Abstract
Group B Streptococcus is the most common cause of bacterial infection in the newborn. Infection in many cases causes persistent pulmonary hypertension, which impairs gas exchange in the lung. We purified the bacterial components causing pulmonary hypertension and identified them as cardiolipin and phosphatidylglycerol. Synthetic cardiolipin or phosphatidylglycerol also induced pulmonary hypertension in lambs. The recognition that bacterial phospholipids may cause pulmonary hypertension in newborns with Group B streptococcal infection opens new avenues for therapeutic intervention.
Group B Streptococcus (GBS) is the most frequent cause of sepsis and meningitis in the human newborn (1). Despite prompt treatment with antibiotics, neonates with GBS infection are often quite ill and the fatality rate is 5% (2). Respiratory distress, a prominent sign in these babies, is caused by pulmonary hypertension induced by the GBS infection (3). The pulmonary hypertension reflects an increase in pulmonary vascular resistance, which impairs exchange of oxygen and carbon dioxide. Infusion of live or heat-killed GBS into sheep promptly induces pulmonary hypertension, with little or no effect on systemic pressure (4). This response has been reproduced by many investigators, who also established that the pulmonary hypertension is not a consequence of simple embolization because infusion of latex beads of the same size as GBS caused no change in pulmonary physiology (5). The hypertension is mediated by an increase in thromboxane A2, so that treatment with cyclooxygenase inhibitors such as indomethacin or with thromboxane synthesis inhibitors prevents or reverses the increase in pulmonary pressure (5, 6). However, the component(s) of GBS that activate thromboxane synthesis are unknown. Guided by bioassays performed in neonatal lambs (7), we undertook the purification and identification of these components, using standard biochemical techniques.
Materials and Methods
Purification and Analysis of Pulmonary Hypertensive Compounds from GBS.
Biochemical fractionation procedures were linked in series to develop an initial purification protocol, which was modified to give the final protocol described here. At each step, fractions were assayed for pulmonary arterial hypertensive activity in 3- to 12-day-old lambs of ≈5 kg, as described (7). We previously demonstrated that pulmonary arterial pressure measurements were a valid surrogate for measurement of pulmonary vascular resistance in this animal model (7). Fractions were infused peripherally into the femoral vein, not directly into the pulmonary artery. Fractions were considered active if they increased pulmonary arterial pressure by at least 50% in at least two lambs tested on different days.
GBS type III, strain 878 was grown to mid- or late-log phase (7), washed with sterile normal saline, heat-killed by incubation in a 60°C water bath for 90 min, pelleted by centrifugation, and resuspended in saline to an OD of 2.0 at 650 nm. Heat-killed GBS (3-gm wet weight) were extracted with 40 ml of 2:1 chloroform/methanol by stirring in a glass-stopped flask at 4°C for 5–6 days. The organic solvent was removed by vacuum centrifugation (Thermo Savant, Holbrook, NY) and the resulting pellet was redissolved in methanol. The solution was applied to an anion exchange column (TosoHaas DEAE-5PW, Tosoh Bioscience, Montgomeryville, PA) in an Agilent model 1100 HPLC (Agilent Technologies, Palo Alto, CA) with methanol running at 0.5 ml/min. Then the solvent was changed to 45:45:10 chloroform/methanol/water. The chloroform/methanol was held constant and a linear sodium chloride gradient to 150 mM was developed over 4 min. The active material was eluted by the salt gradient, dried in the vacuum centrifuge, and redissolved in 10% methanol. This solution was applied to a C18 reverse-phase column (Vydac 218TP5205, Hesperia, CA) run at 0.2 ml/min. A gradient to 100% methanol was run over 9 min, and then another gradient to 100% chloroform over 10 min. The contents of the eluted fractions were monitored by negative ion mode electrospray MS (8) (Agilent model 1946). The structure of the active compounds was established by using both positive and negative ion mode tandem MS (LCQ Classic and TSQ 700, Finnigan-MAT, San Jose, CA).
Synthetic Lipids.
The dioleoyl forms of diacylglycerol, phosphatidic acid, phosphatidylglycerol, and cardiolipin were obtained from Avanti Polar Lipids. Their purity was confirmed by RP-HPLC and their identity by MS. Multilamellar liposomes were formed by drying from chloroform and rehydration with GBS buffer with vigorous vortexing. Unilamellar vesicles of defined size were prepared with the Avanti miniextruder. Mass spectrometric analysis established that extrusion did not change the phospholipid content.
