Abstract
The cytotoxicity of several important antitumor drugs depends on formation of the covalent topoisomerase–DNA cleavage complex. However, cellular processes such as DNA replication are necessary to convert the cleavage complex into a cytotoxic lesion, but the molecular mechanism of this conversion and the precise nature of the cytotoxic lesion are unknown. Using a bacteriophage T4 model system, we have previously shown that antitumor drug-induced cleavage complexes block replication forks in vivo. In this report, we show that these blocked forks can be cleaved by T4 endonuclease VII to create overt DNA breaks. The accumulation of blocked forks increased in endonuclease VII-deficient infections, suggesting that endonuclease cleavage contributes to fork processing in vivo. Furthermore, purified endonuclease VII cleaved the blocked forks in vitro close to the branch points. These results suggest that an indirect pathway of branched-DNA cleavage contributes to the cytotoxicity of antitumor drugs that target DNA topoisomerases.
Several important classes of antitumor drugs, as well as the antibacterial quinolones, inhibit type II topoisomerases by a common mechanism of action (1–3). These drugs alter the equilibrium of the reaction cycle to favor an otherwise transient reaction intermediate called the cleavage complex. Within the cleavage complex, the enzyme is covalently linked, by means of phosphotyrosine bonds, to the cleaved DNA at two staggered positions on the opposing strands of the helix. Camptothecin and related anticancer drugs attack eukaryotic topoisomerase I by a related mechanism, trapping a cleavage complex with a single cleaved phosphodiester bond (4).
Various results demonstrate that cytotoxicity of type II topoisomerase inhibitors depends on formation of the cleavage complex, and not just inhibition of enzyme activity (2, 5, 6). However, the cleavage complex itself is reversible, and is thus unlikely to be the cytotoxic lesion (7). Instead, cellular processes apparently convert the cleavage complex into a cytotoxic lesion (8, 9). DNA replication appears to play an important role in this conversion because S phase cells are more sensitive to topoisomerase inhibitors than G1 cells, and because the replication inhibitor aphidicolin can abrogate drug sensitivity (8–11). Despite the implication that DNA replication is involved in cytotoxicity, the exact nature of the cytotoxic lesion and the pathway of its formation are not clear.
A drug-induced cleavage complex with a type II topoisomerase contains a latent double-strand DNA break (DSB), but in vitro this break is revealed only when the protein is denatured with a strong detergent such as SDS (see ref. 5). Nonetheless, overt DNA breaks are apparently generated from the drug-induced cleavage complexes in vivo, and these breaks are probably important in cytotoxicity. Several types of indirect evidence support this view. First, mutants deficient in DSB repair are hypersensitive to topoisomerase inhibitors that induce the cleavage complex (12–15). Second, cleavage-inducing inhibitors lead to gross DNA rearrangements, which have been implicated in the generation of secondary tumors that arise after treatment with anticancer agents that target topoisomerases (16–19). Third, the inhibitors also stimulate homologous recombination, and the presence of a single strong topoisomerase cleavage site increases homologous recombination in the presence of one of the antitumor drugs (12, 15, 18).
Two studies provide more direct evidence for overt DNA breaks. Caldecott et al. (14) detected long-lived protein-free DNA breaks after CHO xrs-1 (repair-deficient) mutant cells were washed free of etoposide or 4′-(9-acridinylamino)methanesulfon-m-anisidide (m-AMSA), but wild-type CHO cells showed no such breaks. In the same experiment, protein-linked DNA breaks disappeared after washout in both wild-type and mutant cells, which is consistent with simple reversal of cleavage complexes. The authors concluded that protein-free breaks were generated at a low frequency from cleavage complexes and were repaired only in the wild-type cells. Drlica and Zhao (6) found that the structure of the bacterial nucleoid was markedly disturbed after cells were treated with quinolones, and the physical analysis suggested that the nucleoids had sustained overt DNA breaks. This analysis did not distinguish whether the DNA ends had residual topoisomerase attached.
The pathway(s) for generating overt DNA breaks from type II topoisomerase cleavage complexes have not been elucidated, but they could be highly relevant for cancer and antibacterial chemotherapy. An oft-discussed model is that the replication fork or the fork-associated helicase disrupts the cleavage complex, releasing a broken lagging-strand template arm free of protein. The leading-strand template would presumably remain covalently attached to the topoisomerase, perhaps still associated with the template DNA ahead of the fork by means of the topoisomerase dimer interface. Support for a similar model has been obtained in the case of drug-stabilized type I topoisomerase cleavage complexes (21). This model is supported by the finding that a purified helicase can disrupt a type II topoisomerase cleavage complex in vitro, although the helicase used in that study was not a replicative helicase (22). Notably, the replicative helicase DnaB from Escherichia coli failed to disrupt a drug-stabilized (topoisomerase IV) cleavage complex (23), raising some doubt about the applicability of this model to type II topoisomerase inhibitors.
