Abstract
Voltage-dependent (VD) inhibition of N-type Ca2+ channels is mediated primarily by neurotransmitter receptors that couple to pertussis toxin (PTX)-sensitive G proteins (such as Go and Gi). To date, however, the composition of heterotrimeric complexes, i.e., specific Gαβγ combinations, capable of coupling receptors to N-type Ca2+ channels has not been defined. We addressed this question by heterologously expressing identified Gαβγ combinations in PTX-treated rat sympathetic neurons and testing for reconstitution of agonist-mediated VD inhibition. The heterologously expressed Gα subunits were rendered PTX-insensitive by mutating the codon specifying the ADP ribosylation site. The following results were obtained from this approach. (i) Expression of GαoA, GαoB, and Gαi2 (along with Gβ1γ2) reconstituted VD inhibition mediated by α2-adrenergic, adenosine, somatostatin, and prostaglandin E2 receptors. Conversely, expression of Gαi1 and Gαi3 was ineffective at restoring coupling. (ii) Coupling efficiency, as determined from the magnitude of reconstituted Ca2+ current inhibition, depended on both the receptor and Gα subtype. The following rank order of coupling efficiency was observed: GαoA = GαoB > Gαi2 for α2-adrenergic receptor; Gαi2 > GαoA = GαoB for adenosine and prostaglandin E2 receptors; and GαoB = Gαi2 > GαoA for the somatostatin receptor. (iii) In general, varying the Gβγ composition of GαoA-containing heterotrimers had little effect on the coupling of α2-adrenergic receptors to the VD pathway. Taken together, these results suggest that multiple, diverse Gαβγ combinations are capable of coupling neurotransmitter receptors to VD inhibition of N-type Ca2+ channels. Thus, if exquisite Gαβγ-coupling specificity exists in situ, it cannot arise solely from the inherent inability of other Gαβγ combinations to form functional signaling complexes.
The minimal G protein-signaling pathway is composed of a seven-transmembrane spanning receptor, a heterotrimeric G protein complex (i.e., Gαβγ), and an effector. A specific form of neurotransmitter-mediated N-type Ca2+ channel modulation, termed voltage-dependent (VD) inhibition, appears to require only these elements. The accumulated evidence suggests that VD inhibition is membrane-delimited, does not require an enzymatic component (e.g., kinase or phosphatase) (1), and is mediated by interaction of “free” Gβγ with the α1-subunit (α1B) of the N-type Ca2+ channel (2–5). Moreover, VD inhibition is the most common form of N-type Ca2+ channel modulation, is easily identifiable from distinct biophysical characteristics (e.g., “kinetic slowing” and prepulse facilitation), and most likely plays a crucial role in regulating neurotransmitter release from presynaptic nerve terminals (1, 5). Although general aspects of this signaling pathway have been well studied, the impact of heterotrimeric G protein composition, i.e., the specific Gα, Gβ, and Gγ isoforms, on coupling receptors to VD N-type Ca2+ channel modulation has been explored little.
The question of how G protein heterotrimer composition influences signaling pathways arises because there are a large number of potential discrete Gαβγ combinations. At present, about 20 Gαs, 5 Gβs, and 11 Gγs have been identified in mammals. Consequently, around 1,100 potential G protein heterotrimer combinations are possible. However, several factors serve to limit the number of combinations relevant to a given signaling pathway. First, several of the Gα and Gγ subunits have a very restricted tissue distribution that confines their potential contribution mainly to sensory transduction systems (6). Second, some combinations of Gβ and Gγ do not appear to form functional heterodimers, at least when produced in vitro (7–10). Third, it has been reported that Gβ5-containing heterodimers couple exclusively to Gαq/11 family subunits (11). Finally, agents such as pertussis toxin (PTX) modify a distinct subset of Gα subunits (12). Thus, if the PTX-sensitivity of a signaling pathway is known, the number of possible combinations is reduced further. Unfortunately, even after improbable heterotrimer combinations are eliminated, there often can remain >100 candidate heterotrimer compositions.
For the simplest systems, i.e., those composed of receptor, G protein, and effector, coupling specificity can be examined at three different junctions. First, the ability of a defined G protein heterotrimer composition to interact with a receptor can be examined. This usually is investigated in heterologous systems in which the ability of defined G protein heterotrimers to affect ligand affinity is measured (13, 14). Successful coupling of the G protein to receptor is indicated by an increase in ligand affinity. Second, the ability of activated G protein components, i.e., GTP-bound Gα or Gβγ subunits, to interact with an effector can be examined. For GIRK-type K+ channels and N-type Ca2+ channels, “free” Gβγ appears to be the component responsible for activation and VD inhibition, respectively (5, 15). G protein/effector interaction thus can be determined by applying in vitro manufactured Gβγ (16) or heterologously overexpressing Gβγ (17) and determining whether tonic channel modulation occurs. Third, one can attempt to express a defined Gαβγ combination and determine whether receptor activation leads to effector modulation. In the following experiments, native G protein coupling was disrupted by treating the sympathetic neurons with PTX. Reconstitution then was attempted by heterologously expressing a PTX-insensitive mutant of Gα (18–21) along with Gβ and Gγ. Successful reconstitution was determined by receptor-mediated VD inhibition of N-type Ca2+ channels. The most parsimonious view of receptor–effector coupling predicts that the intersection of data sets describing receptor/G protein and G protein/effector coupling will dictate the outcome of receptor/G protein/effector reconstitution experiments. Accordingly, because only limited specificity has been observed between receptor/G protein and G protein/effector (e.g., Gβγ and N-type Ca2+ channels), successful reconstitution of coupling by numerous combinations of Gαβγ might be expected. However, antisense knockout of individual G protein subunits in GH3 cells suggests that exquisitely specific Gαβγ combinations mediate native coupling of receptors to L-type Ca2+ channels (22–24). Thus, reconstitution experiments complement the knockout experiments by examining which Gαβγ combinations are capable of supporting coupling rather than which Gαβγ combinations mediate native coupling. Taken together, these data should lend insight into factors regulating receptor–effector-coupling specificity.