Purification of Apolipoprotein H and Thromboxane Assay.
We purified the protein from outdated human plasma (National Institutes of Health Blood Bank) by using a heparin column (9) (TosoHaas 5PW, Tosoh Biosep, Tokyo). It was quantitated from the area of the peak on the HPLC (10), using a molar absorbtivity of 47,000. Thromboxane A2 was analyzed by ELISA as thromboxane B2, its stable degradation product (Amersham Pharmacia).
Results
Purification and Identification of the Active Components.
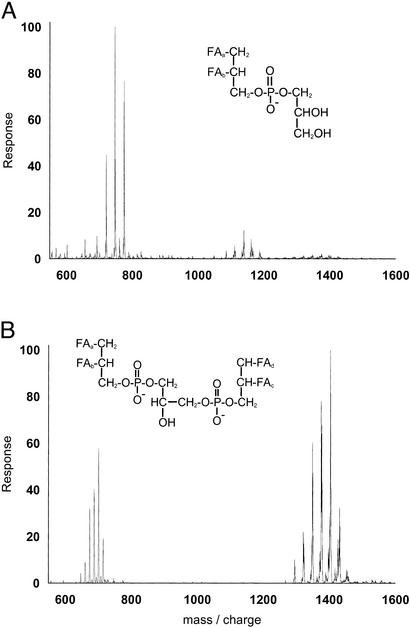
Guided by bioassays performed in neonatal lambs, we undertook the purification of the GBS components which induce pulmonary hypertension. Biologically active material was extracted from GBS by a mixture of chloroform and methanol, and two components were purified by anion exchange. The mass spectrum of the two active fractions established that they were phosphatidylglycerol and cardiolipin (Fig. 1 A and B). Each caused pulmonary hypertension in the lamb assay.
Figure 1.
Mass spectra of the bioactive material purified from GBS. (A) Earlier eluting fraction from the Vydac column. The peaks from 700–800 are singly-charged phosphatidylglycerol, free of cardiolipin. The structure is phosphatidylglycerol with fatty acids represented as FA. (B) Later eluting fraction from the Vydac column. The peaks are singly and doubly charged cardiolipin, free of phosphatidylglycerol. The structure is cardiolipin with fatty acids represented as FA. Both fractions were bioactive in the lamb assay.
The structure of the largest peak in the cardiolipin mass spectrum was established to be dipalmitoyl, dioleoyl cardiolipin by fragmentation in the mass spectrometer. The mass expected from the structure of this cardiolipin is 1,405 Da, equal to the experimentally measured value. The largest peak in the phosphatidylglycerol spectrum is palmitoyl, oleoyl phosphatidylglycerol, with the calculated and observed masses being 748.5 Da. The other peaks in the mass spectra are from phospholipids with fatty acids of different chain lengths. The observed distribution of fatty acids matches that expected for GBS (11).
Synthetic Phospolipids Are Biologically Active.
To assure that these phospholipids were the bioactive material rather than a minor component of the preparation such as peptidoglycan, we tested pure, synthetic compounds by using the dioleoyl species. Both phosphatidylglycerol and cardiolipin triggered an increase in pulmonary arterial pressure within minutes of infusion into the femoral vein of lambs, but diacylglyerol and phosphatiditic acid were not active (Table 1).
Table 1.
Effect of dioleolyl glycerol compounds on pulmonary arterial pressure
| Compound | Change in pressure, mm |
|---|---|
| Dioleoyl glycerol | 0 |
| Dioleoyl phosphatidic acid | 0 |
| Dioleoyl phosphatidylglycerol | +14 |
| Dioleoyl cardiolipin | +21 |
Values are the average from two 5-kg lambs given 35 nmol/kg of each compound.
