Abstract
Glucagon-like peptide (GLP) 1 is produced through posttranslational processing of proglucagon and acts as a regulator of various homeostatic events. Among its analogs, however, the function of GLP-1-(1–37), synthesized in small amounts in the pancreas, has been unclear. Here, we find that GLP-1-(1–37) induces insulin production in developing and, to a lesser extent, adult intestinal epithelial cells in vitro and in vivo, a process mediated by up-regulation of the Notch-related gene ngn3 and its downstream targets, which are involved in pancreatic endocrine differentiation. These cells became responsive to glucose challenge in vitro and reverse insulin-dependent diabetes after implantation into diabetic mice. Our findings suggest that efficient induction of insulin production in intestinal epithelial cells by GLP-1-(1–37) could represent a new therapeutic approach to diabetes mellitus.
At the end of gastrulation, the mouse endoderm exists as a one-cell layer covering the mesoderm and ectoderm of the embryo. Gut development begins with the invagination of the most anterior and posterior endoderm at embryonic day 8.5 (E8.5), leading to the formation of two open-ended tubes. This anterior and posterior migration, in combination with embryonic twisting, closes the midgut and forms a primitive gut tube. Pseudostratified immature epithelium is found in the gut by E15, and subsequent remodeling of the gut endoderm between E15 and E19 leads to the production of nascent villi with a monolayer of epithelial cells. During the first 2 postnatal wk, the intervillus epithelium develops into crypts, which contain a dividing epithelial stem cell population. Crypt cell progenies differentiate into the nonproliferative four principal epithelial cell lineages: enterocytes (columnar cells), goblet cells, enteroendocrine cells, and Paneth cells (1).
Generation of these cell types requires a number of processes regulated by various signaling molecules and transcription factors. Like neuronal development in the central nervous system, the Notch signaling pathway is involved in endodermal endocrine differentiation (2–5). In this signaling system, ligands bind to Notch receptors and activate their intracellular domains, leading to interaction with the DNA-binding protein RBP-Jk. This protein in turn activates expression of the basic helix-loop-helix Hes genes (6, 7), which, in turn, repress expression of downstream target genes, including neurogenin (ngn) and Math-1 (8, 9). Decreased Hes repression results in cell differentiation to a primary fate. In the developing pancreases of mice lacking RBP-Jk or a Notch ligand, Delta-like gene 1 (Dll1), differentiation of pancreatic endocrine cells was accelerated (10, 11). Overexpression of ngn3 or the intracellular domain of Notch3 (a repressor of Notch signaling) (12) also resulted in a similar pancreatic phenotype (2). Deletion of Hes-1 leads to pancreatic hypoplasia caused by increased endocrine differentiation from pancreatic progenitors and to abnormal endocrine differentiation of the stomach and intestine, such as extensive differentiation of progenitors into enteroendocrine and goblet cells (4). Furthermore, in the gut epithelium of mice lacking Math-1, enteroendocrine, goblet, and Paneth cells were depleted (5). These studies strongly suggest that fate-determination of pancreatic and intestinal endocrine-lineage cells is controlled by a common mechanism, the Notch-signaling pathway.
Whereas pancreatic and intestinal endocrine cells share common mechanisms of differentiation and hormone secretion, insulin genes, expressed in pancreatic β cells that reside in the islets of Langerhans, have never been activated in intestinal endocrine cells. However, because these cells share several molecular mechanisms with pancreatic endocrine cells in terms of development, they may possess the potential for insulin expression. To test this idea, we sought to induce insulin-production in intestinal epithelial progenitors by using glucagon-like peptide (GLP) 1.
Materials and Methods
Cell Culture.