Materials and Methods
Dissociation of Sympathetic Neurons.
Superior cervical ganglion (SCG) neurons were enzymatically dissociated from adult male Wistar rats as described previously (25, 26). After washing twice, the dissociated neurons were resuspended in minimum essential medium (MEM) containing 10% FCS, 1% glutamine, and 1% penicillin-streptomycin solution (all from GIBCO). Neurons then were plated onto polystyrene culture dishes (35 mm), coated with poly-l-lysine, and maintained in a humidified atmosphere of 95% air/5% CO2 at 37°C. All neurons were used within 24 hr after intranuclear injection of vectors. PTX-pretreated neurons were incubated overnight (16–20 hr) with 500 ng/ml PTX (List Biological Laboratories, Campbell, CA).
Construction and Expression of PTX-Insensitive Mutants of Gα Subunits.
Vectors encoding wild types of mouse GαoA,B, bovine Gβ1, human Gβ2,3, mouse Gβ4,5, and bovine Gγ2,3 were from M. I. Simon (California Institute of Technology, Pasadena, CA); rat Gαi1–3 were from R. R. Reed (Johns Hopkins Medical School, Baltimore, MD); and mouse Gγ4 was from N. Gautam (Washington University School of Medicine, St. Louis). The coding region for each subunit was subcloned into the mammalian expression vector, pCI (Promega). To generate PTX-insensitive mutants of the Gαo/i subunits, the codon specifying the fourth amino acid, cysteine (C), from the carboxyl terminus was mutated to code for a glycine (G). The coding region of each Gα subunit was amplified by PCR with Pfu polymerase. Site-directed mutagenesis was performed by incorporating the altered base into the reverse primer. For GαoA, cysteine-to-serine (S) or -isoleucine (I) mutations also were generated. The resultant PCR products were subcloned into the mammalian expression vector, pCI. The sequence of each construct was verified with an automated DNA sequencer (ABI 310; PE Applied Biosystems). The PTX-insensitive Gα mutants were coexpressed with different Gβ and Gγ subunits by using intranuclear injection methods as described previously (2, 25). The cDNAs encoding Gα, Gβ, and Gγ subunits (10 mM Tris/1 mM EDTA, pH 7.4) were injected at a ratio (weight) of ≈0.3–0.5:1:1.
Electrophysiology and Data Analysis.
Ca2+ channel currents were recorded by using the whole-cell variant of the patch–clamp technique (27) as described previously (26, 28, 29). To isolate Ca2+ currents, patch electrodes were filled with a solution containing 120 mM N-methyl-d-glucamine (NMG) methanesulfonate (MS), 20 mM TEA-MS, 20 mM HCl, 11 mM EGTA, 1 mM CaCl2, 10 mM Hepes, 4 mM MgATP, 0.3 mM Na2GTP, and 14 mM creatine phosphate (pH 7.2, 297 mOsM/kg H2O). External recording solution contained 145 mM tetraethylammonium (TEA)-MS, 10 mM Hepes, 10 mM CaCl2, 15 mM glucose, and 0.0003 mM tetrodotoxin (TTX) (pH 7.4, 318 mOsM/kg H2O). All experiments were performed at room temperature (21–24°C). Data were presented as means ± SEM where appropriate. Student's t test (unpaired) or ANOVA followed by a posthoc test, as appropriate, was applied to the data to determine statistical significance. P < 0.05 was considered significant.
Results
Reconstitution of α2-Adrenergic Receptor Coupling by PTX-Insensitive Mutants in SCG Neurons.
