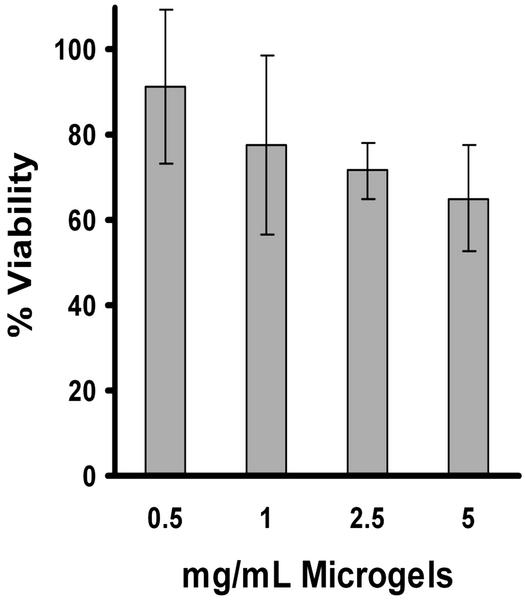
The results of the class I antigen presentation assay are shown in Fig. 4. APCs incubated with free ovalbumin are not able to activate CTLs, indicating that these APCs are unable to present free ovalbumin as a class I antigen. This is presumably because ovalbumin endocytosed by the APCs is sequestered in lysosomes and does not have access to the APC cytoplasm. In contrast, APCs incubated with ovalbumin encapsulated in the microgels can efficiently activate CTLs. Ovalbumin encapsulated in the microgels is several orders of magnitude more efficient than free ovalbumin at inducing the activation of CTLs; for example, 1 μg/ml ovalbumin encapsulated in the microgels gives T cell activation levels that are three times greater than 1 mg/ml free ovalbumin (the UV absorbance resulting from activation with 1 mg/ml free ovalbumin was only 0.037 versus 0.1106 for activation with 1 μg/ml ovalbumin encapsulated in the microgels). Thus the acid-degradable microgels are capable of delivering protein antigens into APCs for class I antigen presentation. Importantly, the acid-degradable microgels show minimal toxicity to RAW 309.1 macrophages at concentrations as high as 5 mg/ml (Fig. 5).
Figure 4.
Class I antigen presentation of phagocytosed ovalbumin-loaded acid-degradable microgels (1.6% crosslinked). The LacZ class I antigen presentation assay was performed with ovalbumin-loaded acid-degradable microgels. RAW 309.CR1 APCs were incubated with ovalbumin-loaded microgels for 6 h and then incubated with B3Z T cells for 24 h. The activation of B3Z T cells was determined by quantifying β-galactosidase activity (absorbance at 595 nm). See Materials and Methods for details. Data points represent the average of three experiments; error bars represent the standard deviation.
Figure 5.
Toxicity of ovalbumin-loaded acid-degradable microgels to RAW 309.1CR cells. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was performed to determine the toxicity of ovalbumin-loaded acid-degradable microgels (1.6% crosslinked). The microgels were incubated with RAW 309.1CR cells for 16 h in 10% FBS and removed from the cells by washing with DMEM. The RAW cells were then grown for another 48 h and harvested with the MTT reagent to quantify toxicity (see Supporting Text for details). Data points represent the average of three experiments; error bars represent the standard deviation.
Conclusions
In this article, an acid-degradable protein-loaded microgel was synthesized for the development of protein-based vaccines. The main building block of this system consists of crosslinking monomer 6, which provides the acid-labile component of the microgels. Ovalbumin was successfully encapsulated in microgels made with 6 by using the neutral biocompatible surfactants Tween 80/Span 80. It was demonstrated that the rate of protein release from these microgels was pH-sensitive, with 80% release of encapsulated ovalbumin observed in 6 h at pH 5.0, but only 10% at pH 7.4. APCs that phagocytosed the acid-degradable ovalbumin-loaded microgels were capable of activating ovalbumin-specific CTLs, demonstrating that the acid-degradable microgels can deliver protein antigens to APCs for class I antigen presentation. Since class I antigen presentation is generally observed with cytoplasmic proteins, we speculate that the rapid breakdown of the microgels into multiple smaller molecules causes disruption of the phagosomal membrane, leading to release of intact protein antigens into the cytoplasm. We believe that this disruption is the direct result of the large change in osmotic pressure occurring within the phagosome as a result of the hydrolysis of the encapsulating microgel. The microgels developed in this article therefore show promise as carriers for a variety of biological payloads, in particular, protein vaccines targeted against tumors and viruses, where the activation of CTLs is critical for successful immunization.