Cell suspensions of pancreatic, liver, stomach, duodenal, and intestinal (including jejunum, ileum, and colon) tissue were prepared from C57BL/6 E17.5 fetal mice (CLEA Japan, Tokyo). These organs were carefully dissected under the microscope and placed in Ca2+-free Hanks' balanced salt solution (GIBCO/BRL) containing 5 mmol/liter CaCl2 (pH 7.4) and 0.1% collagenase (Sigma). Digestion was carried out by pipetting after cutting of tissues by a surgical blade and incubation for 10 to 20 min at 37°C. Triturated cells were washed in standard medium (13) before beginning cell culture. Our standard culture medium is a 1:1 mixture of Dulbecco's modified Eagle medium and F-12 (Sigma) with 10% FBS (Serologicals Corporation, Norcross, GA), γ-insulin (1 μg/ml; Wako, Tokyo), dexamethasone (1 × 10−7 mol/liter; Sigma), nicotinamide (10 mmol/liter; Sigma), l-glutamine (2 mmol/liter; GIBCO/BRL), 2-mercaptoethanol (50 μmol/liter; Sigma), Hepes (5 mmol/liter; Wako), and penicillin/streptomycin (GIBCO/BRL). For monolayer cultures, dissected cells were plated on six-well tissue culture plates (Becton Dickinson) at a density of 5 × 104 cells per cm2 and cultured in fresh standard medium. Growth factors, such as GLP-1-(1–37) (Sigma), activin A (generous gift from Y. Eto, Ajinomoto Corp.), betacellulin (Sigma), hepatocyte growth factor (Sigma), and vascular endothelial growth factor (VEGF; Sigma), were added 24 h after culture initiation.
Immunostaining.
Cultured monolayer cells were washed three times with PBS and fixed first with 4% phosphate-buffered paraformaldehyde for 5 min at room temperature (rt) and then with 25% acetone in methanol for 1 min at rt. After fixation, the cells were washed in PBS containing 0.05% polyoxyethylene (20) sorbitan monolaurate (Tween 20; Wako) and treated with 0.2% Triton X-100 (Sigma) for 1 h at rt. After washing in PBS-Tween 20 and blocking, fixed cells were incubated with primary goat anti-insulin antibody (Santa Cruz Biotechnology) and mouse anti-glucagon (Sigma) in a moist chamber for 16 h at 4°C. After washing and blocking, they were incubated with Alexa 488-conjugated donkey anti-goat IgG (Molecular Probes) and Cy3-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch) for 4 h at 4°C, respectively. After final washing, stained cells were viewed by using a Zeiss LSM510 laser scanning microscope.
Measurement of Insulin Protein.
Secreted insulin in the culture medium was measured by using a mouse insulin detection kit (Shibayagi, Gunma, Japan) according to the manufacturer's instructions.
RT-PCR Analysis.
We prepared total RNA by using an RNeasy Mini Kit (Qiagen, Tokyo) according to the manufacturer's instructions and synthesized cDNA from total RNA (4 μg) as described (13). After various dilutions of template cDNA, we optimized their concentration for each primer. In these concentrations, amplification by PCR did not reach plateau but could be used for semiquantitative analysis. There was no amplification of examined genes when prepared RNA itself was used as PCR template. Primer sequences are as follows [many were presented in our previous papers (13, 14); those that were not are in parentheses]: GLP-1R (5′-TGA ACC TGT TTG CAT CCT TCA-3′ and 5′-ACT TGG CAA GCC TGC ATT TGA-3′); insulin I, insulin II, glucagon, pdx-1, ngn3 (5′-AGT GCT CAG TTC CAA TTC CAC-3′ and 5′-AAG AAG TCT GAG AAC ACC AGT-3′); neuroD (4), pax-4 (5′-TCC TGA GTG AAG GCT CTG TGA A-3′ and 5′-AAC CTT AAG GCT CCG TGA AAT-3′); Nkx6.1 (4), and HNF-6 (5′-ATG ACC ATG GCC TGT GAA ACT-3′ and 5′-ATT CAG GTG GGC ATG AGG AT-3′); and hypoxanthine phosphoribosyltransferase (HPRT) as a positive control. PCR cycles were as follows: initial denaturation at 95° for 4 min followed by 30 to 45 cycles (as to a kind of primer) of 94° for 1 min, 55° for 1 min, 72° for 1 min, and final extension at 72° for 10 min. PCR products were separated in 2% agarose gel.
Injection of GLP-1-(1–37).
GLP-1-(1–37) was injected i.p. into C57BL/6 pregnant mice (E10.5) or 10-wk-old adult mice (CLEA Japan) at 10 nmol/day for 8 or 9 days, respectively. After daily injection, jejunum, ileum, and colon were analyzed by immunohistochemistry.
Organ Culture.