Norepinephrine (NE) produces VD inhibition of N-type Ca2+ currents via α2-adrenergic receptors (α2-ARs) in SCG neurons (30). The signaling pathway underlying the modulation is PTX-sensitive (31) and likely involves Gαo (32). We thus examined the ability of PTX-insensitive Gαo mutants to couple α2-ARs to N-type Ca2+ channels. The PTX-insensitive mutant (C351G) codes for glycine instead of cysteine at position 351, the site for PTX-catalyzed ADP ribosylation in the Gαo (33). Ca2+ currents were evoked from a holding potential of −80 mV with a double-pulse protocol consisting of two identical test pulses to +10 mV separated by a large, depolarizing conditioning pulse to +80 mV (see Fig. 1A). In control neurons expressing only GFP, 10 μM NE inhibited Ca2+ currents by 62 ± 2% (n = 26) (Fig. 1 A and E). NE-induced Ca2+ current inhibition displayed the hallmarks of VD inhibition (see ref. 5), i.e., slowed activation kinetics in the prepulse and an enhanced postpulse amplitude (Fig. 1 A and D). Facilitation, defined as the ratio of the postpulse to prepulse current amplitude, increased from 1.17 ± 0.01 to 2.36 ± 0.07 (n = 26) after NE application. Overnight treatment of the neurons with PTX (500 ng/ml) greatly attenuated NE-induced Ca2+ current inhibition (Fig. 1 B and E), implicating the involvement of a PTX-sensitive G protein(s) in the signaling pathway. Coexpression of GαoA(C351G) with Gβ1 and Gγ2 subunits (denoted Gβ1γ2 hereafter) reconstituted the NE-induced Ca2+ current inhibition in PTX-treated neurons (Fig. 1C). The success of the reconstitution experiments was critically dependent on establishing a functional stoichiometric match between the Gα and Gβγ subunits expressed in SCG neurons. For example, if expression of Gα greatly exceeded that of Gβγ, basal facilitation and NE-induced Ca2+ current inhibition were ablated. This result presumably reflects sequestration of free Gβγ subunits (both endogenous and exogenous) by the GDP-bound Gα (2, 34). In contrast, if expression of Gβγ exceeded that of Gα, a significant tonic inhibition (as indicated by increased basal facilitation) was observed. This result presumably reflects interaction of “free” or excess Gβγ with N-type Ca2+ channels (2, 3, 17). To obtain criteria for judging the “balanced condition,” we examined the relationship between Ca2+ current inhibition (%) and basal facilitation ratio (BFR) for 49 cells expressing GoA(C351G)β1γ2 (data not shown). When the BFR was <1, reconstitution did not occur. In cells showing successful reconstitution, the BFR was always >1 (ranging from 1.02 to 1.46). Although reconstitution was apparent in cells with a BFR >1.5 (1.66–2.59), we did not include these neurons in the analysis. Thus, we defined a basal facilitation range of 1.02–1.5 as indicating adequate “balance” between Gα and Gβγ subunits. In neurons expressing GαoA(C351G)β1γ2, only about 20% of the neurons (9 of 49) fulfilled this criteria. For these neurons, NE application inhibited Ca2+ currents by 52 ± 3% and increased prepulse facilitation from 1.15 ± 0.05 to 1.90 ± 0.09 (n = 9) after PTX treatment (Fig. 1 D and E).
Figure 1.
Reconstitution of α2-AR coupling to N-type Ca2+ channels by expression of PTX-insensitive GαoA mutants. Time course of current inhibition and superimposed current traces in the absence (1 and 3) or presence (2 and 4) of 10 μM NE recorded from control neurons (no PTX) (A), control (500 ng/ml PTX, overnight) (B), PTX-pretreated neurons coexpressing GαoA(C351G) and Gβ1γ2 (C). The cDNAs encoding PTX-insensitive Gα mutants and Gβ1γ2 were injected directly into nuclei of SCG neurons. The Ca2+ current was evoked every 10 s by a double-pulse voltage protocol (see Inset in A) consisting of two identical test pulses (+10 mV from a holding potential of −80 mV) separated by a large, depolarizing conditioning pulse to +80 mV. The amplitudes of currents generated by pre- (●) and post- (○) pulses were plotted. (D) Summary of prepulse facilitation in the absence or presence of NE. Prepulse facilitation was calculated by the ratio of the postpulse to prepulse current amplitude measured isochronally at 10 ms after the start of the test pulses. (E) Summary of NE-induced Ca2+ current inhibition. The C-terminal cysteine residue of GαoA also was mutated to serine or isoleucine. Inhibition (%) was calculated by using the amplitudes of currents determined isochronally 10 ms after the start of the prepulse. In both D and E, data are presented as mean ± SEM and numbers in parentheses indicate the number of neurons tested.
The nature of amino acid residue at the site of PTX-catalyzed ADP ribosylation has been shown to influence the maximal stimulation of Gαi1 by α2-ARs (35, 36). In addition, Gαz, a naturally occurring PTX-insensitive Gα (which contains an isoleucine at the equivalent site), has been shown to efficiently couple α2-ARs to N-type Ca2+ channels in SCG neurons (26). Accordingly, we tested whether reconstitution of NE-induced Ca2+ current inhibition was altered by the expression of GαoA(C351I) or GαoA(C351S) when compared with GαoA(C351G). Coexpression of GαoA(C351I) or GαoA(C351S) with Gβ1γ2 resulted in a 31 ± 4% (n = 9) and 24 ± 7% (n = 5) inhibition of Ca2+ current, respectively, after NE application (Fig. 1E). These data indicated that the C→G mutation, under the experimental conditions utilized in these studies, produced the greatest current inhibition and, thus, presumably, coupling efficiency. Therefore, the C→G mutation was used to render all other Gαs used in the study as PTX-insensitive. When compared with native coupling, NE-induced Ca2+ current inhibition via GαoA(C351G) occurred with about 2-fold less potency [EC50 of 2.35 ± 0.51 (n = 3) vs. 1.25 ± 0.38 μM (n = 6), data not shown] but similar maximal inhibition [50 ± 9% (n = 3) vs. 59 ± 4% (n = 6), data not shown].
Effect of Gβγ Composition on Coupling Between α2-ARs and Ca2+ Channels.
