Abstract
RNA interference is an effective method to silence specific gene expression. Its application to mammalian cells, however, has been hampered by various shortcomings. Recently, it was reported that introduction of 22-bp double-stranded RNAs (dsRNAs) would specifically suppress expression of endogenous and heterogeneous genes in various mammalian cell lines. However, using this method, we failed to knock out proteins of interest effectively. Here we report the development of a stable and controllable method for generating dsRNA intracellularly. Tetracycline-responsive transactivator-containing cells were transfected with a vector capable of tetracycline-induced bidirectionally overexpressing sense and antisense RNA to form dsRNA in vivo. With this method, glutaredoxin, monitored by Western blot, was knocked out by overexpressing 290-base sense and antisense RNA in NIH 3T3 cells controlled by tetracycline or doxycycline. By using these glutaredoxin knocked-out cells, we have demonstrated that actin deglutathionylation plays a key role in growth factor-mediated actin polymerization, translocalization, and reorganization near the cell periphery.
When double-stranded RNA (dsRNA) is introduced into animal or plant cells, mRNA corresponding to that dsRNA is cleaved. This posttranscriptional gene silencing is referred to as RNA interference (RNAi; refs. 1–3). The phenomenon was documented >10 years ago and has aroused great interest in recent years not only because new insights into its mechanism have started to unfold, but also because it has great value in applications in cellular studies such as protein knockout. The process is believed to occur in cytosol and requires ATP for completion (4). The dsRNA is first cleaved into ≈22 nucleotide fragments (4, 5), which then bind to RNA-induced silencing complex (RISC) and are probably unwound (6, 7). Annealing of the antisense strands to mRNA is then facilitated, and the mRNA molecules are subsequently cleaved. Evidence suggests that an RNA polymerase uses these antisense strands as primers to synthesize longer dsRNA by using mRNA as a template (8–10). Such signal amplification explains why small quantities of dsRNA can effectively silence corresponding gene products in cells. In Drosophila, it has been shown that an RNase III homology Dicer is responsible for the cleavage of dsRNA (11). The downstream event is the formation of RISC in which elF2C1 and elF2C2 are also implicated as crucial players (6, 7).
Most successful RNAi-induced protein knockout studies have been carried out in the fruit fly Drosophila (12, 13) and nematode Caenorhabditis elegans (14, 15). In vertebrate cells, apoptosis and nonspecific knockout have been reported, although the reasons are unknown (16, 17). It was reported, however, that introduction of dsRNA as short as 22 bp can overcome the problem of nonspecificity in mammalian cells (18). Nevertheless, mouse cells, such as NIH 3T3 cells, have been used to achieve complete gene silence by using full-length dsRNA without adverse effects (19).
The effectiveness of protein knockout by RNAi depends on the efficiency of cellular uptake of dsRNA and the half-life of dsRNA inside cells as well as the half-life of the protein to be knocked out. Although introduction directly into cells of 22-bp dsRNA by transfection methods leads to specific gene silencing, this knockout is transient and usually is not sustained for the full period during the remaining gene product degradation inside the cells. Methods were therefore developed to transfect cells with vectors overexpressing dsRNA (22-mer) to sustain long-term gene silencing (20–22). The limit of 22-mer dsRNA interference is such that its efficacy depends on the selection of the most effective target site within the target mRNA to achieve complete gene silencing (23). The objective of this report is to establish an effective, specific, and controllable RNAi method by using full-length dsRNA generated intracellularly. This method was used to reveal the role of actin glutathionylation, regulated in part by glutaredoxin (GRx), in growth factor-induced actin polymerization, translocation, and reorganization near the cell periphery.
Materials and Methods
Materials.
Monoclonal antiglutathione Ab was obtained from ViroGen (Watertown, MA). Polyclonal anti-GRx Ab was obtained from American Diagnostica (Greenwich, CT), and polyclonal anti-actin Ab was obtained from Sigma. Doxycycline, mouse cDNA library, pBI, pYFP-actin (YFP-actin, yellow fluorescent protein actin), and pTet-On vectors were purchased from BD Biosciences Clontech. Fibroblast growth factors (FGFs) FGFa and FGFb were obtained from Sigma. Cell culture medium, DMEM, was purchased from GIBCO. FuGENE 6 and geneticin disulfide were purchased from Roche Applied Sciences and Mediatech (Herndon, VA), respectively.