Organ cultures of E14.5 fetal mouse-derived jejunums were performed after embedding in type I collagen gels (Nitta Gelatin, Osaka) with or without GLP-1-(1–37) (50 nmol/liter), and, when they were transplanted, we added VEGF (50 ng/ml) in gels to induce the migration of blood vessels. To analyze them, they were fixed in 4% phosphate-buffered paraformaldehyde overnight at 4°C and embedded in OCT compound. Cryostat sections from these tissues were dried and fixed in acetone for 10 min at rt. Sections were dried at −20°C overnight, washed with PBS-Tween 20 and treated with 0.2% Triton X-100 for 1 h at rt. Immunohistochemical staining of insulin and glucagon was then conducted.
Implantation of GLP-1-Induced Insulin-Producing Intestinal Epithelial Cells.
Two days after the initiation of organ culture, gel-embedded jejunums were implanted into the i.p. space of recipient mice. Recipient mice were injected with streptozotocin (STZ; 200 mg/kg) 3 days pretransplant (pre-TP) to induce insulin-dependent diabetes, and were selected on the basis of elevated fasting blood glucose level (300 to 350 mg/dl; n = 5).
Results
GLP-1-(1–37) Induces Insulin Expression in Intestinal Epithelial Cells.
GLP-1 is synthesized by posttranslational processing of proglucagon in the intestine and pancreas. Tissue-specific processing leads to the formation of several similar analogs. GLP-1-(7–36) and GLP-1-(7–37), secreted from enteroglucagon-producing cells (L cells) in the intestinal mucosa during hyperglycemia (15–17), are potent intestinal insulinotropic hormones that augment insulin secretion (18) and acutely lower glucose levels in rodents and in both type 1 and 2 human diabetic subjects (19–22). In contrast to these well characterized molecules, GLP-1-(1–37), formed in very small amounts by pancreatic glucagon-producing α cells, has little effect on insulin release (23). However, its function is still largely unknown. Because the receptor for GLP-1 (GLP-1R) is expressed in the E17.5 developing pancreas in addition to the stomach, duodenum, and intestine (jejunum, ileum, and colon; Fig. 1A), it was speculated that GLP-1-(1–37) may elicit unexpected responses from these tissues, including insulin expression.
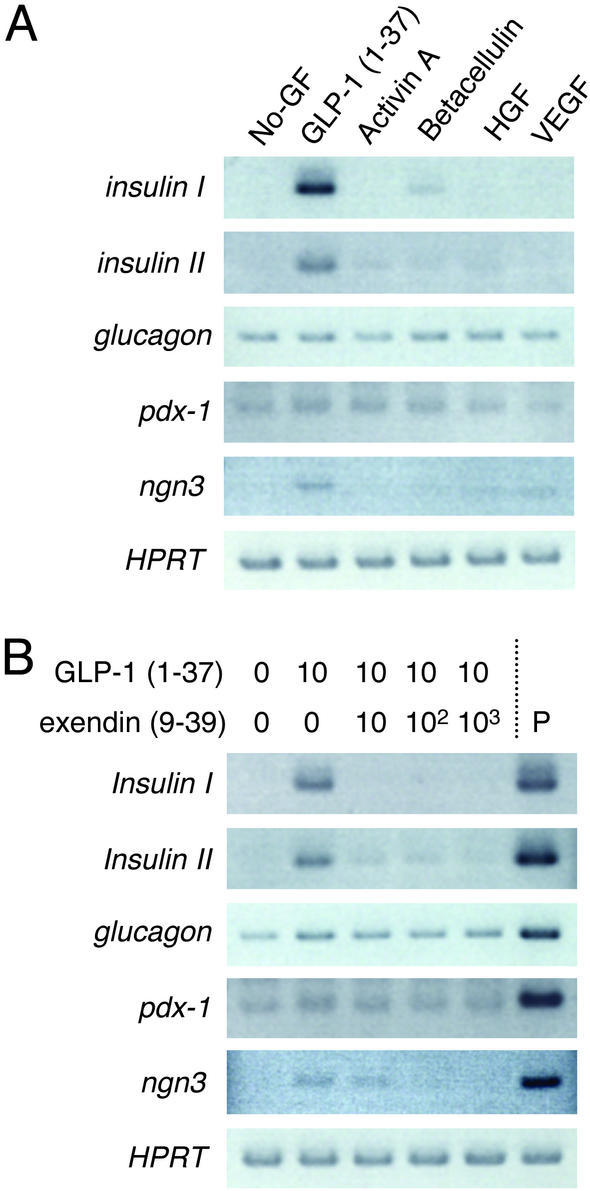
Figure 1.
