Abstract
DNA polymerase η (Polη) functions in the proficient bypass of a variety of DNA lesions. Relative to the replicative polymerases, Polη has a greater tolerance for distorted DNA geometries and possesses a low fidelity. X-ray crystal structures and studies with nucleotide analogs have implicated interactions with the DNA minor groove as being crucial for the high fidelity of replicative DNA polymerases. To determine whether Polη also makes such functionally important contacts with the DNA minor groove, here we examine the effects on Polη-catalyzed nucleotide incorporation when 3-deazaguanine, a base analog that lacks the ability to form minor-groove hydrogen bonds with the protein, is substituted for guanine at various positions in the DNA. From these studies, we conclude that Polη makes only a single functional contact with the DNA minor groove at the position of the incoming nucleotide; in this regard, Polη differs from high-fidelity DNA polymerases that are unable to replicate through DNA lesions. These results help explain the proficient ability of Polη for bypassing distorting DNA lesions.
Eukaryotic DNA polymerase (Pol)η is a member of the recently discovered Y family of DNA polymerases. Both yeast and human Polη replicate efficiently and accurately through a cis-syn thymine–thymine (TT) dimer by incorporating As opposite the two Ts of the dimer (1–3), and genetic studies in yeast have also indicated the requirement of Polη for the error-free bypass of cyclobutane dimers formed at TC and CC sequences (4). Polη efficiently bypasses a 7,8-dihydro-8-oxoguanine by preferentially incorporating a C opposite the lesion (5), and it is able to replicate through a variety of other distorting DNA lesions (6–8) but with reduced efficiencies. Mutational inactivation of Polη in humans results in the variant form of xeroderma pigmentosum (9, 10), a genetic disorder characterized by a high incidence of UV-induced skin cancers.
Based on the ability of Polη to efficiently incorporate nucleotides opposite distorting DNA lesions, we hypothesized that Polη possesses an active site that is unusually tolerant of geometric distortions in the DNA (11). Because an intolerance of geometric distortions in the DNA plays a crucial role in the high fidelity of replicative DNA polymerases, we predicted that Polη would synthesize DNA with a low fidelity. In fact, steady-state kinetics analyses have shown that both yeast and human Polη misincorporate nucleotides with a frequency of 10−2 to 10−3 (3, 11), and consistent with this low fidelity, in an in vitro reaction human Polη was found to synthesize DNA in a highly mutagenic manner (12).
Crystal structures of ternary complexes of high-fidelity DNA polymerases consisting of the polymerase, double-stranded DNA, and a nucleotide substrate in the active site have revealed specific hydrogen-bonding interactions between the polymerase and the N3 (purine) and O2 (pyrimidine) hydrogen-bonding acceptors in the minor groove of duplex DNA (13–18). Furthermore, biochemical studies with nucleotide analogs lacking hydrogen-bonding acceptors in the DNA minor groove have been carried out to map functionally important hydrogen bonds between DNA polymerases and the DNA minor groove (19–21). Because the hydrogen-bond acceptors lie in the same position for all four correct base pairs but occupy different positions for incorrect base pairs, polymerases may use these minor-groove interactions to test for the correctness of the geometry of the newly formed base pair in DNA.
The high-resolution structures of several members of the Y family of DNA polymerases have been solved, including the structure of the catalytic core of yeast Polη in the absence of DNA and the incoming nucleotide (22) and the structures of related DinB homologs Dbh and Dpo4 from Sulfolobus solfataricus (23–25). The structure of Dpo4 has been solved in complex with DNA and the incoming nucleotide (25). Interestingly, in the structure of Dpo4 ternary complex, no hydrogen bonds are observed between the protein and the N3 or O2 atoms of the DNA minor groove (25). The structure of yeast Polη modeled with DNA and the incoming dNTP also suggests that there are no amino acid residues that are in close-enough proximity to form direct minor-groove interactions with DNA (22); however, that may be because in the model the enzyme does not adopt the appropriate conformational state.