Construction of Bidirectional dsRNA Producing Vector pBI-dsGRx.
Sense and antisense DNA sequences corresponding to bases 11–300 of mouse GRx cDNA were obtained from PCR by using the BD Biosciences Clontech mouse cDNA library as template and were cloned into pBI vector MCS I and II regions, respectively. Primers for sense DNA contained MluI/NheI restriction sites (5′-ggccacgcgtAgtttgtgaactgcaagatccagt-3′ and 5′-ccgggctagcCttcagccgagtcatcagctcccc-3′), and primers for antisense DNA contained NotI/SalI restriction sites (5′-gacgcggccgcCttcagccgagtcatcagctcccc-3′ and 5′-ggccgtcgacAgtttgtgaactgcaagatccagt-3′).
Establishing NIH 3T3 Cell Line Harboring Tetracycline-Controlled Reverse Transactivator.
NIH 3T3 cells grown in DMEM (2 ml in each well) with 10% (vol/vol) FBS to 50% confluency in a six-well plate were infected with pTet-On vector by using FuGENE 6 as a transfecting agent. Cells were selected with 400 μg/ml geneticin disulfide until stable resistant colonies formed.
GRx Knockout.
NIH 3T3 cells stably expressing tetracycline-controlled reverse transactivator were grown in DMEM with 10% FBS and 400 μg/ml geneticin disulfide to 50% confluency before transfection with pBI-dsGRx (1 μg in each well) by using FuGENE 6 (6 μl in each well) according to the FuGENE 6 protocol of Roche Applied Sciences. Doxycycline with a final concentration of 2 μg/ml was added to the culture ≈12 h after the transfection. Typically, during a 6-day knockout, the cell cultures were split twice, with one more transfection on day 2 with pBI-dsGRx to ensure a high transfection rate. Doxycycline concentration was maintained by replenishing culture medium at least once every 2 days. To determine the GRx level, cells in each well (≈1.0 × 106) were lysed in 100 μl of 2× SDS sample buffer at the designated times. After all of the samples were collected, they were subjected to SDS/PAGE (4–20% gel, 35 μl in each lane) and poly(vinylidene difluoride) membrane blotting. Western blot analysis was performed by using GRx Ab. Actin level was monitored by Western blot with anti-actin Ab.
Actin Deglutathionylation Assay.
NIH 3T3 cells with or without (cells transfected with pBI-dsGRx but not treated with doxycycline) GRx knockout were grown on six-well plates and starved in DMEM (without serum) for 3 h before adding FGFa and FGFb to achieve a final concentration of 25 ng/ml each. After incubation at 37°C for 15 min, the culture medium was removed and the cells were lysed with 2× SDS/PAGE sample buffer (100 μl in each well) without reducing agents. The cell lysates were separated on a 12% SDS/PAGE gel (30 μl in each lane) and blotted to a poly(vinylidene difluoride) membrane for Western blot analysis by using antiglutathione Ab.
Confocal Electromicroscopy.
In DMEM with 10% FBS, NIH 3T3 cells with or without the GRx knockout were grown to ≈30% confluency on 25-mm-diameter circular glass plates coated with gelatin, and they were transfected with both pBI-dsGRx and pYFP-actin vectors 12 h before doxycycline treatment for GRx knockout and no treatment for GRx nonknockout cells as described. After starvation in DMEM without serum for 3 h, FGFa and FGFb were added to the cells to a final concentration of 25 ng/ml each. Confocal images were taken with a Zeiss LSM 510 confocal microscope before FGF treatment and every 20 s after the treatment.
Results and Discussion
Construction of Bidirectional dsRNA Producing Vector pBI-dsGRx.