GLP-1-(1–37) induces expression and production of insulin in intestinal epithelial cells. (A) Semiquantitative RT-PCR using RNA from E17.5 pancreas (P), stomach (S), liver (L), duodenum (Du), and intestine (I). GLP-1R was expressed in each of these tissues except the liver. Note that HNF-6 gene expression was found to be lowest in the intestine. (B) On culture of dissociated cells from these developing organs with GLP-1-(1–37) (10 nmol/liter), a significant change in insulin immunoreactivity was observed only for cells derived from intestine, where many were seen to turn into insulin-immunoreactive cells that coexpressed glucagon. Scale bar = 50 μm (P) and 100 μm (S, L, Du, and I). (C) After 8 days incubation, intestine-derived cells were washed and then stimulated with 25 mM glucose for 2 h. GLP-1-treated cells with glucose secreted more insulin than cells treated with glucose or GLP-1-(1–37) alone. Bar shows mean ± SD (n = 3). SM, standard medium. *, P < 0.01; **, P < 0.03. (D) In addition to insulin gene induction, culture with GLP-1-(1–37) (5, 10, and 50 nmol/liter) led to up-regulation of ngn3, neuroD, pax-4, Nkx6.1, and HNF6 expression as the concentration of GLP-1-(1–37) was raised. In contrast, expression of glucagon, pdx-1, and GLP-1R was not affected.
To examine the effect of GLP-1-(1–37), dissociated cells from E17.5 pancreas, stomach, liver, duodenum, and intestine were cultured for 8 days in the presence of this peptide and analyzed by immunocytochemistry. As previously reported, GLP-1-(1–37) was not able to enhance insulin production in our pancreas cultures. In addition, none of the cells from the stomach, liver, and duodenum were positive for insulin after culture. Surprisingly, however, insulin and glucagon double-positive cells did emerge from the cultures of intestinal cells (Fig. 1B). Stimulation with 25 mM glucose for 2 h after 8 days incubation led to induction of insulin release from these insulin-producing cells (Fig. 1C). Compared with normal islets that contain ≈10 μg of insulin per million cells, the average insulin content of these induced cultures (88.9 ± 15.1 ng per well, n = 3) is low because all of the intestinal epithelial cells could not be insulin-positive. However, this result shows that insulin secretion from insulin-producing intestinal epithelial cells occurs in response to physiologically appropriate glucose concentration. Furthermore, semiquantitative RT-PCR revealed that, synchronous with insulin I and II expression, genes involved in pancreatic endocrine differentiation, such as ngn3 and its downstream targets neuroD (24) and pax-4 (25), and the pancreatic β cell-specific gene Nkx6.1 (26), were highly expressed after culture with GLP-1-(1–37) in a dose-dependent manner (Fig. 1D). However, little change was detected in the expression of pancreas duodenal homeobox 1 (pdx-1), known to be a strong inducer of insulin gene expression (27). During pancreatic development, hepatocyte nuclear factor 6 (HNF-6) regulates endocrine cell differentiation by controlling ngn3 expression (28). Although HNF-6 was observed to be expressed at low levels in precultured intestinal cells (Fig. 1A), its expression was stimulated by GLP-1-(1–37) in culture (Fig. 1D). These data suggest that the insulin expression in intestinal epithelial progenitors depends on up-regulation of ngn3 expression mediated by HNF-6 via the GLP-1 signaling pathway.
Insulin Induction in Intestinal Epithelial Cells Is Due to a Specific and Direct Effect of GLP-1-(1–37).
Previous studies showed that activin A, betacellulin, hepatocyte growth factor, and VEGF could induce differentiation of pancreatic β cells (29–31). Culture of E17.5 intestinal epithelial cells with such molecules, however, leads only to very low expression of the insulin gene compared with GLP-1-(1–37) (Fig. 2A). RT-PCR analysis also showed that other analogs of GLP-1, such as GLP-1-(7–36) and GLP-1-(7–37), as well as exendin-4, a long-acting GLP-1 agonist that stimulates both proliferation and differentiation of β cells (32), have no effect on insulin gene expression (data not shown). The results show that GLP-1-(1–37) specifically induces insulin gene expression in intestinal epithelial progenitors, and that this conversion occurs by a separate pathway during pancreatic β cell differentiation.
Figure 2.