Caldecott et al. (14) proposed an alternative model for formation of DNA breaks at cleavage complexes. According to this model, overt DSBs are created by a repair nuclease that recognizes the cleavage complex and cleaves the DNA nearby to release a broken chromosome end free of protein. However, this model does not readily explain why DNA replication is connected to cytotoxicity, and no evidence in favor of this model has emerged.
More recent research has demonstrated that drug-stabilized type II topoisomerase cleavage complexes can block replication forks. In vitro studies with the E. coli replication system demonstrated that quinolone-stabilized cleavage complexes blocked completion of DNA replication on a linear template (24). Overt DNA breaks were not generated from the cleavage complexes, but the collision events converted the normally reversible cleavage complexes to an irreversible form (24). This result led to the proposal that the cytotoxic DNA lesion is created by denaturation of the topoisomerase in the irreversible complex during a subsequent repair event (24).
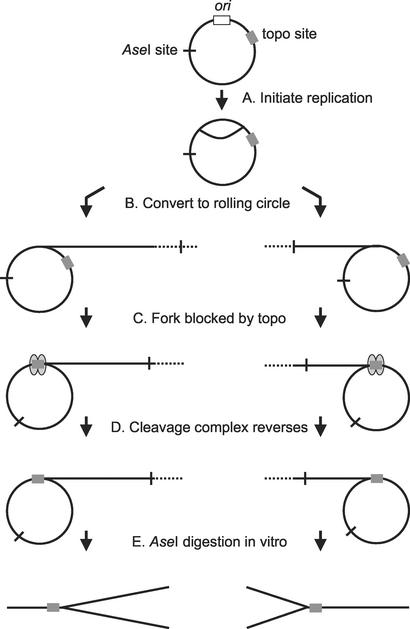
Another study, using a bacteriophage T4 model system, has shown directly that cleavage complexes block replication forks in vivo (25). Phage T4 encodes a type II topoisomerase that is sensitive to many of the antitumor drugs that inhibit mammalian topoisomerase, and the mechanism of inhibition appears to be conserved (26, 27). We found that antitumor drug-induced cleavage complexes block replication forks in a site-specific manner in vivo (25). Even though DNA within a cleavage complex contains a latent DSB, the arrested forks were intact, even when the DNA was extracted from cells in the presence of SDS (25). Therefore, the blocking cleavage complex had not been converted into an irreversible form. Instead, this and other results implied that the cleavage complex responsible for fork arrest had reversed in vivo before cell lysis (Fig. 1). The stalled fork was apparently unable to resume synthesis immediately after the cleavage complex reversed, suggesting that one or more components of the replication machinery had become inactivated or dissociated from the arrested fork (25).
Figure 1.
Generation of Y-form DNA from blocked replication forks on a plasmid. See text for description of the replication fork blockage in vivo (steps A–D) and the DNA analysis in vitro (step E).
If replication forks in vivo are simply blocked by cleavage complexes, which then reverse, how is the cytotoxic DNA lesion created? Recent work on replication fork restart may be relevant to this question. Stalled replication forks can be restarted by multiple pathways. Direct restart pathways, presumed to occur frequently, reload the replication machinery directly onto the stalled fork without any DNA breakage (28, 29). However, several studies indicate that stalled forks are cleaved by recombination nucleases when direct restart pathways fail. In E. coli, the Holliday junction resolution system RuvABC is implicated in cleavage of replication forks that are arrested because of mutationally altered replication proteins (30). In Saccharomyces cerevisiae, the elimination of the ATR/ATM homolog Mec1 leads to replication fork breakage in specific regions of the genome that replicate slowly (ref. 31; also see refs. 32–34). Additional evidence that blocked forks are susceptible to cleavage comes from studies of E. coli Tus and S. cerevisiae Fob1 proteins, which block forks at specific sites and thereby coordinate the termination of replication or the interplay of transcription and replication (35, 36). In both cases, homologous recombination is stimulated by the programmed fork blockage, arguing that DNA breaks are generated at some frequency (37–39). The prevailing view is that cleavage of stalled replication forks represents a last-ditch effort to restart the forks when direct restart pathways fail (28, 29). Homologous recombination can restart such broken forks by directing strand invasion followed by DNA replication from the invading 3′ end. This recombination pathway is dangerous, because if it fails, unrepaired breaks and/or genome rearrangements are generated.
These results on replication fork restart suggest a hypothesis for the generation of the cytotoxic lesion from topoisomerase cleavage complexes, namely that the DNA damage is created indirectly by recombination nucleases that attack topoisomerase-blocked forks. In this report, we provide, to our knowledge, the first evidence that supports this hypothesis. We demonstrate that the phage T4-encoded Holliday junction-cleaving enzyme (endonuclease VII) can cleave topoisomerase-arrested replication forks in vitro, and that the arrested forks accumulate at a higher level in vivo when this enzyme is mutationally inactivated.