In GH3 cells, somatostatin and muscarinic receptors couple to L-type Ca2+ channel modulation via very specific Gαβγ compositions as indicated by antisense experiments (22–24). Likewise, α2-ARs have been shown to discriminate among Gαi heterotrimers containing different Gβγ combinations (14). Thus, we tested whether various Gβγ combinations influenced the ability to reconstitute coupling between α2-ARs and N-type Ca2+ channels in PTX-treated neurons. For these experiments, five different Gβγ combinations (Gβ1γ2, Gβ1γ3, Gβ2γ2, Gβ3γ4, and Gβ4γ4) were selected based on previous studies done by Schultz and colleagues (37). When these Gβγ combinations were coexpressed with GαoA(C351G) in PTX-treated SCG neurons, the VD inhibition of Ca2+ currents by NE was robust (Fig. 2A). On average, GoA(C351G) heterotrimers containing Gβ1γ2, Gβ1γ3, Gβ2γ2, Gβ3γ4, or Gβ4γ4 produced 52 ± 3% (n = 9), 49 ± 9% (n = 5), 43 ± 4% (n = 10), 45 ± 4% (n = 5), and 45 ± 9% (n = 6) inhibition of Ca2+ currents, respectively (Fig. 2B). The NE-induced Ca2+ current inhibition was not significantly different among the tested Gβγ combinations (P > 0.05). These results suggest that a specific Gβγ combination is not required for coupling between α2-ARs and N-type Ca2+ channels. As a negative control, we tested Gβ5γ2, a Gβγ combination thought to selectively interact with Gαq subunits (11). Expression of β5γ2 alone produced a modest basal facilitation, supporting the functional expression of this Gβγ (V. Ruiz-Velasco and S.R.I., unpublished data). Coexpression of GαoA(C351G) with β5γ2 produced a tonic inhibition (as judged by kinetic slowing and basal facilitation) similar to that produced by the expression of β5γ2 alone and failed to reconstitute NE-induced Ca2+ current inhibition (n = 6, Fig. 2). These data suggest that, under the conditions used here, heterologously expressed GαoA(C351G) does not, to a significant extent, form functional heterotrimers with natively expressed Gβγ.
Figure 2.
Specific Gβγ combinations are not required for coupling between α2-ARs and N-type Ca2+ channels. (A) Superimposed current traces in the absence or presence of 10 μM NE recorded from neurons expressing GαoA(C351G) and different Gβγ combinations (β1γ2, β1γ3, β2γ2, β3γ4, β4γ4, β5γ2). (B) Summary of VD Ca2+ current inhibition by NE under conditions described above. Note that tonic inhibition is apparent in neurons expressing GαoA(C351G) β5γ2. Data are presented as mean ± SEM, and numbers in parentheses indicate the number of neurons tested.
Specificity of Interaction Between Gα Subunits and α2-ARs for Ca2+ Channel Modulation.
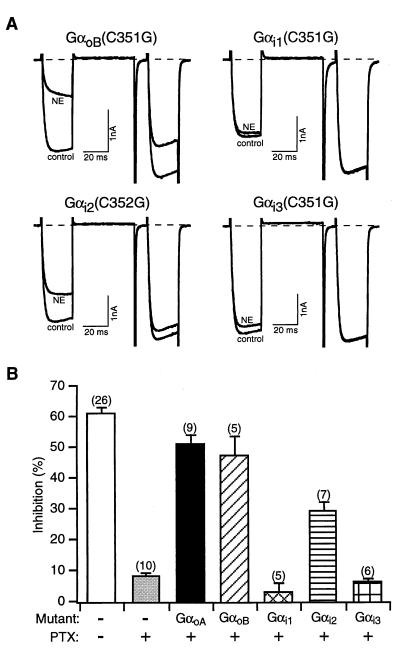
Additional members of the PTX-sensitive Gαi/o family were tested to determine their ability to reconstitute VD inhibition of N-type Ca2+ channels. PTX-insensitive mutants of GαoB, Gαi1, Gαi2, and Gαi3, generated as for GαoA, were coexpressed with Gβ1γ2 subunits in PTX-treated SCG neurons. Fig. 3 illustrates representative traces and summary data acquired from neurons expressing different PTX-insensitive heterotrimer compositions. NE application produced VD inhibition of Ca2+ currents in neurons expressing GαoB(C351G) and Gαi2(C352G) (Fig. 3). In contrast, NE-induced Ca2+ current inhibition was negligible in neurons expressing Gαi1(C351G) or Gαi3(C351G) (Fig. 3). The functional expression of Gαi1(C351G) and Gαi3(C351G) was indirectly confirmed by observing a lack of basal facilitation when these constructs were expressed without Gβγ subunits (data not shown, n = 3 for each mutant type of Gαi). Furthermore, the Gαi1(C351G) and Gαi3(C351G) constructs used in this study are capable of coupling m2-muscarinic receptors to GIRK channel activation in Xenopus oocytes (C. Doupnik, personal communication). On average, NE produced 48 ± 6% (n = 5) and 30 ± 3% (n = 7) inhibition of Ca2+ currents in neurons expressing GαoB(C351G) and Gαi2(C352G), respectively (Fig. 3B). Taken together, these results suggest that GαoA/B and Gαi2, when coexpressed with Gβ1γ2, are capable of coupling α2-ARs to VD N-type Ca2+ channel inhibition. Under the present experimental conditions, expression of Gαo isoforms resulted in larger NE-induced inhibitions when compared with neurons expressing Gαi.