The induction of insulin gene expression in intestinal epithelial cells was a specific and direct effect of GLP-1-(1–37). (A) Several inducers of pancreatic β cell differentiation, such as activin A, betacellulin, hepatocyte growth factor, and VEGF, induced far less insulin and ngn3 gene expression than GLP-1-(1–37). (B) Blocking the biological effect of GLP-1-(1–37) (10 nmol/liter) with the receptor-specific GLP-1 antagonist exendin (9–39) (10, 102, and 103 nmol/liter) dramatically inhibited insulin and ngn3 gene expression in intestinal epithelial cells by competitive suppression. P, pancreatic cells used as a control.
We next used the receptor-specific GLP-1 antagonist exendin-(9–39) to inhibit the biological effects of GLP-1 (33). Exendin-(9–39) was found to compete with GLP-1-(1–37), inhibiting GLP-1-induced insulin expression while interfering with up-regulation of ngn3 in intestinal cell cultures (Fig. 2B). This examination clearly shows that the insulin production displayed by these intestinal epithelial cells is due to a direct effect of GLP-1 working through GLP-1R.
GLP-1-(1–37) Converts Developing and Adult Intestinal Epithelial Cells into Insulin-Producing Cells in Vivo.
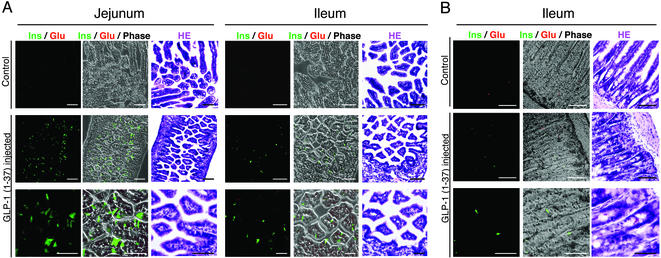
To determine whether GLP-1-(1–37) could bring about the differentiation of intestinal epithelial progenitors into insulin-producing cells in vivo, we injected it i.p. into pregnant mice every day between E10.5 and E17.5. Parental mice and newborns seemed normal after sequential injections, but the average date of birth was 1 day earlier than controls, suggesting that excess GLP-1-(1–37) may affect hormonal balance during mouse development. Immunostaining of neonatal jejunum, ileum, and colon revealed that insulin-, but not glucagon-, producing cells did indeed develop in the jejunal and ileal epithelium. These insulin-producing cells seemed to be produced in much larger numbers in the jejunum than in the ileum (Fig. 3A). We also injected GLP-1-(1–37) into 10-wk-old adult mice everyday for 9 days. Unlike neonates, insulin immunoreactivity was observed in only ileal intestinal epithelia, but in much lower numbers (Fig. 3B). These data indicate that, similar to pancreatic β cells, the potential for insulin production is endowed not only on developing, but also on adult intestinal epithelial cells. However, the much greater efficiency of conversion found in the developing intestines suggests that GLP-1-(1–37) can exert its effects only on actively proliferating and differentiating progenitors residing in the pseudostratified and intervillus epithelia, rather than on differentiating cells derived from adult crypt stem cells.
Figure 3.
GLP-1-(1–37) allowed the generation of insulin-producing cells in both developing and adult intestinal epithelia in vivo. (A) Daily injection of GLP-1-(1–37) into pregnant mice (E10.5–E17.5) led to differentiation of intestinal epithelial progenitors residing in the neonatal jejunum and ileum into insulin-producing cells. The frequency of observed insulin-producing cells was higher in jejunal than in ileal and colonic epithelia. (B) Insulin-producing cells also appeared in adult ileal epithelia after direct injection of GLP-1-(1–37) for 9 days. In contrast with the in vitro findings, few developing or adult epithelial cells produced both glucagon and insulin in vivo. Scale bar = 100 μm (Top and Middle) and 50 μm (Bottom).
Implantation of GLP-1-Induced Insulin-Producing Intestinal Epithelial Cells Restores Glucose Homeostasis in Diabetic Mice.