Methods
Materials.
The Drug Synthesis and Chemistry Branch, National Cancer Institute (Bethesda), provided m-AMSA. Nylon-blotting membrane was obtained from Schleicher & Schuell, random-primed labeling kit from Roche Molecular Biochemicals, restriction enzymes from New England Biolabs, low-melt agarose from FMC, and T4 endonuclease VII from Pharmacia Biotech.
Bacterial Phage and Strains.
The bacterial host for phage infections was a derivative of E. coli CAG12135 with the following additional mutations: acrA∷Tn10-kan, recA∷Tn10-cam, and recD. The acrA mutation eliminates a multidrug efflux pump so that the infected cells are more sensitive to m-AMSA (J. George and K.N.K., unpublished data). Phage strains used in this study were T4 K10 [amB262 (gene 38), amS29 (gene 51), nd28 (denA), and rIIPT8 (denB-rII deletion); ref. 40], T4 K10-49am [K10 with amE727 (gene 49); ref. 41], and T4 K10-49ts [K10 with the temperature-sensitive C9 mutation in gene 49; which was constructed as described by Kreuzer et al. (42)].
Analysis of Arrested Forks in Vivo.
Bacterial cells harboring plasmid pGH2-01 (25) were grown at 37°C or 43°C to a cell density of 4 × 108 per ml, and then infected with the indicated T4 strain at a multiplicity of three plaque-forming units per cell. After 4 min without shaking for adsorption, cells were incubated for 2 min with shaking, and then m-AMSA (5 μg/ml) was added. The infected cells were incubated an additional 18 min with vigorous shaking at the indicated temperature. Infected cells (1 ml) were collected by centrifugation, and then were frozen and thawed in 300 μl of lysis buffer (50 mM Tris⋅HCl, pH 7.8/10 mM Na2EDTA/100 mM NaCl/0.2% SDS). Proteinase K was added to 0.5 mg/ml and the suspension was incubated at 65°C for 2 h. Nucleic acid was extracted sequentially with phenol and chloroform/isoamyl alcohol (24:1), and then dialyzed overnight at 4°C against TE buffer (10 mM Tris⋅HCl, pH 7.8/1 mM Na2EDTA). The DNA was treated with restriction enzymes AseI (which cleaves the T4-replicated plasmid DNA once) and HaeIII (which destroys any plasmid DNA that has not been replicated by T4; see ref. 25). Two-dimensional agarose gels were run and Southern blotted as described by Hong and Kreuzer (ref. 25; also see ref. 43).
T4 Endonuclease VII Reactions.
DNA was treated with T4 endonuclease VII in a reaction mix consisting of 10 mM MgCl2, 50 mM Tris⋅HCl at pH 8.1, 1 mM DTT, and 100 μg/ml BSA for 30 min at 37°C. The substrate for the reactions consisted of total nucleic acid extracted from an m-AMSA-treated 49am mutant infection (Fig. 3) or gel-purified branched DNA from the same infection (Fig. 4). The reaction products were visualized by Southern blotting with a radioactive plasmid probe.
Figure 3.
Cleavage of blocked replication forks by purified endonuclease VII. Total DNA from an m-AMSA-treated 49am mutant infection was treated with 0, 5, 50, or 500 units of endonuclease VII as described in Methods (A, B, C, and D, respectively). Two-dimensional gel electrophoresis and visualization of the plasmid DNA were as described in the legend to Fig. 2.
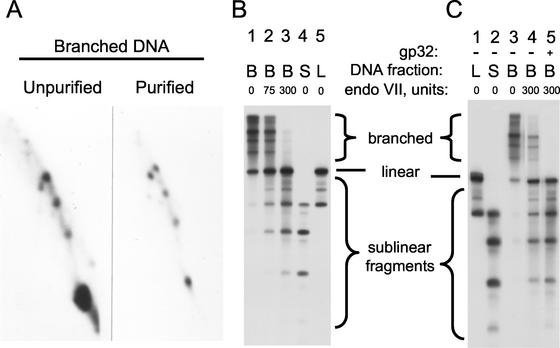
Figure 4.