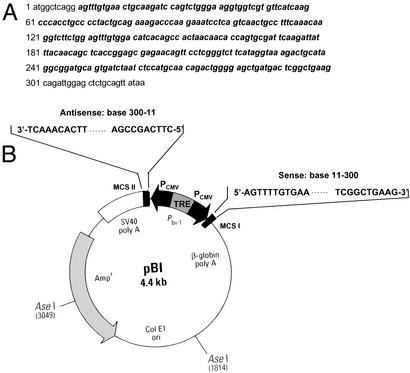
To develop a controllable protein knockout method in mammalian cells by using full- or nearly full-length dsRNA, we constructed a bidirectional overexpression vector (pBI-Tet-On) for sense and antisense DNA sequences corresponding to bases 11–300 of mouse GRx cDNA with strong cytomegalovirus promoters (Fig. 1). This vector was introduced into mouse fibroblast NIH 3T3 cells by using FuGENE 6 as a transfecting agent. When both strands of RNA (290 bases long in this case) are synthesized, they will anneal to form dsRNA in vivo. In concert with this notion, no significant decrease in the GRx level was observed 5 days after doxycycline treatment in a parallel experiment in which only antisense RNA was synthesized (data not shown). The vector also contains a tetracycline-responsive element so that expression of sense and antisense RNA starts only when tetracycline-responsive cells (those stably expressing tetracycline-controlled reverse transactivator) are subjected to tetracycline (or doxycycline) treatment. This vector allows us to knock out proteins at a desired stage of cellular processes. In addition to NIH 3T3 cells, this method has been successfully applied to human embryonic kidney 293 cells (J.W. and P.B.C., unpublished results).
Figure 1.
(A) cDNA of mouse GRx. Sequence in bold corresponds to that of the dsRNA used in RNAi. (B) Construction of pBI-dsGRx. Sense and antisense DNA sequences corresponding to bases 11–300 of mouse GRx DNA sequences were inserted into MCS I and II regions, respectively. Primers for sense DNA contained MluI/NheI restriction sites: 5′-ggccacgcgtAgtttgtgaactgcaagatccagt-3′ and 5′-ccgggctagcCttcagccgagtcatcagctcccc-3′. Primers for antisense DNA contained NotI/SalI restriction sites: 5′-gacgcggccgcCttcagccgagtcatcagctcccc-3′ and 5′-ggccgtcgacAgtttgtgaactgcaagatccagt-3′.
GRx Knockout.
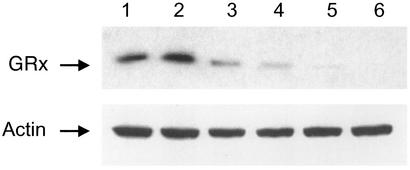
By using this controllable RNAi method, we successfully knocked out GRx in NIH 3T3 cells. Protein glutathionylation plays a major role in cell signaling and cellular response to oxidative stress (24–28). GRx specifically and efficiently catalyzes the deglutathionylation of proteins. Thus, it is an important enzyme in cellular redox regulation (29–31). The effect of long-term deletion of this enzyme is not well documented. However, we have shown that human epidermal A431 cells can sustain temporal deactivation of this enzyme (31). In our knockout experiments, the pBI-dsGRx vector was designed so that the sense and antisense RNA of GRx cover bases 11–300 of its mRNA. This vector would allow 290-bp dsRNA to be synthesized inside cells to ensure effective mRNA silencing (Fig. 1). After the vector was introduced into NIH 3T3 cells stably expressing tetracycline-controlled reverse transactivator by FuGENE 6, doxycycline was added to start dsRNA production. As shown in Fig. 2, a Western blot using anti-GRx Ab indicates that GRx starts to disappear in cells after doxycycline treatment. Although at 2 and 4 days after doxycycline treatment GRx was still present in cells (Fig. 2, lanes 3 and 4), after 6 days GRx was not detectable by Western blot (Fig. 2, lanes 5 and 6). These results are consistent with our estimate of 1.5 days for the degradation half-life of GRx. [Note: Actin was used to ensure equal loading (Fig. 2).] That an equal amount of actin is shown in all lanes that contain an equal quantity of total proteins indicates that the presence of this 290-bp dsRNA did not alter actin expression.
Figure 2.