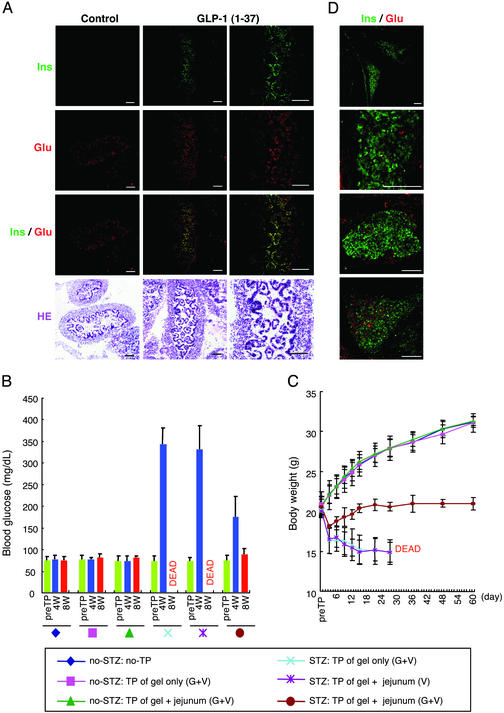
Pancreas and islet cell transplantation for type-1 diabetic patients restores β cell-mediated glucose homeostasis (34). To test whether insulin-producing intestinal epithelial cells could reverse insulin-dependent diabetes after implantation into STZ-induced diabetic mice, we transplanted E14.5 jejunums into these mice after collagen gel-based organ cultures with GLP-1-(1–37). Immunohistochemical staining of the cultured jejunums demonstrated the appearance of insulin and glucagon double-positive intestinal epithelial cells (Fig. 4A). Similar to monolayer cultures, the expression of glucagon was also stimulated without GLP-1-(1–37), suggesting that the relatively higher observed expression of glucagon resulted from exposure to culture medium-derived factors. Within 8 wk post-TP of GLP-1-treated jejunums, the implanted diabetic mice showed a decrease in fasting blood glucose levels (Fig. 4B). The body weights of these implanted mice remained ≈20 g for 8 wk, whereas the weights of nonimplanted mice or mice that received jejunums that had been cultured without GLP-1-(1–37) decreased gradually, leading to death in a maximum of 5 wk (Fig. 4C). Clearly, the transplanted tissue was capable of responding adequately to blood glucose in vivo, and could mitigate diabetic status. Moreover, in explanted donor-derived tissues at 60 days post-TP, many insulin-positive cells formed islet-like structures with glucagon-positive cells (Fig. 4D). This result indicates that GLP-1-induced insulin-producing cells could proliferate in gels after transplantation, and function as aggregate structures for at least 2 mo.
Figure 4.
Amelioration of diabetes after implantation of GLP-1-induced insulin-producing intestinal cells. (A) Insulin immunoreactivity was detected in intestinal epithelial cells after collagen gel cultures of E14.5 jejunums with GLP-1-(1–37). Most insulin-positive cells also expressed glucagon, which was most likely not a specific effect of GLP-1-(1–37) because glucagon's expression was also observed after culture without GLP-1-(1–37). (B and C) Gel-embedded E14.5 jejunums and their culture for 2 days with GLP-1-(1–37) and VEGF were implanted into the i.p. cavity of recipient mice that had been treated with STZ 3 days pre-TP to promote insulin-dependent diabetes. (B) Fasting blood glucose levels were determined pre-TP and at 4 and 8 wk post-TP after 12-h starvation for each mouse (n = 5). Bar shows mean ± SD. At or before 5 wk post-TP, all untransplanted mice or those implanted jejunums that had been cultured without GLP-1-(1–37) have died. (C) The body weights of the recipient mice were also measured pre-TP, and at 3, 6, 9, 12, 15, 21, 27, 36, 48, and 60 days post-TP (mean ± SD). (D) At 60 days post-TP, implanted tissues were analyzed by immunohistochemistry. Many insulin-positive cells formed islet-like structures with glucagon-positive cells. G, GLP-1-(1–37) (50 nmol/liter); V, VEGF (50 ng/ml). Scale bar = 100 μm.