Cleavage of purified branched DNA by endonuclease VII. Three DNA fractions were purified from an m-AMSA-treated 49am mutant infection by using an agarose gel after cleavage with AseI plus HaeIII. Shown are the branched DNA (fraction B) from the region above the unit-length linear plasmid; linear DNA (fraction L) from the region containing unit-length linear plasmid; and sublinear DNA fragments (fraction S) from the region below the unit-length linear plasmid. The starting DNA and purified branched DNA (fraction B) were analyzed by 2D gel electrophoresis (A), as described in the legend to Fig. 2. All three DNA fractions were also analyzed on a 1D agarose gel (B; lanes 1, 4, and 5 represent the untreated DNA fractions). The branched DNA fraction was also treated with 75 or 300 units of T4 endonuclease VII (B, lanes 2 and 3, respectively) and analyzed on the 1D agarose gel. Plasmid DNA was visualized by Southern hybridization with a plasmid probe. The amounts of various DNA forms in lanes 1 and 3 were quantitated by using a direct radioisotope counting system (AMBIS) by drawing boxes around the regions indicated as branched, linear, and sublinear fragments; the values were as follows (lane 1 versus lane 3): branched DNA, 33,900 and 8,700 cpm; linear DNA, 5,800 and 12,000 cpm; and sublinear DNA, 13,800 and 31,300 cpm. The experiment in C used a second set of similarly purified fractions containing mostly unit-length linear plasmid DNA (L), sublinear fragments (S), and branched DNA (B). The branched DNA substrate was treated with endonuclease VII (300 units) with or without the T4 single-strand binding protein gp32 (0.3 μM) for 30 min at 37°C (lanes 4 and 5).
Results
Accumulation of Blocked Replication Forks in Gene 49 Mutant Infections.
Blocked replication forks were previously detected after treatment of phage T4-infected cells with the antitumor drug m-AMSA by using 2D gel electrophoresis (25). The blocked forks were detected within a plasmid that contained a T4 replication origin, a cloned recognition site for the T4 type II topoisomerase, and plasmid vector sites that were also recognized by the topoisomerase. After T4 infection, the phage replication machinery induced rolling-circle replication of the plasmid, and forks initiated from the T4 origin were sometimes blocked near the topoisomerase recognition site when m-AMSA was present. Generation of the blocked forks depended on the plasmid-borne T4 replication origin, the phage-encoded topoisomerase, and m-AMSA (25). Because replication forks travel in either direction on the plasmid during the rolling-circle replication, each topoisomerase recognition site generated two branched DNA forms (Fig. 1). After treatment with the restriction enzyme AseI, which cleaves the plasmid once, the branched DNA forms fall on the Y-arc of a 2D agarose gel (Fig. 2 A and E). Prominent spots along the Y-arc were generated from forks blocked at the cloned topoisomerase recognition site and at the strong topoisomerase sites present within the vector sequence (25). Deletion of the cloned topoisomerase recognition site from the plasmid eliminated the two spots corresponding to forks blocked at that site, but did not affect the forks blocked at the topoisomerase recognition sites in the vector (25).
Figure 2.
Accumulation of blocked forks in endonuclease VII-deficient infections. Bacterial cells harboring plasmid pGH2-01 were infected with T4 K10 (wild type; A and C), T4 K10-49am (B), or T4 K10-49ts (D) at the indicated temperature. The infected cells were treated with m-AMSA, and the DNA was extracted, purified, and cleaved with restriction enzymes AseI and HaeIII. The first dimension of the gel was run from left to right and the second dimension from top to bottom. The plasmid DNA was visualized by Southern hybridization with a plasmid probe. A schematic of the observed pattern is shown in E.
The topoisomerase cleavage complexes that blocked the replication forks have apparently reversed in vivo before cell lysis (see introduction and ref. 25). Thus, the forked DNA is intact even though we extracted the DNA in the presence of SDS, which reveals DNA breaks within cleavage complexes. Nonetheless, plasmid cleavage complexes are also present in these infections, as revealed by protein-linked plasmid DNA breaks (see Supporting Text and Fig. 5, which are published as supporting information on the PNAS web site, www.pnas.org).
The major enzyme responsible for cleaving Holliday junctions and other recombinational branches in the phage T4 system is endonuclease VII, the product of gene 49 (44, 45). This enzyme also cleaves synthetic Y structures composed of three complementary oligonucleotides (46, 47), suggesting that it could play a role in the processing of blocked replication forks in vivo. To begin to test this hypothesis, we compared blocked forks induced by m-AMSA in T4 wild-type versus T4 gene 49 amber-mutant (T4 49am) infections of a nonsuppressing host. The accumulation of simple Y-form DNA containing blocked forks was substantially increased in the endonuclease VII-deficient infection, which was consistent with the stated hypothesis (Fig. 2 A and B). As previously reported for T4 wild-type infections (25), the accumulation of strong spots along the Y-arc depended on m-AMSA in the T4 49am infection (data not shown).
More complex branched forms were also overproduced in the mutant infection (Fig. 2B). We suspect that most or all of these more complex branched forms result from blockage of multiple replication forks in a single molecule. The most prominent feature of the more complex branched region was a strong X-arc, which, like the Y-arc, contained discrete spots. These X-shaped molecules might result from two opposing replication forks converging on a single topoisomerase cleavage complex, either within a θ replication intermediate or within a rolling circle in which replication has reinitiated at one or more of the replicated origins. Alternatively, cleavage complexes might possibly arrest branch migration during plasmid recombination reactions, leading to the strong X-arc.