Presteady-state kinetic analyses with yeast Polη have indicated a selectivity of ≈150-fold for the correct base pair at the nucleotide-incorporation step (kpol), and this selectivity is achieved by an induced-fit conformational change mechanism (26). This observation has suggested that, as for the replicative polymerases, Polη also may form functionally important hydrogen-bonding interactions with the DNA minor groove to assess the geometry of the incipient base pair. Here we determine whether Polη in fact makes such hydrogen-bonding interactions with the DNA minor groove by using 3-deazaguanine (3DG), which is a base analog of guanine but lacks the ability to form minor-groove hydrogen bonds with the polymerase. We find that by contrast to the DNA polymerases examined to date, Polη only makes a functionally important contact with the DNA minor groove at the incoming nucleoside triphosphate. This finding of a functional contact with the incoming nucleotide and not with the templating base helps to explain the ability of Polη to incorporate nucleotides opposite distorting DNA lesions in the template strand.
Materials and Methods
Purification of Yeast Polη.
Full-length yeast Polη fused to an N-terminal GST tag was expressed in yeast strain BJ5464 as described (1). The GST–Polη fusion protein was purified, and the GST tag was cleaved with PreScission protease (Amersham Pharmacia) as described (26) to generate the full-length yeast Polη protein fused to a 7-aa leader peptide. The protein was stored in 10-μl aliquots at −70°C.
Nucleotides.
The 3DG-containing nucleoside triphosphate (d3DGTP) was prepared from the nucleoside (27) by the method of Ludwig (28). Solutions of each of the four dNTPs (100 mM) were purchased from Roche Molecular Biochemicals, and their concentrations were confirmed by measuring A260.
Oligodeoxynucleotide Substrates.
The oligodeoxynucleotides containing 3DG were synthesized, PAGE-purified, and reverse-phase HPLC-purified as described (29). Three synthetic oligodeoxynucleotides (24-mer-C/G, 24-mer-C/3DG, and 24-mer-G/G) were used as template strands in the various DNA substrates. These templates had the sequence 5′-CTGCG AXTYC TGCGT CTGCG GTGC, where in 24-mer-C/G the X is a C and the Y is a G; in 24-mer-C/3DG, the X is a C and the Y is a 3DG; and in 24-mer-G/G, the X is a G and the Y is a G. Twelve synthetic oligodeoxynucleotides (15-mer-G, 15-mer-3DG, 16-mer-G, 16-mer-3DG, 17-mer-G, 17-mer-3DG, etc.) were used as primer strands in the various DNA substrates. The 15-mer-G had the sequence 5′-GCACC GCAGA CGCAZ, where Z is a G, and the 15-mer-3DG had the same sequence, where Z is a 3DG. The 16-mer-G and 16-mer-3DG had the same sequences as the 15-mer-G and 15-mer-3DG with an additional C at their 3′ ends. The 17-, 18-, 19-, and 20-mers had the identical sequences as the 15-mers with an additional CA, CAG, CAGT, and CAGTC on their 3′ ends, respectively.
The primer strands were 5′ 32P end-labeled by using polynucleotide kinase (Roche Molecular Biochemicals) and [γ-32P]ATP [≈6,000 Ci/mmol (1 Ci = 37 GBq)] (Amersham Pharmacia) and were subsequently purified by using a BioGel P30 spin column. The 32P end-labeled primer (0.5 μM) was annealed to the template (0.8 μM) in 50 mM Tris⋅HCl, pH 7.5/100 mM NaCl by incubating at 90°C for 2 min and slowly cooling to room temperature over several hours. To generate the P1 substrates, either the 15-mer-G or 15-mer-3DG primers were annealed to the 24-mer-C/G template, and to generate the P2 substrates, either the 16-mer-G or 16-mer-3DG primers were annealed to the 24-mer-C/G template. Similarly, to generate the T0 substrates, the 15-mer-G primer was annealed to either the 24-mer-C/G or 24-mer-C/3DG templates, and to generate the T1 substrates, the 16-mer-G primer was annealed to either the 24-mer-C/G or 24-mer-C/3DG templates. The annealed substrates are shown in Fig. 1C. Finally, to generate the P0 substrates, the 17-mer-G primer was annealed to either the 24-mer-C/G or 24-mer-G/G templates.