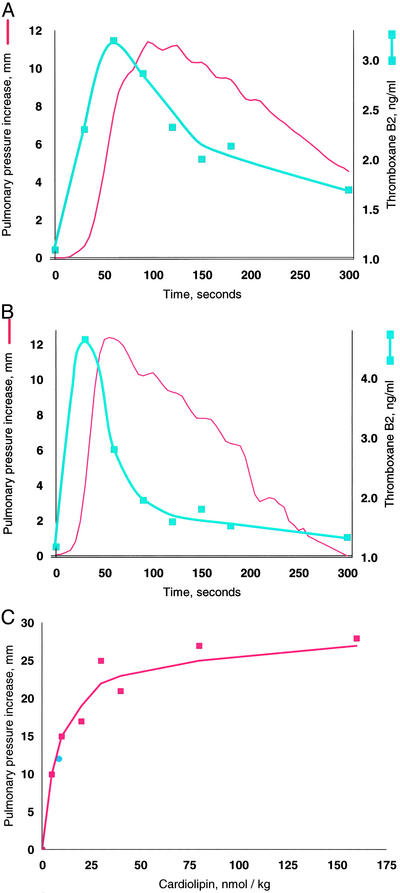
Although both phosphatidylglycerol and cardiolipin were bioactive, the increase in pulmonary arterial blood pressure increase was more reproducible with cardiolipin, perhaps because of the ease of rehydrating the dried preparations to form liposomes. We therefore used cardiolipin for subsequent studies. As mentioned, GBS infusion is known to induce pulmonary hypertension by stimulating the production of thromboxane A2, and the increase in pulmonary pressure closely tracks that of the plasma thromboxane concentrations (Fig. 2A). We established that synthetic cardiolipin has the same activity (Fig. 2B). A dose-response curve shows half-maximal response at ≈10 nmol/kg (Fig. 2C).
Figure 2.
Thromboxane production and pulmonary hypertension induced by GBS and cardiolipin. Pulmonary pressure is plotted in red by using the left axis and thromboxane on the right. (A) GBS, 3 × 109 heat-killed cells. (B) Cardiolipin, 75 nmol. The same quantitative responses in pulmonary pressure and thromboxane were observed when we infused 75 nmol of cardiolipin preincubated with 75 nmol of purified human apolipoprotein H (12). (C) Cardiolipin dose–response. Doses were injected in a randomized order into a 5-kg lamb, and the line was fit by linear regression of a double reciprocal plot. The blue circle was the response in a different lamb to cardiolipin purified from GBS. Cardiolipin liposomes prepared by simple rehydration are multilamellar, but because a liposome size effect has been observed by some investigators (29), we also prepared unilamellar liposomes with diameters ranging from 0.1 to 5.0 microns; all were active.
Effect of Apolipoprotein H.
Apolipoprotein H is the major phospholipid binding protein in plasma, with particular affinity for cardiolipin (12). The concentration of apolipoprotein H in adult serum is 170 mg/liter (13), and we found similar concentrations in the neonatal lamb and piglet as well as FCS. Infusion of apolipoprotein H should bind circulating bacterial phosphatidylglycerol or cardiolipin, but the rate of binding may not be fast enough to prevent unliganded phospholipid from reaching the pulmonary circulation (14). Binding could inhibit the hypertensive effect, although it might actually promote the hypertensive effect because apolipoprotein H binds to endothelial cells (15). We therefore tested the effect of cardiolipin, which was mixed with a equimolar concentration of purified apolipoprotein H and allowed to stand overnight before testing, assuring that the binding reaction had gone to completion (14). The peak hypertensive effect and increase in plasma thromboxane were equivalent to that of the cardiolipin infused without apolipoprotein H (as in Fig. 2B). Thus, circulating apoplipoprotein H appears sufficient to rapidly bind all of the infused cardiolipin, and the bound form is bioactive. We conclude that apolipoprotein H is unlikely to be useful in treating GBS-induced pulmonary hypertension.
Inhibition of Cyclooxygenase Prevents Pulmonary Hypertension.
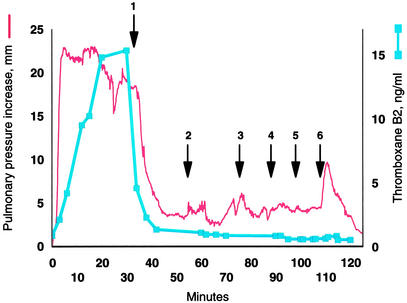
Cyclooxygenase inhibitors such as indomethacin consistently block thromboxane synthesis and the pulmonary hypertensive effect of infused GBS in animals (6, 16–19). If the bacterial phospholipid is the bioactive molecule of GBS, then indomethacin should also prevent the pulmonary hypertensive response to cardiolipin. Administration of a single dose of 5 mg/kg indomethacin prevented the increase in pulmonary arterial blood pressure caused by either GBS or cardiolipin. We then determined whether indomethacin could reverse persistent pulmonary hypertension caused by cardiolipin. A continuous infusion of cardiolipin clamped the pulmonary arterial blood pressure at an increased level (Fig. 3). Treatment with indomethacin lowered the pressure, bringing it close to the control basal level even though the cardiolipin infusion was continued. Increasing the infusion rate or even administering a bolus of >100 nmol/kg cardiolipin did not increase the pulmonary pressure.