We also compared a T4 wild-type versus T4 gene 49 temperature-sensitive (T4 49ts) mutant infection at the restrictive temperature of 43°C. The accumulation of blocked forks in the wild-type infection was markedly decreased at this higher temperature (Fig. 2C). One possible explanation is that translation of gene 49 mRNA is thought to be increased at higher temperatures because of melting of the secondary structure in the Shine–Delgarno region (48). Thus, T4 infections likely contain more endonuclease VII at higher temperatures, potentially leading to more efficient cleavage of blocked forks in vivo. In any case, the T4 49ts mutant infection clearly resulted in much larger amounts of blocked forks than did the wild-type infection at 43°C (compare Fig. 2 C and D). The increased accumulation of blocked forks in the two different endonuclease VII-deficient infections argues that endonuclease VII-dependent cleavage provides a major pathway for processing blocked forks in wild-type infections.
Cleavage of Blocked Replication Forks in Vitro with Purified T4 Endonuclease VII.
We next asked whether purified endonuclease VII cleaves blocked forks in vitro. DNA containing blocked forks (from the T4 49am infection) was treated with increasing amounts of endonuclease VII and subjected to 2D gel electrophoresis. The branched DNA forms were indeed cleaved by endonuclease VII (Fig. 3 A–D). The linear restriction fragment, which serves as an internal control, was not cleaved even with excess endonuclease VII (Fig. 3D).
We purified branched DNA forms from a T4 49am infection by using an agarose gel so that we could directly analyze the products of endonuclease VII cleavage. The nature of the purified fraction was verified by 2D gel electrophoresis. As expected, most of the purified DNA molecules that hybridized to the plasmid probe consisted of Y-form DNA, i.e., the topoisomerase-blocked replication forks (Fig. 4A; purified branched DNA). This purified branched DNA fraction was contaminated by some full-length linear DNA-restriction fragment.
At the same time, we also purified two additional DNA fractions, one containing the bulk of the full-length AseI linear restriction fragment and the other containing sublinear fragments. The sublinear fragments consist of the cleaved DNA portion of m-AMSA-induced topoisomerase cleavage complexes, after the covalently attached topoisomerase has been removed by proteinase K (see Supporting Text). These bona fide topoisomerase cleavage products will serve as size standards when we analyze cleavage of the blocked forks below.
All three DNA fractions were compared on a 1D agarose gel, using Southern hybridization with a plasmid probe. As expected from the 2D analysis in Fig. 4A, the branched DNA fraction (fraction B) showed mostly slowly migrating branched forms (Fig. 4B, lane 1). The full-length linear fraction (fraction L) contained mostly full-length linear DNA, along with the largest prominent sublinear band, and the sublinear fraction (fraction S) contained all sublinear plasmid fragments (Fig. 4B).
When the purified branched DNA substrate was treated with purified endonuclease VII and analyzed by 1D gel electrophoresis, the slowly migrating branched DNA forms were cleaved into linear DNA and sublinear fragments (Fig. 4B, lanes 2 and 3). The sublinear fragments essentially comigrated with the bona fide topoisomerase cleavage products of the same DNA (compare lanes 2 and 3 with lanes 4 and 5). Therefore, the branch points of the blocked replication forks must have been very close to the topoisomerase recognition site, and the endonuclease VII must have cleaved very close to these branch points.
We quantitated lanes 1 and 3 of Fig. 4B with a direct radioisotope imaging system, dividing each lane into three boxes: branched forms, full-length linear DNA, and all sublinear fragments. On endonuclease VII cleavage, radioactivity in the branched form box decreased by 25,200 cpm, while radioactivity in the full-length and sublinear boxes increased by 6,200 and 17,500 cpm, respectively (see legend to Fig. 4). Because the amount of full-length linear DNA increased with endonuclease VII treatment, some of the branched Y-form DNA molecules had only one arm amputated by the nuclease. Nonetheless, the sublinear forms were produced in excess of full-length linear DNA, and therefore some of the Y branches were also cleaved into three fragments.
We repeated the above experiment with a second preparation of purified branched DNA, one that contained less contaminating full-length linear DNA (Fig. 4C). The generation of full-length linear DNA on endonuclease VII cleavage was confirmed (compare lanes 3 and 4), as was the comigration of the sublinear fragments with topoisomerase cleavage fragments (compare lanes 1, 2, and 4).
In vivo, the single-stranded portions of blocked replication forks should be covered by the T4 single-strand DNA-binding protein gp32 (49). Therefore, if endonuclease VII cleaves blocked forks in vivo, it should be able to cleave in the presence of gp32. This prediction was confirmed by using the purified blocked forks as substrate; gp32 even showed an apparent slight stimulation of cleavage by endonuclease VII (Fig. 4C, lane 5).