Figure 1.
DNA substrates containing 3DG. (A) The structure of guanine (G) and 3DG. (B) The designation of the base positions in the DNA substrates. P0 is the position of the incoming dNTP, P1 is the position of the primer-terminal residue, and P2, P3, P4, etc., are the positions of the consecutive bases in the primer in the 5′ direction. T0 is the position of the template base, and T1, T2, T3, etc., are the positions of the consecutive bases in the template in the 3′ direction. (C) The DNA substrates used in this study. The bold, underlined G is either a guanine or a 3DG.
Steady-State Kinetics Assays.
Yeast Polη (1 nM) was incubated with the DNA substrate (50 nM) and various concentrations of either the correct nucleotide or the incorrect nucleotide for 4 or 10 min at 25°C in 25 mM Tris⋅HCl, pH 7.5/5 mM MgCl2/5 mM DTT/100 μg/ml BSA/10% glycerol. Reactions were quenched with 10 volumes of formamide loading buffer (80% deionized formamide/10 mM EDTA, pH 8.0/1 mg/ml xylene cyanol/1 mg/ml bromophenol blue), boiled for 2 min, and kept on ice. The extended product was separated from the unextended substrates on a 15% polyacrylamide sequencing gel containing 8 M urea, and the intensity of each gel band was quantified by using a PhosphorImager (Molecular Dynamics). The rate of nucleotide incorporation was graphed as a function of nucleotide concentration, and the kcat and Km steady-state parameters were obtained from the best fit of these data to the Michaelis–Menten equation (30). The efficiency of nucleotide incorporation (kcat/Km) when 3DG is substituted for G in the DNA substrate then was calculated. Each measurement of the efficiency of nucleotide incorporation was repeated at least three times to ensure reproducibility, and the kcat/Km values obtained from these experiments were in close agreement. In addition, we carried out time-course experiments over a broad range of dNTP concentrations to ensure that nucleotide incorporation was linear over the time periods used.
Analysis of the Polymerase Structures.
To examine the hydrogen-bonding interactions of various DNA polymerases with the DNA minor groove, we used the SWISS-PDBVIEWER 3.7B2 software (Glaxo Wellcome) with the parameters set at their default values to calculate potential hydrogen bonds. This analysis was performed for Thermus aquaticus PolI (PDB ID code 1QTM) (17), T7 DNA polymerase (PDB ID code 1T7P) (15), human Polβ (PDB ID code 1BPY) (14), HIV reverse transcriptase (RT) (PDB ID code 1RTD) (31), and S. solfataricus Dpo4 protein (PDB ID code 1JX4) (25).
Results
It is widely held that the ability of DNA polymerases to sense geometric distortions in the DNA arises from hydrogen-bonding interactions between the protein and specific positions along the DNA minor groove. To determine whether Polη makes any such functionally important contacts with the DNA minor groove, we examined the ability of yeast Polη to incorporate nucleotides on DNA substrates containing 3DG, which is identical to guanine except that it has a CH substituted for the N3 minor-groove hydrogen-bond acceptor (Fig. 1A). Thus, the 3DG analog cannot participate in forming a minor-groove hydrogen bond with the polymerase. The DNA substrates in which 3DG was substituted for G at various positions along the primer strand, designated P0–P5, or the template strand, designated T0–T5 (Fig. 1B), are shown in Fig. 1C. In this scheme, P0 refers to the base of the incoming dNTP, P1 refers to the primer-terminal base, and P2–P5 refer to the consecutive bases in the primer strand in the 5′ direction (Fig. 1B). Likewise, T0 refers to the template base, and T1–T5 refer to the consecutive bases in the template strand in the 3′ direction (Fig. 1B).
Effects of 3DG Substitution at P0, the Incoming dNTP.