Figure 3.
Cyclooxygenase inhibition blocks the effect of a continuous infusion of cardiolipin. The red line plots the increase in mean pulmonary arterial pressure, while the blue squares and line show the plasma thromboxane B2 concentration. Beginning at time 0, 13 nmol/kg/min cardiolipin was infused through the femoral vein. A 5 mg/kg dose of indomethacin was then administered over 10 min (arrow 1). Next, the cardiolipin was increased to 27 nmol/kg per min, and then to 40 nmol/kg per min (arrows 2 and 3). Finally boluses of 67 mg/kg, 133 mg/kg, and 667 mg/kg were infused (arrows 4, 5, and 6). Pulmonary arterial pressure at time 0 was 22 mm.
Discussion
Phosphatidylglycerol and cardiolipin are the dominant phospholipids of GBS (20, 21), located mainly in the cell wall, although their topography has not been established. Because infusion of intact, heat-killed GBS cells induces the pulmonary hypertensive effect, some of the molecules are presumably surface exposed and available for binding to pulmonary endothelial cell receptors. In addition many bacteria, especially the Streptococci, normally secrete some of the phospholipids, which they synthesize. In log phase this is 15% of the total synthesized, increasing to 30% during stationary phase (22). Thus, patients infected with GBS (23) may receive an endogenous infusion of the phospholipids capable of inducing pulmonary hypertension.
Because GBS is sensitive to the penicillin class of antibiotics, neonates suspected to have sepsis usually receive such antibiotics. However exposure of Streptococci to penicillin induces an immediate secretion of phospholipids (22, 24, 25). Then over the next several hours penicillin causes an increase in lipid synthesis, all of which is excreted (26). This might also occur with other Gram-positive and negative infections in patients of any age because excretion of phospholipids is a common response of bacteria to certain antibiotics (22–25). Lipid excretion by Streptococci may increase 15-fold with more than one-half of the material excreted being phosphatidylglycerol and cardiolipin (25). Thus, antibiotic treatment effects an increase in production and excretion of the molecules which we showed cause pulmonary hypertension.
In bacteria, both phosphatidylglycerol and cardiolipin synthesis require phosphatidylglycerol synthase. Mutants lacking this enzyme will be useful in identifying any other GBS components capable of inducing pulmonary hypertension (27). One such known molecule is CM101, an extracellular polysaccharide produced by GBS, which is capable of inducing pulmonary hypertension. However, it was present only in the culture media and not in the GBS cells (28).
Indomethacin is available for i.v. administration in the newborn to induce closure of a patent ductus arteriosus. It has not yet been studied as a treatment for pulmonary hypertension, but given that it prevents the pulmonary hypertensive effect of cardiolipin, it seems appropriate to test its efficacy in neonatal pulmonary hypertension.
The key finding in our study is that bacterial phospholipids cause pulmonary hypertension. Identification of the receptors and pathways that are activated by these phospholipids may allow discovery of therapeutic interventions.
Acknowledgments
We thank Dr. Henry Fales for obtaining mass spectra. J.C. thanks Dr. Russell Moores, Jr., for guidance in the laboratory. J.C. was supported in part by grants from the National Naval Medical Center and by the Chief, Navy Bureau of Medicine and Surgery, Washington, DC, Clinical Investigation Program (no. B00–022). The research reported herein was conducted according to the principles set forth in the Guide for Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, Health and Human Services publication no. (NIH) 85-23, revised 1985.