Knockout of GRx. NIH 3T3 cells stably expressing tetracycline-responsive reverse transactivator were transfected with pBI vector (control) and pBI-dsGRx vector as described in Materials and Methods. Knockout started after 2 μg/ml doxycycline was introduced into the culture media. Cells were collected on the indicated days, and their GRx levels were determined by Western blot with anti-GRx Ab. Cells infected with empty vector pBI with doxycycline (lane 1) or with pBI-dsGRx in the absence of doxycycline (lane 2) showed no decrease in GRx levels in 5 days. Cells transfected with pBI-dsGRx and treated with doxycycline for 2 days (lane 3), 4 days (lane 4), 6 days (lane 5), and 8 days (lane 6). (Lower) Actin level.
GRx Catalyzes Actin Deglutathionylation.
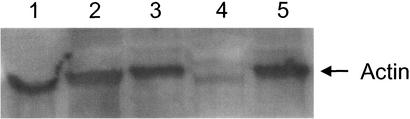
We and others have previously shown (31) that cellular globular actin (G-actin) is moderately glutathionylated at the C374 position under normal growth conditions. The glutathionylation level increases when cells are starved and decreases when they are fed with growth factors. Deglutathionylation at C374 facilitates G-actin polymerization to form filamentous actin (F-actin). Intracellularly, Takagi et al. (30) also previously revealed, both in batch and at the single cell level, that epidermal growth factor-induced actin polymerization correlates with the decrease in glutathionylation of the penultimate cysteine. GRx catalyzed the deglutathionylation of G-actin, and Cd2+, a nonspecific inhibitor of GRx in cells (29), inhibited the deglutathionylation of actin intracellularly. When GRx is knocked out by RNAi in NIH 3T3 cells, a similar phenomenon is observed (Fig. 3). After 3 h of serum deprivation in DMEM, G-actin remained glutathionylated in NIH 3T3 cells transfected either with pBI vector (Fig. 3, lane 1) or with pBI-dsGRx vector (Fig. 3, lanes 2 and 3) and then treated with doxycycline to knock out GRx (Fig. 3, lane 3). However, when FGF was introduced into the pBI-dsGRx-transfected cells after serum deprivation, actin was deglutathionylated in the GRx nonknockout cells (Fig. 3, lane 4) while remaining glutathionylated in the GRx knocked-out cells (Fig. 3, lane 5). The 42-kDa band depicted in Fig. 3 crossreacted with both antiglutathione Ab and anti-actin Ab (data not shown). These results clearly show that GRx is the enzyme catalyzing the deglutathionylation of actin and thus plays a vital role in regulating the state of actin glutathionylation.
Figure 3.
Actin glutathionylation in GRx nonknockout and knockout cells. NIH 3T3 cells with (lanes 3 and 5) or without (lanes 2 and 4) GRx knockout were starved for 3 h in DMEM without serum before treatment with FGF (lanes 4 and 5) to achieve a final concentration of 25 ng/ml each. Cells were lysed after 15 min, and actin glutathionylation levels were monitored by Western blot using antiglutathione Ab. Lane 1, control cells transfected with pBI vector were starved for 3 h.
Effect of GRx Knockout on Growth Factor-Induced Actin Polymerization.