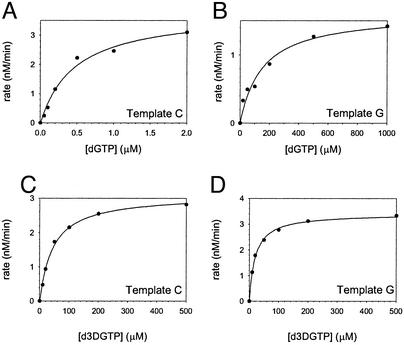
We examined the effects on both correct and incorrect nucleotide incorporation of a 3DG substitution for G at the P0 position, which is the position of the incoming nucleotide. To do this, we measured the steady-state kinetic parameters of G and 3DG incorporation opposite the correct template residue C and an incorrect template residue G. To assay incorporation opposite template C, we used the DNA substrate P3 (Fig. 1C), and to assay incorporation opposite template G, we used the identical sequence except that G was substituted for C at the position of the template base. Fig. 2 A and B show the rate of G incorporation opposite a template C and G, respectively, graphed as a function of [dGTP], and from the best fit to the Michaelis–Menten equation we obtained the kcat and Km values and used them to calculate the efficiency (kcat/Km) of G incorporation opposite these templates. As shown in Table 1, yeast Polη misincorporates a G opposite template G with a frequency of 1.4 × 10−3. Next, we examined the incorporation of 3DG opposite templates C and G (Fig. 2 C and D). Compared with the incorporation of a G opposite template C, the incorporation of 3DG opposite template C was reduced by ≈120-fold. 3DG, however, was incorporated opposite template G ≈15-fold more efficiently than the incorporation of G opposite G (Table 1).
Figure 2.
Steady-state kinetics of nucleotide incorporation by yeast Polη with a G or 3DG at P0, the position of the incoming dNTP. (A) The rate of dGTP incorporation opposite a correct template C graphed as a function of [dGTP]. The solid line represents the best fit to the Michaelis–Menten equation, and the kcat and Km steady-state parameters are listed in Table 1. (B) The rate of dGTP incorporation opposite an incorrect template G graphed as a function of [dGTP]. (C) The rate of d3DGTP incorporation opposite a correct template C graphed as a function of [d3DGTP]. (D) The rate of d3DGTP incorporation opposite an incorrect template G graphed as a function of [d3DGTP].
Table 1.
Steady-state kinetic parameters for nucleotide incorporation by yeast Polη with 3DG substitutions in the primer strand
| DNA substrate | dNTP/template | kcat, min−1 | Km, μM | kcat/Km, μM−1⋅min−1 | Relative efficiency |
|---|---|---|---|---|---|
| P0-G | G/C | 3.8 ± 0.03 | 0.47 ± 0.009 | 8.1 | 1.0 |
| G/G | 1.6 ± 0.1 | 150 ± 40 | 0.011 | 1.4 × 10−3 | |
| P0-3DG | 3DG/C | 3.1 ± 0.07 | 44 ± 3 | 0.070 | 8.6 × 10−3 |
| 3DG/G | 3.4 ± 0.05 | 20 ± 1 | 0.17 | 2.1 × 10−2 | |
| P1-G | C/G | 1.8 ± 0.08 | 0.76 ± 0.10 | 2.4 | 1.0 |
| G/G | 0.12 ± 0.01 | 54 ± 18 | 2.2 × 10−3 | 9.2 × 10−4 | |
| P1-3DG | C/G | 2.4 ± 0.04 | 1.1 ± 0.06 | 2.2 | 0.92 |
| G/G | ND | ND | <1 × 10−3 | <5 × 10−4 |
ND, no nucleotide incorporation detected.
Effects of 3DG Substitutions at Other Positions on the Primer Strand.
Next we examined the effects of 3DG substitution at P1, the position of the primer-terminal residue, by measuring the efficiency of C and G incorporation opposite the template G residue using the P1 DNA substrate (Fig. 1C). As shown in Table 1, when 3DG is substituted for G at the P1 position, the efficiency of correct nucleotide incorporation is not affected. We also examined the effects of a 3DG-for-G substitution at the other positions, P2–P5, along the primer strand, but found no significant effect on the efficiency of correct nucleotide incorporation (Fig. 3A) or on fidelity (data not shown).