Discussion
The results presented here demonstrate that topoisomerase-blocked replication forks can be cleaved by a recombination nuclease. In vitro, purified endonuclease VII from phage T4 cleaved the Y-branched DNA resulting from in vivo fork arrest, sometimes cutting off one arm and sometimes cleaving the Y form into three pieces. One might argue that purification of the Y-branched DNA had removed some critical protein or proteins blocking access to endonuclease VII and thereby protecting the fork in vivo. However, arrested forks accumulated at higher levels in two different endonuclease VII-deficient infections, strongly suggesting that this pathway of fork cleavage also occurs in vivo.
One could also argue that the increased accumulation of blocked forks is an indirect result of the gene 49 mutations, but we have thought of no model for such an indirect effect. In particular, the increased yield of blocked forks does not appear to be the result of altered replication pathways (e.g., θ versus rolling circle) in the 49-mutant infections. A priori, one might expect that endonuclease VII is involved in the conversion of plasmid θ forms to rolling circles. However, neither the yield of replicated plasmid nor the amount of Y-form DNA is significantly affected by the gene 49 mutation, in the absence of the antitumor drug (data not shown). Also, the total yield of replicated plasmid DNA in the presence of m-AMSA is not significantly affected by the gene 49 mutation (see Supporting Text and Fig. 5).
While this work was in progress, Gruber et al. (50) also found that endonuclease VII could cleave replication fork DNA in vitro, by using forks that had been blocked at the replication fork barrier in S. cerevisiae. In that case, the authors were using endonuclease VII as a diagnostic reagent rather than exploring the biology of fork cleavage.
The results of this study provide evidence supporting the hypothesis that nuclease cleavage of topoisomerase-blocked forks is important for the chemotherapeutic potential of topoisomerase inhibitors. Indeed, because DNA replication is required for most of the cytotoxicity in mammalian cells (8–11), enzymatic cleavage of arrested forks could be the major cause of cytotoxicity. Mutational inactivation of recombination proteins causes hypersensitivity in mammalian cells (13, 14), suggesting that enzymatically cleaved forks can sometimes be repaired by DSB repair pathways (see below), but that cytotoxicity results when this process fails. There is also evidence that topoisomerase inhibitors can lead to genome instability, which is presumably important in the secondary tumors that result after chemotherapy (16–19). Incorrect recombinational repair after enzymatic cleavage of the arrested fork can now be considered as a possible pathway for this genome instability. It seems likely that direct restart pathways frequently restart topoisomerase-blocked forks without DNA breakage, and that the potentially cytotoxic lesion is generated only when direct restart fails. The experiments reported here do not address this important issue.
Is a bacteriophage model system relevant for understanding the cytotoxicity of antitumor agents? In a strict sense, the word cytotoxicity is clearly inappropriate for a phage infection, where the host bacterial cell is in the process of dying from the parasite infection. Nonetheless, induction of the cleavage complex by antitumor drugs has a detrimental effect on phage T4 DNA replication and overall yield (12), just as it is important in cytotoxicity in mammalian cells. Furthermore, inactivation of functionally conserved recombination proteins in T4 and in eukaryotic cells leads to hypersensitivity, and homologous recombination is stimulated by induction of the cleavage complex in both systems (12–15, 18, 51). The molecular aspects of cleavage-complex formation and drug resistance are likewise conserved (26, 27, 52). We therefore propose that our results might presage a similar pathway of cytotoxicity in mammalian cells. Undoubtedly, the mammalian system is more complex, with cell-cycle checkpoints and induction of apoptosis playing important roles in cytotoxicity in some cells (53).
Whereas cleavage of the arrested replication fork may create DNA lesions that inhibit phage T4 growth and replication, the cleaved forks can apparently be rescued by recombinational repair at some frequency. As alluded to above, mutations in T4 recombination genes cause hypersensitivity to m-AMSA, and recombination is stimulated by drug-induced cleavage complexes (12, 51). Furthermore, continued DNA replication in the presence of topoisomerase inhibitor depends on phage-encoded recombination proteins (25). Recombinational repair in phage T4 is closely related to a very active system of recombination-dependent replication (RDR). T4 RDR allows the phage to restart replication forks that have reached a genome end, and also allows DSB repair by a replication-coupled pathway (for reviews, see refs. 20 and 54).
Additional experiments using a plasmid model system support the view that T4 RDR provides a pathway for fork restart after breakage of topoisomerase-blocked replication forks. The presence of a single, strong, m-AMSA-inducible topoisomerase cleavage site increased recovery of plasmid repair products that had been replicated by the phage (51). These results argued that drug-induced cleavage complexes created some kind of DNA lesion that could be repaired by RDR. The nature of the lesion was suggested to be a DSB because the gene products required for repair matched those required for repair of endonuclease-generated DSBs. Notably, a mutation in gene 49 reduced, but did not abolish, generation of plasmid repair products (51). Cleavage by endonuclease VII is apparently not strictly required for generating the recombinogenic lesion and some additional cleavage pathway may exist.