Figure 3.
Contribution of both Gαo and Gαi to coupling between α2-ARs and N-type Ca2+ channels with some degree of specificity. (A) Superimposed current traces in the absence or presence of 10 μM NE recorded from neurons coexpressing PTX-insensitive mutants (C351G) of different Gα subunits (GαoB and Gαi1-i3) and Gβ1γ2. (B) Summary of VD Ca2+ current inhibition by NE. Data are presented as mean ± SEM, and numbers in parentheses indicate the number of neurons tested.
Differential Coupling Between Gα Subunits and Other Receptors.
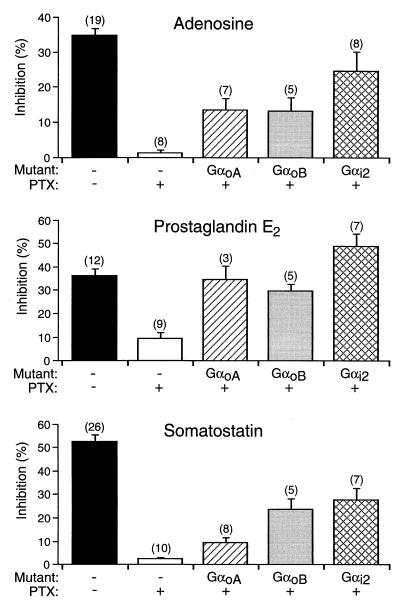
In SCG neurons, it is well established that several different neurotransmitters produce VD inhibition of N-type Ca2+ channels via a pathway utilizing PTX-sensitive G proteins (1). To determine the specificity of coupling between other neurotransmitter receptors and Gαo/i-containing heterotrimers, we carried out experiments analogous to those described above. We selected adenosine (38), prostaglandin E2 (PGE2) (39), and somatostatin (40) because of their large and robust effects on N-type Ca2+ channels. Fig. 4 summarizes the effects of adenosine (10 μM), PGE2 (1 μM), and somatostatin (300 nM) on Ca2+ currents in neurons expressing GαoA(C351G)β1γ2, GαoB(C351G)β1γ2, and Gαi2(C352G)β1γ2. Inhibition of Ca2+ currents was 35 ± 2% (n = 19) after adenosine administration and was prevented by PTX pretreatment, as reported previously (38). In PTX-treated neurons, expression of Gαi2(C352G) partially reconstituted the VD inhibition of Ca2+ currents (25 ± 5%, n = 8). Expression of GαoA(C351G) and GαoB(C351G) had smaller effects than Gαi2(C352G), reconstituting inhibition to 14 ± 3% (n = 7) and 14 ± 4% (n = 5), respectively. PGE2-induced Ca2+ current inhibition was 37 ± 3% (n = 12) and was prevented mostly by the PTX pretreatment. When GαoA(C351G), GαoB(C351G) and Gαi2(C352G) were expressed in PTX-pretreated neurons, PGE2 inhibited the Ca2+ current by 35 ± 6% (n = 3), 30 ± 3% (n = 5), and 50 ± 5% (n = 7), respectively. It should be noted that the PGE2-induced Ca2+ current inhibition in Gαi2(C352G)-expressing cells (PTX-treated) exceeded the inhibition occurring through native G proteins. Activation of somatostatin receptor produced 53 ± 3% and 3 ± 1% inhibition (n = 26) of Ca2+ currents in uninjected control and PTX-treated neurons, respectively. Expression of GαoB(C351G) and Gαi2(C352G) reconstituted the Ca2+ current inhibition to 24 ± 5% (n = 5) and 28 ± 5% (n = 7), respectively. However, the somatostatin-induced Ca2+ current inhibition was only marginally restored (10 ± 2%, n = 8) by expression of GαoA(C351G). Expression of either Gαi1(C352G)β1γ2 or Gαi3(C352G)β1γ2 failed to restore coupling of all tested receptors to N-type Ca2+ channels (data not shown).
Figure 4.
Summary of differential couplings between Gα subunits and other receptors. The coupling specificity was assessed in PTX-pretreated neurons coexpressing Gα(C351G) and Gβ1γ2. The VD inhibition of N-type Ca2+ channels was induced by application of adenosine (10 μM), PGE2 (1 μM), and somatostatin (0.3 μM) by using the voltage protocol described in Fig. 1. Data are presented as mean ± SEM, and numbers in parentheses indicate the number of neurons tested.
Taken together, these data suggest that neurotransmitter receptors natively expressed in SCG neurons are capable of coupling to N-type Ca2+ channels via heterotrimers containing GαoA, GαoB, and Gαi2, but not Gαi1 or Gαi3.