Figure 3.
The effect of 3DG substitution on the efficiency of correct nucleotide incorporation by yeast Polη. (A) Bar graph showing the efficiency (kcat/Km) of correct nucleotide incorporation when G (black) or 3DG (gray) is present at primer positions P0–P5. (B) The efficiency of correct nucleotide incorporation when G (black) or 3DG (gray) is present at template positions T0–T5.
Effects of 3DG Substitution at T0, the Templating Base.
We also examined the effects of a 3DG substitution for G at the T0 position, which is the position of the templating base, on the efficiency of correct and incorrect nucleotide incorporation by using the DNA substrate T0 (Fig. 1C). Fig. 4 A and B show the kinetics of C and G incorporation, respectively, opposite template G, and Fig. 4 C and D show the kinetics of C and G incorporation, respectively, opposite template 3DG. The efficiency of correct nucleotide incorporation when 3DG is substituted for G at position T0 was reduced by ≈2.5-fold, and the efficiency of incorrect nucleotide incorporation when 3DG is substituted for G at T0 was increased ≈5-fold (Table 2). Overall, the substitution of 3DG for G at T0 has little effect on the efficiency of correct nucleotide incorporation and only a minor effect on fidelity.
Figure 4.
Steady-state kinetics of nucleotide incorporation by yeast Polη with G or 3DG at T0, the position of the template base. (A) The rate of correct dCTP incorporation opposite a template G graphed as a function of [dCTP]. The solid line represents the best fit to the Michaelis–Menten equation, and the kcat and Km steady-state parameters are listed in Table 2. (B) The rate of incorrect dGTP incorporation opposite a template G graphed as a function of [dGTP]. (C) The rate of correct dCTP incorporation opposite a template 3DG graphed as a function of [dCTP]. (D) The rate of incorrect dGTP incorporation opposite a template 3DG graphed as a function of [dGTP].
Table 2.
Steady-state kinetic parameters for nucleotide incorporation by yeast Polη with 3DG substitutions in the template strand
| DNA substrate | dNTP/template | kcat, min−1 | Km, μM | kcat/Km, μM−1⋅min−1 | Relative efficiency |
|---|---|---|---|---|---|
| T0-G | C/G | 1.8 ± 0.08 | 0.76 ± 0.10 | 2.4 | 1.0 |
| G/G | 0.12 ± 0.01 | 71 ± 15 | 1.7 × 10−3 | 7.1 × 10−4 | |
| T0-3DG | C/3DG | 0.63 ± 0.02 | 0.69 ± 0.07 | 0.91 | 0.38 |
| G/3DG | 0.48 ± 0.005 | 53 ± 2 | 9.1 × 10−3 | 3.8 × 10−3 | |
| T1-G | A/T | 2.0 ± 0.1 | 0.45 ± 0.8 | 4.4 | 1.0 |
| T/T | 2.9 ± 0.2 | 150 ± 30 | 1.9 × 10−2 | 4.3 × 10−3 | |
| T1-3DG | A/T | 0.65 ± 0.005 | 0.49 ± 0.10 | 1.3 | 0.30 |
| T/T | 0.48 ± 0.04 | 220 ± 50 | 2.2 × 10−3 | 5.0 × 10−4 |
Effects of 3DG Substitutions at Other Positions on the Template Strand.
Next, we examined the effects of a 3DG-for-G substitution at the other positions, T1–T5, along the template strand (Fig. 1C), and in all these cases no significant changes were observed in the efficiency of either correct or incorrect nucleotide incorporation when 3DG was substituted for G (Table 2, Fig. 3B, and data not shown).
In summary, whereas the decrease for the P0 (the incoming dNTP) substitution was 120-fold, the decreases in the efficiency of correct nucleotide incorporation resulting from 3DG substitutions at the other primer and template positions were all <10-fold (Tables 1 and 2 and Fig. 3). Thus, the most significant decrease in the efficiency of correct nucleotide incorporation occurs when 3DG is substituted for G at the P0 position, the incoming dNTP.