In a general sense, we view the arrested replication forks studied here as a site-specific model system for other arrested forks. Arrested replication forks are now thought to be a critical impediment to completing cellular DNA replication, and pathways for restarting forks are thereby important in completing DNA replication and in maintaining genome stability (for recent review, see ref. 28). Recent evidence suggests that enzymatic cleavage of arrested forks could be particularly important when cells are stressed by faulty replication, nucleotide deprivation, or DNA damage (30–33).
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health Grant CA60836.
Abbreviations
- DSB
double-strand break
- m-AMSA
4′-(9-acridinylamino)methanesulfon-m-anisidide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Reece R J, Maxwell A. CRC Crit Rev Biochem Mol Biol. 1991;26:335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- 2.Chen A Y, Liu L F. Annu Rev Pharmacol Toxicol. 1994;34:191–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- 3.Froelich-Ammon S J, Osheroff N. J Biol Chem. 1995;270:21429–21432. doi: 10.1074/jbc.270.37.21429. [DOI] [PubMed] [Google Scholar]
- 4.Pommier Y, Pourquier P, Fan Y, Strumberg D. Biochim Biophys Acta. 1998;1400:83–106. doi: 10.1016/s0167-4781(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 5.Liu L F. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- 6.Drlica K, Zhao X L. Microbiol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsiang Y-H, Liu L F. J Biol Chem. 1989;264:9713–9715. [PubMed] [Google Scholar]
- 8.D'Arpa P, Beardmore C, Liu L F. Cancer Res. 1990;50:6919–6924. [PubMed] [Google Scholar]
- 9.Nitiss J L, Wang J C. Mol Pharmacol. 1996;50:1095–1102. [PubMed] [Google Scholar]
- 10.Wilson W R, Whitmore G F. Radiat Res. 1981;87:121–136. [PubMed] [Google Scholar]
- 11.Holm C, Covey J M, Kerrigan D, Pommier Y. Cancer Res. 1989;49:6365–6368. [PubMed] [Google Scholar]
- 12.Neece S H, Carles-Kinch K, Tomso D J, Kreuzer K N. Mol Microbiol. 1996;20:1145–1154. doi: 10.1111/j.1365-2958.1996.tb02635.x. [DOI] [PubMed] [Google Scholar]
- 13.Jeggo P A, Caldecott K, Pidsley S, Banks G R. Cancer Res. 1989;49:7057–7063. [PubMed] [Google Scholar]
- 14.Caldecott K, Banks G, Jeggo P. Cancer Res. 1990;50:5778–5783. [PubMed] [Google Scholar]
- 15.Nitiss J, Wang J C. Proc Natl Acad Sci USA. 1988;85:7501–7505. doi: 10.1073/pnas.85.20.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson R D, Berger N A. Mutat Res. 1994;309:109–142. doi: 10.1016/0027-5107(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson L R, Baguley B C. Environ Mol Mutagen. 1994;24:245–261. doi: 10.1002/em.2850240402. [DOI] [PubMed] [Google Scholar]
- 18.Pommier Y, Kerrigan D, Covey J M, Kao-Shan C-S, Whang-Peng J. Cancer Res. 1988;48:512–516. [PubMed] [Google Scholar]
- 19.Felix C A. Biochim Biophys Acta. 1998;1400:233–255. doi: 10.1016/s0167-4781(98)00139-0. [DOI] [PubMed] [Google Scholar]
- 20.Mosig G. Annu Rev Genet. 1998;32:379–413. doi: 10.1146/annurev.genet.32.1.379. [DOI] [PubMed] [Google Scholar]
- 21.Hsiang Y-H, Lihou M G, Liu L F. Cancer Res. 1989;49:5077–5082. [PubMed] [Google Scholar]
- 22.Howard M T, Neece S H, Matson S W, Kreuzer K N. Proc Natl Acad Sci USA. 1994;91:12031–12035. doi: 10.1073/pnas.91.25.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shea M E, Hiasa H. J Biol Chem. 1999;274:22747–22754. doi: 10.1074/jbc.274.32.22747. [DOI] [PubMed] [Google Scholar]
- 24.Hiasa H, Yousef D O, Marians K J. J Biol Chem. 1996;271:26424–26429. doi: 10.1074/jbc.271.42.26424. [DOI] [PubMed] [Google Scholar]
- 25.Hong G, Kreuzer K N. Mol Cell Biol. 2000;20:594–603. doi: 10.1128/mcb.20.2.594-603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreuzer K N. In: DNA Topoisomerases: Topoisomerase-Targeting Drugs. Liu L F, editor. San Diego: Academic; 1994. pp. 171–186. [Google Scholar]