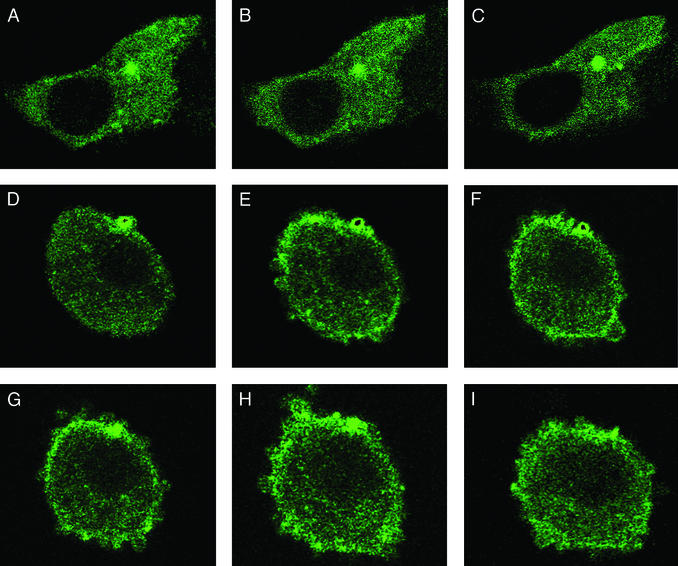
Actin polymerization and active reorganization induced by growth factor treatment can be observed with a confocal microscope by expressing YFP-actin or GFP-linked actin. We reported that within 3 min of growth factor treatment, actin polymerizes, translocates to the periphery of the cells, and undergoes active reorganization as reflected by transient membrane ruffling (31). At the time, we hypothesized that this observation was regulated by the deglutathionylation of actin. This notion is now verified by the results obtained in the GRx knocked-out NIH 3T3 cells shown in Fig. 4. In these experiments, we transfected cells with both pBI-dsGRx and a vector expressing YFP-actin. Before FGF treatment, most YFP-actin was evenly distributed in the cytosol for both GRx nonknockout and knockout cells (Fig. 4 A and D). Twenty seconds after FGF treatment, actin in the GRx nonknockout cells underwent polymerization and active reorganization around the periphery of the cell, and membrane ruffling was also observed (Fig. 4E). This phenomenon proceeded for at least 380 s (Fig. 4I). However, when the GRx knockout cells were treated with FGF in a parallel maneuver, no actin reorganization was detected, and cell shape remained relatively unchanged throughout the experiment (Fig. 4 B and C). Because actin reorganization is highly active in the FGF-treated non-GRx knockout cells and the confocal microscope provides images of a given surface at a given time, the GRx-knockout effect, with respect to actin reorganization and membrane ruffling, can be clearly portrayed with motion pictures. Short of video, five images recorded at 20, 160, 220, 300, and 380 s after FGF addition are shown in Fig. 4 E–I, respectively, to depict actin reorganization observed at various times. Together, these observations demonstrate that actin glutathionylation, regulated in part by GRx, plays a key role in growth factor-induced actin polymerization, translocation, and reorganization near the cell periphery, which occurs in <20 s after the growth factor treatment.
Figure 4.
Lack of actin reorganization in GRx-knocked-out cells. NIH 3T3 cells stably expressing tetracycline-responsive reverse transactivator were cotransfected with YFP-actin and pBI-dsGRx and were grown on 25-mm-diameter circular glass plates coated with gelatin. Cells with or without GRx knockout (with or without doxycycline treatment, respectively) were starved in DMEM without serum for 3 h before treatment with FGF. Fluorescent images were monitored by using a Zeiss LSM 510 confocal microscope with a 488-nm laser and ×63 objective. Similar image changes were observed in >90% of the YFP-actin-transfected cells. (A) YFP-actin and pBI-dsGRx-transfected cells treated with doxycycline for GRx knockout before FGF treatment. B and C were recorded 20 and 380 s after FGF treatment, respectively. (D) YFP-actin and pB1-dsGRx-transfected cells before FGF treatment. E–I were recorded 20, 160, 220, 300, and 380 s after FGF treatment, respectively.
In summary, we have shown an effective and controllable protein knockout method by RNAi. The bidirectional overexpression of sense and antisense RNA allows dsRNA longer than 22-mer to be generated in vivo. This procedure ensures more complete gene silencing because different small interfering RNAs of the same gene have variable silencing capacities. Our method provides stable expression of dsRNA and eliminates the necessity for uptake of extracellular dsRNA, a process we believe is, in part, responsible for the failure to achieve dsRNAi in a number of mammalian cells, particularly when longer dsRNAs are used. The controllable overexpression also allows the knockout to be performed at any desirable stage in cellular processes, particularly when it is used to generate transgenic animals for studying physiological functions of proteins lethal in animal knockouts. Thus, this method holds great promise for investigating protein function in cultured cells and animals. By using this method, we demonstrated that actin glutathionylation plays a key role in regulating growth factor-mediated actin polymerization, translocation to the cell periphery, and reorganization.