Discussion
In these experiments, we investigated the contribution of G protein heterotrimer composition to functional coupling between neurotransmitter receptors and N-type Ca2+ channels. The impetus for the experiments arises from two seemingly paradoxical observations. On one hand, the combinatorial potential for different G protein heterotrimer compositions is quite large. On the other hand, receptor-mediated Ca2+ channel modulation has been proposed to occur with exquisite coupling selectivity. For example, Kleuss et al. (22–24), using antisense knockout techniques, have provided evidence that inhibition of L-type Ca2+ channels in GH3 cells occurs via coupling to GαoAβ3γ4 and GαoBβ1γ3 for M4-muscarinic and somatostatin receptors, respectively. In regard to these studies, it should be noted that the modulatory pathway underlying inhibition of L-type Ca2+ channels in GH3 cells has not been defined and may not be analogous to the pathway mediating VD modulation of N-type Ca2+ channels. The strategy utilized in our experiments relied on uncoupling natively expressed G proteins with PTX followed by heterologous expression of a defined G protein heterotrimer composition. The approach was adopted from earlier studies in which PTX-insensitive Gα mutants were stably transfected into clonal cell lines (18–21). In stably transfected cells, there apparently is a reestablishment of Gα:Gβγ stoichiometry in which the heterologously expressed PTX-insensitive Gα pairs with native and, thus, undefined Gβγ. Hence, these studies evaluated only the contribution of Gα to receptor/effector coupling. In contrast, we have shown previously that transient (ca. 24 hr) expression of either Gα or Gβγ in SCG neurons results in functional alterations consistent with a stoichiometric mismatch between Gα and Gβγ (2, 34). Apparently, the compensatory changes that occur in clonal cells stably transfected with Gα subunits do not occur in neurons, at least during the time scale of our experiments (see ref. 41), therefore allowing us to examine the contribution of the G protein heterotrimer to coupling.
There were several advantages to the strategy we employed. First, coupling was investigated against a neuronal background and, thus, only G protein subunits required heterologous expression. Receptors and channels were natively expressed and presumably existed in a physiologically relevant environment. The level of receptor expression, in particular, has been shown to influence G protein-coupling specificity (42). Second, measurement of N-type Ca2+ channel current facilitation provided a functional indicator of Gα:Gβγ stoichiometry. This ability was crucial for the successful reconstitution of responses. In addition, the ability to express either Gα or Gβγ alone and to determine functional consequences (decreased or increased basal facilitation, respectively) provided a positive control for subunit expression. Third, SCG neurons are a well characterized and standard model system for studying the VD form of N-type Ca2+ channel inhibition (1). Numerous receptor systems have been shown to produce robust and reproducible Ca2+ channel inhibition via a PTX-sensitive pathway. Thus, we were able to compare G protein coupling with several different receptors under identical experimental conditions.
As with all experimental methods, there are potential limitations that need to be addressed as well. First, we had no way of determining the absolute level of G protein subunit expression. As mentioned above, basal Ca2+ current facilitation can be used as a functional measure of relative Gα:Gβγ expression. We attempted to equalize expression of the G protein subunits by subcloning only the ORF of Gα and Gβ subunits into the expression vector, thereby avoiding the potential influence of untranslated regions on gene expression (e.g., ref. 43). Additionally, the start codon was placed in a Kozak consensus sequence (44) when possible. Nonetheless, differences in translational efficiency (e.g., codon bias) or protein half-life would result in different expression levels. Second, in regard to effects on coupling specificity, the consequences of mutating the site of ADP ribosylation are not known. Clearly, the carboxyl terminus of Gα is a major determinant of receptor/G protein-coupling specificity. For example, altering the last 3 aa residues of Gα has dramatic effects on Gα coupling (45). Milligan and colleagues (46) have shown that C→G mutants of Gαi couple to α2A-ARs with specificity similar to wild-type Gαi. However, agonist potency and efficacy were decreased in the Gαi mutant-expressing cells. Thus, some caution is required when interpreting data derived from cells expressing PTX-insensitive Gα subunits. It seems likely that no single experimental approach to coupling specificity will provide definitive answers—hence, data from multiple diverse approaches will be required to reach a consensus view of the process.