- 27.Kreuzer K N. Biochim Biophys Acta. 1998;1400:339–347. doi: 10.1016/s0167-4781(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 28.Klein H L, Kreuzer K N. Mol Cell. 2002;9:471–480. doi: 10.1016/s1097-2765(02)00493-8. [DOI] [PubMed] [Google Scholar]
- 29.Gregg A V, McGlynn P, Jaktaji R P, Lloyd R G. Mol Cell. 2002;9:241–251. doi: 10.1016/s1097-2765(02)00455-0. [DOI] [PubMed] [Google Scholar]
- 30.Seigneur M, Bidnenko V, Ehrlich S D, Michel B. Cell. 1998;95:419–430. doi: 10.1016/s0092-8674(00)81772-9. [DOI] [PubMed] [Google Scholar]
- 31.Cha R S, Kleckner N. Science. 2002;297:602–606. doi: 10.1126/science.1071398. [DOI] [PubMed] [Google Scholar]
- 32.Tercero J A, Diffley J F. Nature. 2001;412:553–557. doi: 10.1038/35087607. [DOI] [PubMed] [Google Scholar]
- 33.Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M, Newlon C S, Foiani M. Nature. 2001;412:557–561. doi: 10.1038/35087613. [DOI] [PubMed] [Google Scholar]
- 34.Kaliraman V, Mullen J R, Fricke W M, Bastin-Shanower S A, Brill S J. Genes Dev. 2001;15:2730–2740. doi: 10.1101/gad.932201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuempel P L, Pelletier A J, Hill T M. Cell. 1989;59:581–583. doi: 10.1016/0092-8674(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi T, Horiuchi T. Genes Cells. 1996;1:465–474. doi: 10.1046/j.1365-2443.1996.d01-256.x. [DOI] [PubMed] [Google Scholar]
- 37.Horiuchi T, Fujimura Y, Nishitani H, Kobayashi T, Hidaka M. J Bacteriol. 1994;176:4656–4663. doi: 10.1128/jb.176.15.4656-4663.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johzuka K, Horiuchi T. Genes Cells. 2002;7:99–113. doi: 10.1046/j.1356-9597.2001.00508.x. [DOI] [PubMed] [Google Scholar]
- 39.Ivessa A S, Zhou J Q, Zakian V A. Cell. 2000;100:479–489. doi: 10.1016/s0092-8674(00)80683-2. [DOI] [PubMed] [Google Scholar]
- 40.Selick H E, Kreuzer K N, Alberts B M. J Biol Chem. 1988;263:11336–11347. [PubMed] [Google Scholar]
- 41.Kreuzer H W E, Kreuzer K N. Genetics. 1994;138:983–992. doi: 10.1093/genetics/138.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreuzer K N, Engman H W, Yap W Y. J Biol Chem. 1988;263:11348–11357. [PubMed] [Google Scholar]
- 43.Friedman K L, Brewer B J. Methods Enzymol. 1995;262:613–627. doi: 10.1016/0076-6879(95)62048-6. [DOI] [PubMed] [Google Scholar]
- 44.Mizuuchi K, Kemper B, Hays J, Weisberg R A. Cell. 1982;29:357–365. doi: 10.1016/0092-8674(82)90152-0. [DOI] [PubMed] [Google Scholar]
- 45.Flemming M, Deumling B, Kemper B. Virology. 1993;196:910–913. doi: 10.1006/viro.1993.1557. [DOI] [PubMed] [Google Scholar]
- 46.Jensch F, Kemper B. EMBO J. 1986;5:181–189. doi: 10.1002/j.1460-2075.1986.tb04194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pottmeyer S, Kemper B. J Mol Biol. 1992;223:607–615. doi: 10.1016/0022-2836(92)90977-r. [DOI] [PubMed] [Google Scholar]
- 48.Barth K A, Powell D, Trupin M, Mosig G. Genetics. 1988;120:329–343. doi: 10.1093/genetics/120.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nossal N G. In: Molecular Biology of Bacteriophage T4. Karam J D, editor. Washington, DC: Am. Soc. Microbiol.; 1994. pp. 43–53. [Google Scholar]
- 50.Gruber M, Wellinger R E, Sogo J M. Mol Cell Biol. 2000;20:5777–5787. doi: 10.1128/mcb.20.15.5777-5787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stohr B A, Kreuzer K N. Genetics. 2001;158:19–28. doi: 10.1093/genetics/158.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freudenreich C H, Chang C, Kreuzer K N. Cancer Res. 1998;58:1260–1267. [PubMed] [Google Scholar]
- 53.Kaufmann S H. Biochim Biophys Acta. 1998;1400:195–211. doi: 10.1016/s0167-4781(98)00136-5. [DOI] [PubMed] [Google Scholar]
- 54.Kreuzer K N. Trends Biochem Sci. 2000;25:165–173. doi: 10.1016/s0968-0004(00)01559-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.