Abbreviations
- RNAi
RNA interference
- dsRNA
double-stranded RNA
- GRx
glutaredoxin
- FGF
fibroblast growth factor
- YFP-actin
yellow fluorescent protein actin
References
- 1.Sharp F A. Genes Dev. 2001;15:485–490. doi: 10.1101/gad.880001. [DOI] [PubMed] [Google Scholar]
- 2.Zamore P D. Nat Struct Biol. 2001;8:746–750. doi: 10.1038/nsb0901-746. [DOI] [PubMed] [Google Scholar]
- 3.Hannon G J. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 4.Zamore P D, Tuschl T, Sharp P A, Bartel D P. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 5.Elbashir S M, Lendeckel W, Tuschl T. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond S M, Bernstein E, Beach D, Hannon G J. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 7.Martinez J, Patkaniowska A, Urlaub H, Lührmann R, Tuschl T. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 8.Lipardi C, Wei Q, Paterson B M. Cell. 2001;107:297–307. doi: 10.1016/s0092-8674(01)00537-2. [DOI] [PubMed] [Google Scholar]
- 9.Nykänen A, Haley B, Zamore P D. Cell. 2001;107:309–321. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 10.Sijen T, Fleenor J, Simmer F, Thijssen K L, Parrish S, Timmons L, Plasterk R H A, Fire A. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein E, Caudy A A, Hammond S M, Hannon G J. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 12.Kennerdell J R, Carthew R W. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- 13.Misquitta L, Paterson B M. Proc Natl Acad Sci USA. 1999;96:1451–1456. doi: 10.1073/pnas.96.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser A G, Kamath R S, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- 15.Gonczy P, Echeverri C, Oegema K, Coulson A, Jones S J, Copley R R, Duperon J, Oegema J, Brehm M, Cassin E, et al. Nature. 2000;408:331–336. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- 16.Hunter T, Hunt T, Jackson R J, Robertson H D. J Biol Chem. 1975;250:409–417. [PubMed] [Google Scholar]
- 17.Zhao Z, Cao Y, Li M, Meng A. Dev Biol. 2001;229:215–223. doi: 10.1006/dbio.2000.9982. [DOI] [PubMed] [Google Scholar]
- 18.Elbashir S M, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 19.Oubrahim H, Chock P B, Stadtman E R. J Biol Chem. 2002;277:20135–20138. doi: 10.1074/jbc.C200226200. [DOI] [PubMed] [Google Scholar]
- 20.Brummelkamp T R, Bernard R, Agami R. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 21.Patrick P J, Caudy A A, Hannon G J. Proc Natl Acad Sci USA. 2002;99:1443–1448. doi: 10.1073/pnas.032652399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sui G, Soohoo C, Affar El B, Gay F, Shi Y, Forrester W C, Shi Y A. Proc Natl Acad Sci USA. 2002;99:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawasaki H, Suyama E, Iyo M, Taira K. Nucleic Acids Res. 2003;31:981–987. doi: 10.1093/nar/gkg184. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Sies H. Free Radical Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 25.Cooper A J L. In: The Molecular and Genetic Basis of Neurological Diseases. Rosenberg R N, Prusiner S B, DiMauro S, Barchi R L, Kunk L, editors. Boston: Butterworth–Heinemann; 1997. pp. 1195–1230. [Google Scholar]
- 26.Barrett W C, DeGnore J P, Keng Y-F, Zhang Z-Y, Yim M B, Chock P B. J Biol Chem. 1999;274:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 27.Klatt R, Lamas S. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan D M, Wehr N B, Fergusson M M, Levine R L, Finkel T. Biochemistry. 2000;39:11121–11128. doi: 10.1021/bi0007674. [DOI] [PubMed] [Google Scholar]
- 29.Chrestensen C A, Starke D W, Mieyal J J. J Biol Chem. 2000;275:26556–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- 30.Takagi Y, Nakamura T, Nishiyama A, Nozaki K, Tanaka T, Hashimoto N, Yodoi J. Biochem Biophys Res Commun. 1999;258:390–394. doi: 10.1006/bbrc.1999.0646. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Boja E S, Tan W, Tekle E, Fales H M, English S, Mieyal J J, Chock P B. J Biol Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]