Abbreviations
- GBS
group B streptococcus
- GBS buffer
50 mM sodium phosphate, 150 mM sodium chloride, pH 7.4
References
- 1.Schuchat A. Lancet. 1999;353:51–56. doi: 10.1016/S0140-6736(98)07128-1. [DOI] [PubMed] [Google Scholar]
- 2.Schrag S J, Zywicki S, Farley M M, Reingold A L, Harrison L H, Lefkowitz L B, Hadler J L, Danila R, Cieslak P R, Schuchat A. N Engl J Med. 2000;342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 3.Rojas J, Stahlman M. Clin Perinatol. 1984;11:591–599. [PubMed] [Google Scholar]
- 4.Rojas J, Green R S, Hellerqvist C G, Olegard R, Brigham K L, Stahlman M T. Pediatr Res. 1981;15:899–904. doi: 10.1203/00006450-198106000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Gibson R L, Redding G J, Truog W E, Henderson W R, Rubens C E. Pediatr Res. 1989;26:241–245. doi: 10.1203/00006450-198909000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Philips J B, III, Lyrene R K, Godoy G, Graybar G, Barefield E, Sams J E, Gray B M. Pediatr Res. 1988;23:81–85. doi: 10.1203/00006450-198801000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter D, Larkin H, Chang A, Morris E, O'Neill J, Curtis J. Pediatr Res. 2001;49:181–188. doi: 10.1203/00006450-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Han X, Gross R W. Proc Natl Acad Sci USA. 1994;91:10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wurm H. Int J Biochem. 1984;16:511–515. doi: 10.1016/0020-711x(84)90168-x. [DOI] [PubMed] [Google Scholar]
- 10.Levine R L, Williams J A, Stadtman E R, Shacter E. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 11.Fischer W. Biochim Biophys Acta. 1977;487:89–104. doi: 10.1016/0005-2760(77)90046-7. [DOI] [PubMed] [Google Scholar]
- 12.Steinkasserer A, Barlow P N, Willis A C, Kertesz Z, Campbell I D, Sim R B, Norman D G. FEBS Lett. 1992;313:193–197. doi: 10.1016/0014-5793(92)81442-o. [DOI] [PubMed] [Google Scholar]
- 13.Hoeg J M, Segal P, Gregg R E, Chang Y S, Lindgren F T, Adamson G L, Frank M, Brickman C, Brewer H B., Jr Atherosclerosis. 1985;55:25–34. doi: 10.1016/0021-9150(85)90163-7. [DOI] [PubMed] [Google Scholar]
- 14.Schousboe I. Thromb Res. 1980;19:225–237. doi: 10.1016/0049-3848(80)90421-1. [DOI] [PubMed] [Google Scholar]
- 15.Ma K, Simantov R, Zhang J C, Silverstein R, Hajjar K A, McCrae K R. J Biol Chem. 2000;275:15541–15548. doi: 10.1074/jbc.275.20.15541. [DOI] [PubMed] [Google Scholar]
- 16.Rojas J, Larsson L E, Ogletree M L, Brigham K L, Stahlman M T. Pediatr Res. 1983;17:107–110. doi: 10.1203/00006450-198302000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Gibson R L, Truog W E, Henderson W R, Jr, Redding G J. Pediatr Res. 1992;31:222–227. doi: 10.1203/00006450-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Hemming V G, O'Brien W F, Fischer G W, Golden S M, Noble S F. Pediatr Res. 1984;18:266–269. doi: 10.1203/00006450-198403000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Huddleston K W, Lyrene R K, Dew A, Gray B M, Philips III J B. Dev Pharmacol Ther. 1986;9:260–265. doi: 10.1159/000457101. [DOI] [PubMed] [Google Scholar]
- 20.Fischer W. Biochim Biophys Acta. 1977;487:74–88. doi: 10.1016/0005-2760(77)90045-5. [DOI] [PubMed] [Google Scholar]
- 21.Filgueiras M H, Op den Kamp J A. Biochim Biophys Acta. 1980;620:332–337. doi: 10.1016/0005-2760(80)90215-5. [DOI] [PubMed] [Google Scholar]
- 22.Cabacungan E, Pieringer R A. Infect Immun. 1980;27:556–562. doi: 10.1128/iai.27.2.556-562.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowfoot P D, Esfahani M, Wakil S J. J Bacteriol. 1972;112:1408–1415. doi: 10.1128/jb.112.3.1408-1415.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horne D, Hakenbeck R, Tomasz A. J Bacteriol. 1977;132:704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brissette J L, Shockman G D, Pieringer R A. J Bacteriol. 1982;151:838–844. doi: 10.1128/jb.151.2.838-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brissette J L, Pieringer R A. Lipids. 1985;20:173–179. doi: 10.1007/BF02534250. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi S, Shibuya I, Matsumoto K. J Bacteriol. 2000;182:371–376. doi: 10.1128/jb.182.2.371-376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellerqvist C G, Rojas J, Green R S, Sell S, Sundell H, Stahlman M T. Pediatr Res. 1981;15:892–898. doi: 10.1203/00006450-198106000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Szebeni J, Baranyi L, Savay S, Bodo M, Morse D S, Basta M, Stahl G L, Bunger R, Alving C R. Am J Physiol. 2000;279:H1319–H1328. doi: 10.1152/ajpheart.2000.279.3.H1319. [DOI] [PubMed] [Google Scholar]