Given these caveats, what have these experiments revealed? First, expression of GαoA(C351G) along with Gβ1 and Gγ2 successfully reconstituted NE-induced Ca2+ current inhibition after PTX pretreatment. The GαoA isoform was chosen for the initial trials because previous studies have demonstrated that Gαo is abundant in SCG neurons and involved with NE-induced Ca2+ channel modulation (32). A second finding was that Gα influenced coupling to a greater extent than Gβγ. Although only a limited number of different Gβγ combinations were coexpressed with GαoA(C351G), all but one, Gβ5γ2, resulted in robust reconstitution of NE-induced Ca2+ current inhibition. The inability of Gβ5γ2 to reconstitute coupling is consistent with the finding that Gβ5 containing Gβγ subunits forms heterotrimers exclusively with the Gαq/11 family (11) and provides evidence that the expressed Gα was not significantly recombining with native Gβγ subunits. In addition, the degree of basal facilitation observed in these experiments (Fig. 2A Lower Right) likely reflects the modest ability of “free” Gβ5-containing Gβγ subunits to inhibit the N-type Ca2+ channel (17). Conversely, the findings that Gβ3- and Gβ4-containing heterotrimers reconstituted coupling are at odds with the results of Garcia et al. (17), who reported that overexpression of Gβ3 or Gβ4 (either alone or with Gγ) produced no significant tonic inhibition of N-type Ca2+ channels. Results from our laboratory, however, indicate that both Gβ3- and Gβ4-containing Gβγ subunits produce robust Ca2+ channel modulation when overexpressed in SCG neurons (V. Ruiz-Velasco and S.R.I., unpublished data). It is possible that different levels of expression underlie these discrepant findings. In contrast to the minimal effects of altering the Gβγ composition, changing the Gα subunit resulted in large effects on NE-mediated coupling efficiency. Expression of either isoform of Gαo resulted in Ca2+ channel inhibition similar to that observed under native conditions. Conversely, expression of Gαi2 produced significant but less inhibition (when compared with Gαo) whereas expression of Gαi1 or Gαi3 failed to reconstitute coupling. In general, studies of α2A-ARs in clonal cells lines have shown that the receptor couples to all three Gαi subtypes, GαoA, and even Gαs/Gαq (42, 46, 47). Thus, the apparent Gα specificity demonstrated in sympathetic neurons is of interest. There are two possible junctures at which specificity could occur. First, heterotrimers containing Gαi1/3 may not couple to α2-AR in SCG neurons, suggesting that additional factors present in the neuronal expression system restrict receptor/G protein interactions. Although it is possible that Gαi1/3 requires a Gβγ combination other than Gβ1γ2 to effectively couple to α2-ARs, this seems unlikely because Gαi1β1γ2 effectively couples to α2A-AR in in vitro systems (14). Second, heterotrimers containing Gαi1/3 may couple to receptors but be incapable of producing VD modulation of N-type Ca2+ channels after receptor activation. In support of this idea, Delmas et al. (41) have shown that expression of the PTX-insensitive Gαi mutant, Gαi1(C351I), in SCG neurons failed to reconstitute VD N-type Ca2+ channel modulation by NE. Interestingly, the expression of Gαi1(C351I) coupled adrenergic receptors to a voltage-independent pathway, which probably would not be observed under our experimental conditions (whole-cell vs. perforated patch recordings). Because Gαi1/3 containing heterotrimers did not successfully reconstitute Ca2+ channel inhibition by any receptor tested in our experiments, we were unable to distinguish between these two possibilities.
Finally, we have shown that different, natively expressed receptors couple with varying degrees of efficiency to heterotrimers containing Gβ1γ2 and different PTX-insensitive Gα subunits. Here, the term “efficiency” is used in a descriptive sense and without mechanistic implications—i.e., the observed differences in coupling efficiency might have arisen from alterations in agonist efficacy, potency, or both. Our results demonstrate that the apparent coupling preferences displayed by these receptors could not have arisen solely from differences in the magnitude of G protein expression. Because all of these experiments were performed with Gβ1γ2, it is not clear whether altering Gβγ subunits would convey another level of specificity. However, it is clear from these data that the identity of Gα subunit influences coupling between receptors and N-type Ca2+ channels. Taken together, these data present a picture of G protein-coupling specificity that falls somewhere between the promiscuous coupling observed in biochemical studies and the exquisitely selective coupling reported from antisense knockout studies. It is easy to envision how modest differences in the expression levels of G protein subunits could convey functional selectivity greater than that observed for our heterologous studies. However, if receptor coupling to N-type Ca2+ channels in situ occurs via a specific heterotrimer composition (as has been proposed for L-type channels), then additional elements must exist to convey such specificity.
Acknowledgments
We thank M. King and K. Cobb for technical assistance in making cDNA constructs encoding PTX-insensitive mutants and Drs. V. Ruiz-Velasco and P. Kammermeier for critical reading of an earlier version of the manuscript. We are also grateful to Drs. M. I. Simon, R. Reed, and N. Gautam for providing G protein subunit cDNA clones. This study was supported by National Institutes of Health Grant GM 56180 to S.R.I.
Abbreviations
- α2-AR
α2-adrenergic receptor
- NE
norepinephrine
- PGE2
prostaglandin E2
- PTX
pertussis toxin
- SCG
superior cervical ganglion
- VD
voltage-dependent