Discussion
Although no functional role for GLP-1-(1–37) has previously been identified, our findings in this study clearly establish a previously uncharacterized function for this pancreas-derived hormone. By controlling the expression of ngn3 and HNF-6, mediated by up-regulation of the neuroD, pax-4, and Nkx6.1 genes, GLP-1-(1–37) converts intestinal epithelial progenitors in the small intestine into insulin-producing cells. These Notch-associated ngn signals control primary differentiation of neural (6–8, 12), pancreatic (2, 4), and intestinal-lineage cells (3–5) via a common mechanism. Whereas a number of cells differentiate in these tissues to secrete various homeostatic hormones, these hormones are not all secreted by a single cell type residing in a limited region. For example, glucagon and somatostatin are secreted by both pancreatic and intestinal endocrine cells, and serotonin (5-hydroxytryptamine) is secreted both by intestinal enterochromaffin cells and by a subtype of neuron. Similarly, insulin secretion is not necessarily specific to pancreatic β cells. In Drosophila, insulin-producing cells that coexpress four insulin genes and play critical roles in larval growth and carbohydrate maintenance exist in the brain (35). In addition, a single cell derived from mouse embryonic stem cells could clonally give rise to both insulin-secreting cells and neural-lineage cells (36). All these findings suggest that hormone-secreting cells in the brain, pancreas, and intestine are closely related to each other, and they develop depending on differences in the microenvironment, with Notch signaling playing a central role. Thus, alteration of environmental signals could provide the potential for secretion of a different hormonal product. GLP-1-(1–37), as used here, might alter a balance in the developmental environment of the intestinal epithelia, leading to the induction of insulin-producing cells from intestinal epithelial progenitors.
Normally, GLP-1-(1–37) is produced by posttranslational processing of proglucagon in such small amounts that the effect found in this study is not observed. However, if large quantities of this molecule were secreted accidentally, pancreatic endocrine-like tissues that include insulin-producing cells would emerge in the intestinal epithelia or other endodermal tissues. Such abnormal secretion of GLP-1-(1–37) may be a cause of ectopic pancreas, a clinically observed embryologic anomaly characterized by the observation of pancreatic endocrine and exocrine tissues around epithelial tissues in the intestine and stomach (37–39). Our present data show that GLP-1-induced insulin-producing intestinal epithelial cells proliferate and function as glucose regulators similar to pancreatic β cells after implantation into diabetic mice, suggesting that ectopically generated β-like cells in epithelial tissues possess the potential for ameliorating diabetic status. To realize the strictest possible glucose regulation using this method, future investigations into increasing differentiated cell numbers or maximizing the differentiation efficiency of transplantable insulin-producing cells would be necessary. In conclusion, manipulation of intestinal epithelial cells using GLP-1-(1–37) may be a viable regenerative therapeutic strategy for the treatment of diabetes mellitus.
Acknowledgments
We thank N. Ukawa and Y. Jinzenji for technical support. This work was supported by the Ministry of Education, Science, and Culture of Japan (Grants-in-Aid for Scientific Research 14207046 and 12557096) and a grant from the NISSAN Science Foundation.
Abbreviations
- En
embryonic day n
- ngn
neurogenin
- GLP
glucagon-like peptide
- VEGF
vascular endothelial growth factor
- rt
room temperature
- STZ
streptozotocin
- TP
transplant
- GLP-1R
GLP 1 receptor
- HNF
hepatocyte nuclear factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Schmidt G H, Wilkinson M M, Ponder B A. Cell. 1985;40:425–429. doi: 10.1016/0092-8674(85)90156-4. [DOI] [PubMed] [Google Scholar]
- 2.Apelqvist A, Li H, Sommer L, Beatus P, Anderson D J, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 3.Rindi G, Ratineau C, Ronco A, Candusso M E, Tsai M, Leiter A B. Development. 1999;126:4149–4156. doi: 10.1242/dev.126.18.4149. [DOI] [PubMed] [Google Scholar]
- 4.Jensen J, Pedersen E E, Galante P, Hald J, Heller R S, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen O D. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Bermingham N A, Finegold M J, Zoghbi H Y. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 6.Lewis J. Curr Opin Neurobiol. 1996;6:3–10. doi: 10.1016/s0959-4388(96)80002-x. [DOI] [PubMed] [Google Scholar]