Discussion
The error frequency of nucleotide incorporation for replicative DNA polymerases is quite low. Because the thermodynamics of base-pairing alone cannot account for their remarkable fidelity, much of the accuracy of DNA polymerases must arise from geometric constraints (32). In the correct Watson–Crick G-C and A-T base pairs, the position of minor-groove hydrogen-bonding acceptors, N3 for purines and O2 for pyrimidines, are nearly identical, whereas in mispairs, these hydrogen-bonding acceptors are in different positions. Consequently, a simple way for the polymerase to check the correctness of a base pair is to sense the presence of a hydrogen-bonding acceptor in the proper position in the DNA minor groove.
The structures of a variety of binary complexes of DNA polymerases and DNA and ternary complexes of DNA polymerases, DNA, and dNTP have been determined. Using the structures of the ternary complexes of T. aquaticus PolI (17), T7 DNA polymerase (15), human Polβ (14), HIV RT (31), and S. solfataricus Dpo4 protein (25), we examined potential hydrogen-bonding interactions between the hydrogen-bonding donors in the protein and the hydrogen-bonding acceptors in the DNA minor groove. These structures show a wide range of different hydrogen-bonding interactions between Arg, Asn, Gln, or Lys side chains and the DNA minor groove. In the discussion below we refer to the hydrogen bonds inferred from high-resolution crystal structures as structural contacts and to those inferred from nucleotide analog studies as functional contacts. The polymerase forming the most structural contacts with the DNA minor groove is PolI from T. aquaticus (homologous to the Klenow fragment of PolI from Escherichia coli), which interacts with the minor groove of the primer and template strand bases at positions P1, P3, and T1 (Fig. 5). The other polymerases, T7, Polβ, and HIV RT also show evidence of making structural contacts with the minor groove along the primer and/or template strands (Fig. 5). The polymerase forming the fewest structural contacts with the DNA minor groove is Dpo4 from S. solfataricus, which shows no evidence of structural contacts with the DNA minor groove.
Figure 5.
Structural and functional contacts of various DNA polymerases with the DNA minor groove. White arrows represent structural contacts with the DNA minor groove, and black arrows represent functional contacts. Structural contacts for T7 DNA polymerase, Polβ, and HIV RT were inferred as described in Materials and Methods from the high-resolution structures (14, 15, 31); functional contacts were determined experimentally (20). For PolI, the structural contacts were inferred from the structure of T. aquaticus PolI (Klentaq) (17), and the functional contacts were determined experimentally by using E. coli PolI (Klenow) (20).
Because inferring hydrogen bonds from the positions of hydrogen-bonding donors and acceptors in high-resolution crystal structures is to some degree a matter of interpretation, it is absolutely necessary to determine experimentally whether a particular hydrogen-bonding interaction between the protein and the DNA minor groove is in fact energetically important. Studies with a variety of DNA polymerases using nucleotide analogs have been carried out to identify such interactions. These analogs include 4-methyl-benz-imidazole (Z), an A analog that lacks the ability to form Watson–Crick hydrogen bonding between the bases as well as hydrogen bonding between the protein and the DNA minor groove; 9-methyl-1H-imidazo[(4, 5)-b]pyridine (Q), an A analog that lacks the ability to form Watson–Crick hydrogen bonds between the bases but can form hydrogen bonds between the protein and the DNA minor groove (19, 20); and 3DG, a G analog that can form Watson–Crick hydrogen bonds between the bases but lacks the ability to form hydrogen bonds between the protein and the DNA minor groove (21). Studies with these analogs have shown that DNA polymerases make functional contacts with different primer and template positions in the minor groove (Fig. 5). In the case of E. coli Klenow, T7 DNA polymerase, and Taq polymerase, the primary functional contact between these enzymes and the DNA minor groove is at the position of the primer-terminal base (P1) (19–21). For Polβ, three functional contacts exist between the enzyme and the DNA minor groove, and they include the bases of the incipient base pair (P0 and T0) and the base at the primer terminus (P1) (20). By contrast, HIV RT functionally interacts with the minor groove at T1, and it shows a weak functional interaction at P1 (20).