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hille B. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda S R. Nature (London) 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 3.Herlitze S, Garcia D E, Mackie K, Hille B, Scheuer T, Catterall W A. Nature (London) 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 4.Dolphin A C. J Physiol (London) 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda S R, Dunlap K. Adv Second Messenger Phosphoprotein Res. 1999;33:131–151. doi: 10.1016/s1040-7952(99)80008-1. [DOI] [PubMed] [Google Scholar]
- 6.Hildebrandt J D. Biochem Pharmacol. 1997;54:325–339. doi: 10.1016/s0006-2952(97)00269-4. [DOI] [PubMed] [Google Scholar]
- 7.Pronin A N, Gautam N. Proc Natl Acad Sci USA. 1992;89:6220–6224. doi: 10.1073/pnas.89.13.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt C J, Thomas T C, Levine M A, Neer E J. J Biol Chem. 1992;267:13807–13810. [PubMed] [Google Scholar]
- 9.Ray K, Kunch C, Bonner L M, Robinshaw J D. J Biol Chem. 1995;270:21765–21771. doi: 10.1074/jbc.270.37.21765. [DOI] [PubMed] [Google Scholar]
- 10.Yan K, Kalyanaraman V, Gautam N. J Biol Chem. 1996;271:7141–7146. doi: 10.1074/jbc.271.12.7141. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher J E, Lindorfer M A, DeFilippo J M, Yasuda H, Guilmard M, Garrison J C. J Biol Chem. 1998;273:636–644. doi: 10.1074/jbc.273.1.636. [DOI] [PubMed] [Google Scholar]
- 12.Hepler J R, Gilamn A G. Trends Biochem Sci. 1992;17:383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- 13.Figler R A, Graber S G, Lindorfer M A, Yasuda H, Linden J, Garrison J C. Mol Pharmacol. 1996;50:1587–1595. [PubMed] [Google Scholar]
- 14.Richardson M, Robishaw J D. J Biol Chem. 1999;274:13525–13533. doi: 10.1074/jbc.274.19.13525. [DOI] [PubMed] [Google Scholar]
- 15.Wickman K D, Clapham D E. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- 16.Wickman K D, Iniquez-Lluhi J A, Davenport P A, Taussig R, Krapivinsky G B, Linder M E, Gilman A G, Clapham D E. Nature (London) 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- 17.García D E, Li B, García-Ferreiro R E, Hernández-Ochoa E O, Yan K, Gautam N, Catterall W A, Mackie K, Hille B. J Neurosci. 1998;18:9163–9170. doi: 10.1523/JNEUROSCI.18-22-09163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taussig R, Sanchez S, Rifo M, Gilman A G, Belardetti F. Neuron. 1992;8:799–809. doi: 10.1016/0896-6273(92)90100-r. [DOI] [PubMed] [Google Scholar]
- 19.Hunt T W, Carroll R C, Peralta E G. J Biol Chem. 1994;269:29565–29570. [PubMed] [Google Scholar]
- 20.Senogles S E. J Biol Chem. 1994;269:23120–23127. [PubMed] [Google Scholar]
- 21.Kozasa T, Kaziro Y, Ohtsuka T, Grigg J J, Nakajima S, Nakajima Y. Neurosci Res. 1996;26:289–297. doi: 10.1016/s0168-0102(96)01111-x. [DOI] [PubMed] [Google Scholar]
- 22.Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Nature (London) 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 23.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Nature (London) 1992;358:424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- 24.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Science. 1993;259:832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda S R. In: Methods in Molecular Biology. Challis R A J, editor. Totowa, NJ: Humana; 1997. pp. 191–202. [Google Scholar]
- 26.Jeong S W, Ikeda S R. Neuron. 1998;21:1201–1212. doi: 10.1016/s0896-6273(00)80636-4. [DOI] [PubMed] [Google Scholar]
- 27.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda S R. J Physiol (London) 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda S R, Lovinger D M, McCool B A, Lewis D. Neuron. 1995;14:1029–1038. doi: 10.1016/0896-6273(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 30.Schofield G G. Eur J Pharmacol. 1990;180:37–47. doi: 10.1016/0014-2999(90)90590-3. [DOI] [PubMed] [Google Scholar]
- 31.Schofield G G. Eur J Pharmacol. 1991;207:195–207. doi: 10.1016/0922-4106(91)90031-c. [DOI] [PubMed] [Google Scholar]
- 32.Caufield M P, Jones S, Vallis Y, Buckley N J, Kim G D, Milligan G, Brown D A. J Physiol (London) 1994;477:415–422. doi: 10.1113/jphysiol.1994.sp020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milligan G. Biochem J. 1988;255:1–13. doi: 10.1042/bj2550001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong S W, Ikeda S R. J Neurosci. 1999;19:4755–4761. doi: 10.1523/JNEUROSCI.19-12-04755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahia D S, Wise A, Fanelli F, Lee M, Rees S, Milligan G. Biochemistry. 1998;37:11555–11562. doi: 10.1021/bi980284o. [DOI] [PubMed] [Google Scholar]
- 36.Jackson V N, Bahia D S, Milligan G. J Pharmacol Exp Ther. 1999;55:195–201. doi: 10.1124/mol.55.2.195. [DOI] [PubMed] [Google Scholar]
- 37.Kalkbrenner F, Dippel E, Wittig B, Schultz G. Biochim Biophys Acta. 1996;1314:125–139. doi: 10.1016/s0167-4889(96)00072-9. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, Ikeda S R. J Neurophysiol. 1993;70:610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda S R. J Physiol (London) 1992;458:339–359. doi: 10.1113/jphysiol.1992.sp019421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda S R, Schofield G G. J Physiol. (London) 1989;409:221–240. doi: 10.1113/jphysiol.1989.sp017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delmas P, Abogadie F C, Milligan G, Buckley N J, Brown D A. J Physiol (London) 1999;518:23–36. doi: 10.1111/j.1469-7793.1999.0023r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eason M G, Kurose H, Holt B D, Raymond J R, Liggett S B. J Biol Chem. 1992;267:15795–15801. [PubMed] [Google Scholar]
- 43.Ren H, Stiles G L. Proc Natl Acad Sci USA. 1994;91:4864–4866. doi: 10.1073/pnas.91.11.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozak M. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 45.Conklin B R, Farfel Z, Lustig K D, Julius D, Bourne H R. Nature (London) 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- 46.Wise A, Watson-Koken M, Rees S, Lee M, Milligan G. Biochem J. 1997;321:721–728. doi: 10.1042/bj3210721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chabre O, Conklin B R, Brandon S, Bourne H R, Limbard L E. J Biol Chem. 1994;269:5730–5734. [PubMed] [Google Scholar]