- 7.Beatus P, Lendahl U. J Neurosci Res. 1998;54:125–136. doi: 10.1002/(SICI)1097-4547(19981015)54:2<125::AID-JNR1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Ma Q, Kintner C, Anderson D J. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- 9.Zheng J L, Shou J, Guillemot F, Kageyama R, Gao W Q. Development. 2000;127:4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- 10.Oka C, Nakano T, Wakeham A, de la Pompa J L, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak T W, Honjo T. Development. 1995;121:3291–3301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- 11.Hrabe de Angelis M, McIntyre J, 2nd, Gossler A. Nature. 1997;386:717–721. doi: 10.1038/386717a0. [DOI] [PubMed] [Google Scholar]
- 12.Lardelli M, Williams R, Mitsiadis T, Lendahl U. Mech Dev. 1996;59:177–190. doi: 10.1016/0925-4773(96)00589-8. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki A, Zheng Y W, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H, Taniguchi H. Hepatology. 2000;32:1230–1239. doi: 10.1053/jhep.2000.20349. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Zheng Y W, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H. J Cell Biol. 2002;156:173–184. doi: 10.1083/jcb.200108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holz G G, 4th, Kuhtreiber W M, Habener J F. Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holst J J. Gastroenterology. 1994;107:1848–1855. doi: 10.1016/0016-5085(94)90831-1. [DOI] [PubMed] [Google Scholar]
- 17.Hoyt E C, Lund P K, Winesett D E, Fuller C R, Ghatei M A, Bloom S R, Ulshen M H. Diabetes. 1996;45:434–439. doi: 10.2337/diab.45.4.434. [DOI] [PubMed] [Google Scholar]
- 18.Mojsov S, Weir G C, Habener J F. J Clin Invest. 1987;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutniak M, Orskov C, Holst J J, Ahren B, Efendic S. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 20.Zander M, Madsbad S, Madsen J L, Holst J J. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 21.Greenbaum C J, Prigeon R L, D'Alessio D A. Diabetes. 2002;51:951–957. doi: 10.2337/diabetes.51.4.951. [DOI] [PubMed] [Google Scholar]
- 22.Tourrel C, Bailbe D, Lacorne M, Meile M J, Kergoat M, Portha B. Diabetes. 2002;51:1443–1452. doi: 10.2337/diabetes.51.5.1443. [DOI] [PubMed] [Google Scholar]
- 23.Fehmann H C, Goke R, Goke B. Endocr Rev. 1995;16:390–410. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- 24.Huang H P, Liu M, El-Hodiri H M, Chu K, Jamrich M, Tsai M J. Mol Cell Biol. 2000;20:3292–3307. doi: 10.1128/mcb.20.9.3292-3307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gradwohl G, Dierich A, LeMeur M, Guillemot F. Proc Natl Acad Sci USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen J, Serup P, Karlsen C, Nielsen T F, Madsen O D. J Biol Chem. 1996;271:18749–18758. doi: 10.1074/jbc.271.31.18749. [DOI] [PubMed] [Google Scholar]
- 27.Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 28.Jacquemin P, Durviaux S M, Jensen J, Godfraind C, Gradwohl G, Guillemot F, Madsen O D, Carmeliet P, Dewerchin M, Collen D, et al. Mol Cell Biol. 2000;20:4445–4454. doi: 10.1128/mcb.20.12.4445-4454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mashima H, Ohnishi H, Wakabayashi K, Mine T, Miyagawa J, Hanafusa T, Seno M, Yamada H, Kojima I. J Clin Invest. 1996;97:1647–1654. doi: 10.1172/JCI118591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Ocana A, Vasavada R C, Cebrian A, Reddy V, Takane K K, Lopez-Talavera J C, Stewart A F. Diabetes. 2001;50:2752–2762. doi: 10.2337/diabetes.50.12.2752. [DOI] [PubMed] [Google Scholar]
- 31.Rooman I, Schuit F, Bouwens L. Lab Invest. 1997;76:225–232. [PubMed] [Google Scholar]
- 32.Xu G, Stoffers D A, Habener J F, Bonner-Weir S. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Wang R M, Owji A A, Smith D M, Ghatei M A, Bloom S R. J Clin Invest. 1995;95:417–421. doi: 10.1172/JCI117671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bottino R, Trucco M, Balamurugan A N, Starzl T E. Best Pract Res Clin Gastroenterol. 2002;16:457–474. doi: 10.1053/bega.2002.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rulifson E J, Kim S K, Nusse R. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- 36.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 37.Khomiakov IuS, Firsova T P. Khirurgiia (Mosk) 1970;46:129–131. [PubMed] [Google Scholar]
- 38.Lucaya J, Ochoa J B. J Pediatr Surg. 1976;11:101–102. doi: 10.1016/0022-3468(76)90181-0. [DOI] [PubMed] [Google Scholar]
- 39.Chen C H, Yang C C, Yeh Y H, Chou D A, Kuo C L. Gastrointest Endosc. 2001;53:121–123. doi: 10.1067/mge.2001.111396. [DOI] [PubMed] [Google Scholar]