Here we have examined whether Polη makes any functional contacts with the DNA minor groove by substituting the 3DG for G at various positions along the primer and template strands. The most substantial effect of the 3DG-to-G substitutions occurred at position P0, the incoming dNTP. Compared with the incorporation of a G opposite template C, 3DG incorporation opposite C was reduced by ≈120-fold. This demonstrates that the efficiency of yeast Polη-catalyzed DNA replication is indeed sensitive to disruptions of the minor-groove geometry, but it is so only at the P0 position. We note that the incorporation of both 3DG opposite template G and G opposite template 3DG were slightly more efficient than the incorporation of G opposite G (Tables 1 and 2), which suggests that in the context of certain mispairs Polη may sense the 3DG analog slightly differently than a G. However, because the differences are rather small, they are likely to have little bearing on the implications of our observations.
E. coli PolI (Klenow) and T7 DNA polymerase make functional contacts with the DNA minor groove only at the position of the primer-terminal base P1. Thus, it is likely that functional contacts at the P1 position are required for high-fidelity DNA synthesis by these polymerases. Polβ also functionally interacts with P1 in addition to functionally contacting P0 and T0. Interestingly, HIV RT, a low-fidelity polymerase whose fidelity is still higher than that of Polη, functionally interacts weakly with the P1 position. By contrast, Polη is unique in that it does not functionally interact with the P1 position. Thus, it would seem that the lack of this interaction contributes to the low fidelity of Polη, which makes functional contacts only with P0 (the incoming nucleotide), a position that may not be as sensitive as P1.
The biochemical properties reported here for Polη are consistent with its biological function in DNA-damage bypass. If Polη made functional contacts with the minor groove of the templating base (T0), that would have likely resulted in a substantially reduced efficiency of nucleotide incorporation opposite distorting DNA lesions. Consequently, Polη would have lacked the ability for the efficient bypass of DNA lesions. By contrast, the functional contact with the incoming nucleotide (P0) is unlikely to hinder DNA lesion bypass but still this minor-groove interaction could afford Polη some degree of geometric selectivity for incorporating the correct nucleotide.
Acknowledgments
This work was supported by National Institutes of Health Grants GM19261 and CA75074.
Abbreviations
- Pol
DNA polymerase
- 3DG
3-deazaguanine
- d3DGTP
3DG-containing nucleoside triphosphate
- RT
reverse transcriptase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Johnson R E, Prakash S, Prakash L. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 2.Washington M T, Johnson R E, Prakash S, Prakash L. Proc Natl Acad Sci USA. 2000;97:3094–3099. doi: 10.1073/pnas.050491997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson R E, Washington M T, Prakash S, Prakash L. J Biol Chem. 2000;275:7447–7450. doi: 10.1074/jbc.275.11.7447. [DOI] [PubMed] [Google Scholar]
- 4.Yu S-L, Johnson R E, Prakash S, Prakash L. Mol Cell Biol. 2001;21:185–188. doi: 10.1128/MCB.21.1.185-188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haracska L, Yu S-L, Johnson R E, Prakash L, Prakash S. Nat Genet. 2000;25:458–461. doi: 10.1038/78169. [DOI] [PubMed] [Google Scholar]