Supplementary Material
Acknowledgments
We thank Ann Fischer for help with the cell culture studies. We thank the U.S. Department of Energy (Grant DE-ACO3-76SF00098) for funding for the development of biocompatible acid-cleavable microgels and the National Institutes of Health (Grant RO1GM65361) for funding the biological studies. J.K. is a Research Fellow of the Japan Society for the Promotion of Science. The Center for New Directions in Organic Synthesis is supported by Bristol-Myers Squibb as the Sponsoring Member and Novartis as a Supporting Member.
Abbreviations
- CTL
cytotoxic T lymphocyte
- APC
antigen-presenting cell
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy Sciences elected on May 2, 2000.
References
- 1.Robinson H L. Nat Rev Immunol. 2002;2:239–250. doi: 10.1038/nri776. [DOI] [PubMed] [Google Scholar]
- 2.Berzofsky J A, Ahlers J D, Belyakov I M. Nat Rev Immunol. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 3.Blower S M, Koelle K, Kirschner D E, Mills J. Proc Natl Acad Sci USA. 2001;98:3618–3623. doi: 10.1073/pnas.061029998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jondal M, Schirmbeck R, Reimann J. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- 5.Yewdell W J, Norbury C C, Bennink R J. Adv Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron P, Mortimer O, Paiement J, Bergeron J J M, Desjardins M. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 7.Norbury C C, Chamber B J, Prescott A R, Shastri N, Watts C. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 8.Kleijmeer M J, Escola J M, Uytdehaag F G, Jakobson E, Griffith J M, Osterhaus A D, Stoorvogel W, Melief C J, Rabouille C, Geuze H J. Traffic. 2001;2:124–137. doi: 10.1034/j.1600-0854.2001.020207.x. [DOI] [PubMed] [Google Scholar]
- 9.Oh Y, Harding C V, Swanson J A. Vaccine. 1997;15:511–518. doi: 10.1016/s0264-410x(97)00221-1. [DOI] [PubMed] [Google Scholar]
- 10.Caetano R S, Germain R. J Exp Med. 1995;182:841–851. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynn D M, Mansoor A, Langer R. Angew Chem Int Ed. 2001;40:1707–1710. [PubMed] [Google Scholar]
- 12.Park T G. Biomaterials. 1999;20:517–521. doi: 10.1016/s0142-9612(98)00197-5. [DOI] [PubMed] [Google Scholar]
- 13.Sassi A P, Shaw A J, Han S M, Blanch H W, Prausnitz J M. Polymer. 1996;11:2151–2164. [Google Scholar]
- 14.Murthy N, Thng Y X, Schuck S, Xu M, Fréchet J M J. J Am Chem Soc. 2002;124:12398–12399. doi: 10.1021/ja026925r. [DOI] [PubMed] [Google Scholar]
- 15.Barton J, Kawamoto S, Fujimoto K, Kawaguchi H, Capek I. Polym Int. 2000;49:358–366. [Google Scholar]
- 16.Sanderson S, Shastri N. Int Immunol. 1994;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 17.Loth H, Ulrich F. J Controlled Release. 1998;54:273–282. doi: 10.1016/s0168-3659(97)00218-6. [DOI] [PubMed] [Google Scholar]
- 18.Kriwet B, Walter E, Kissel T. J Controlled Release. 1998;56:149–158. doi: 10.1016/s0168-3659(98)00078-9. [DOI] [PubMed] [Google Scholar]
- 19.Koval M, Preiter K, Adles C, Stahl P D, Steinberg H T. Exp Cell Res. 1998;242:265–273. doi: 10.1006/excr.1998.4110. [DOI] [PubMed] [Google Scholar]
- 20.Ekman B, Lofter C, Sjoholm I. Biochemistry. 1976;15:5115–5120. doi: 10.1021/bi00668a026. [DOI] [PubMed] [Google Scholar]
- 21.Daubresse C, Grandfils C, Jerome R, Teyssie P. J Colloid Interface Sci. 1994;168:222–229. [Google Scholar]
- 22.Franssen O, Stenekes R J H, Hennik W E. J Controlled Release. 1999;59:219–228. doi: 10.1016/s0168-3659(98)00193-x. [DOI] [PubMed] [Google Scholar]
- 23.O'Hagan D T, Palin K, Davis S S, Artursson P. Vaccine. 1989;7:421–424. doi: 10.1016/0264-410x(89)90156-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.