- 6.Haracska L, Prakash S, Prakash L. Mol Cell Biol. 2000;20:8001–8007. doi: 10.1128/mcb.20.21.8001-8007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minko I G, Washington M T, Prakash L, Prakash S, Lloyd R S. J Biol Chem. 2001;276:2517–2522. doi: 10.1074/jbc.M007867200. [DOI] [PubMed] [Google Scholar]
- 8.Levine R L, Miller H, Grollman A, Ohashi E, Ohmori H, Masutani C, Hanaoka F, Moriya M. J Biol Chem. 2001;276:18717–18721. doi: 10.1074/jbc.M102158200. [DOI] [PubMed] [Google Scholar]
- 9.Johnson R E, Kondratick C M, Prakash S, Prakash L. Science. 1999;285:263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- 10.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 11.Washington M T, Johnson R E, Prakash S, Prakash L. J Biol Chem. 1999;274:36835–36838. doi: 10.1074/jbc.274.52.36835. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda T, Bebenek K, Masutani C, Hanaoka F, Kunkel T A. Nature. 2000;404:1011–1013. doi: 10.1038/35010014. [DOI] [PubMed] [Google Scholar]
- 13.Pelletier H, Sawaya M R, Kumar A, Wilson S H, Kraut J. Science. 1994;264:1891–1903. [PubMed] [Google Scholar]
- 14.Sawaya M R, Prasad R, Wilson S H, Kraut J, Pelletier H. Biochemistry. 1997;36:11205–11215. doi: 10.1021/bi9703812. [DOI] [PubMed] [Google Scholar]
- 15.Doublie S, Tabor S, Long A M, Richardson C C, Ellenberger T. Nature. 1998;391:251–258. doi: 10.1038/34593. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Korolev S, Waksman G. EMBO J. 1998;17:7514–7525. doi: 10.1093/emboj/17.24.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Mitaxov V, Waksman G. Proc Natl Acad Sci USA. 1999;96:9491–9496. doi: 10.1073/pnas.96.17.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin M C, Wang J, Steitz T A. Cell. 2001;105:657–667. doi: 10.1016/s0092-8674(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 19.Morales J C, Kool E T. J Am Chem Soc. 1999;121:2323–2324. doi: 10.1021/ja983502+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morales J C, Kool E T. Biochemistry. 2000;39:12979–12988. doi: 10.1021/bi001578o. [DOI] [PubMed] [Google Scholar]
- 21.Spratt T E. Biochemistry. 2001;40:2647–2652. doi: 10.1021/bi002641c. [DOI] [PubMed] [Google Scholar]
- 22.Trincao J, Johnson R E, Escalante C R, Prakash S, Prakash L, Aggarwal A K. Mol Cell. 2001;8:417–426. doi: 10.1016/s1097-2765(01)00306-9. [DOI] [PubMed] [Google Scholar]
- 23.Silvian L F, Toth E A, Pham P, Goodman M F, Ellenberger T. Nat Struct Biol. 2001;8:984–989. doi: 10.1038/nsb1101-984. [DOI] [PubMed] [Google Scholar]
- 24.Zhou B-L, Pata J D, Steitz T A. Mol Cell. 2001;8:427–437. doi: 10.1016/s1097-2765(01)00310-0. [DOI] [PubMed] [Google Scholar]
- 25.Ling H, Boudsocq F, Woodgate R, Yang W. Cell. 2001;107:91–102. doi: 10.1016/s0092-8674(01)00515-3. [DOI] [PubMed] [Google Scholar]
- 26.Washington M T, Prakash L, Prakash S. Cell. 2001;107:917–927. doi: 10.1016/s0092-8674(01)00613-4. [DOI] [PubMed] [Google Scholar]
- 27.Spratt T E, Campbell C R. Biochemistry. 1994;33:11364–11371. doi: 10.1021/bi00203a035. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig J. Acta Biochim Biophys Acad Sci Hung. 1981;16:131–133. [PubMed] [Google Scholar]
- 29.Spratt T E. Biochemistry. 1997;36:13292–13297. doi: 10.1021/bi971253g. [DOI] [PubMed] [Google Scholar]
- 30.Creighton S, Bloom L B, Goodman M F. Methods Enzymol. 1995;262:232–256. doi: 10.1016/0076-6879(95)62021-4. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, Chopra R, Verdine G L, Harrison S C. Science. 1998;282:1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 32.Goodman M F. Proc Natl Acad Sci USA. 1997;94:10493–10495. doi: 10.1073/pnas.94.20.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]