Abstract
Working memory (WM) is the ability to retain and associate information over brief time intervals. Functional imaging studies demonstrate that WM is mediated by a distributed network including frontal and posterior cortices, hippocampus, and cerebellum. In rodents, the presentation of stimuli in a WM task is followed by a reset of the phase of hippocampal theta. In this paper we report the observation of a similar phenomenon in normal human subjects. Neuromagnetic responses were recorded during presentation of a set of digits and a subsequent probe of the retained items. All stimuli were presented with a fixed temporal pattern. We observed phase reset of ≈7 Hz theta in left hippocampus ≈120 ms after probe stimuli, whereas reset of theta in right hippocampus was visible ≈80 ms prior to these anticipated stimuli. The duration of stimulus-locked theta increased with memory load, with a limiting value of ≈600 ms for 5–7 retained items. We suggest that, as in rats, stimulus-locked theta may index involvement of human hippocampal networks in the cognitive processing of sensory input. The anticipatory phase reset of theta indicates involvement of hippocampus in right hemisphere and cerebellar timing networks. Hippocampal structures are essential for orientation to perturbations in the sensory scene, a function that requires use of a context established by a constellation of stimuli. We suggest that the initiation and maintenance of stimulus-locked hippocampal theta observed here may facilitate processing of potentially salient and/or novel input with respect to a context established by the contents of WM.
Coherent oscillatory activity of neuronal populations accompanies an imposing variety of cerebral functions across species. Among the most intensely studied is 4- to 12-Hz rhythmic slow activity (theta) discovered in rodent brain (1). Recordings in rat hippocampus show activity at 4–7 Hz during “cognitive” tasks and movement-related oscillations at 8–12 Hz. These brain rhythms are believed to be highly functional. For example, in the freely moving animal, “place cells” in the hippocampus are identified with specific locations in the environment. As the animal moves through the place field of a cell the firing of the cell correlates with a systematic advancement of the phase of ongoing theta rhythm (2, 3). Theta also participates in the sculpting of hippocampal networks. Stimulation-induced modulation of long-term potentiation (LTP) and long-term depression (LTD) of synaptic strengths within hippocampus is believed to depend on the phase of ongoing theta (4, 5).
Reports of theta oscillations in human hippocampus are rare. Hippocampal theta has been detected with intracranial electrodes in a few patients, although this activity has occurred in association with epileptic disorder (6–10). Movement-related oscillatory activity has not been reported. However, recordings of action potentials show that firing of human hippocampal units is related to various movements, especially those requiring a high degree of effort (11).
Neuronal current flow in in vitro hippocampal slice preparations has been detected by using superconducting magnetic field sensors (SQUIDs) (12–16). These measurements demonstrate that magnetic signals can be recorded from hippocampus. Magnetoencephalographic (MEG) arrays of SQUID sensors are now being used to record noninvasively stimulus-evoked neuronal population responses in human hippocampal structures (17–20). These studies include a report of the detection of ongoing oscillatory 4- to 12-Hz activity in normal human hippocampal structures (21).
We monitored the dynamics of neuronal population activity in normal adults by using a whole-scalp MEG array. We used a working memory (WM) task to elicit theta in hippocampal structures. WM is engaged when subjects retain information in memory for a brief time to perform a cognitive task or to initiate a goal-directed movement. Although the hippocampus is usually associated with consolidation of experiences into long-term memory, presentations of brief WM stimuli are known to induce resetting of the phase of theta in rodent dentate and medial septal areas (22). Phase reset of theta is seen also in recordings from cats during the acquisition of reference memory (23, 24) and in rats during the initial sessions of a classical conditioning task (25). We hypothesized that phase reset may provide a unique window of opportunity for detection and characterization of hippocampal theta in humans. In particular, averaging of responses recorded during hundreds of trials of a WM task may eliminate prominent ongoing background activity while preserving the signal from stimulus-locked hippocampal theta.
Methods
Subjects and Experimental Design.
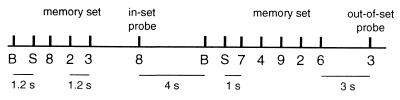
We used a variant of a well-known WM task, the Sternberg paradigm (26). Ten normal right-handed adults were asked to retain in memory for a brief interval a set of the integers (1, 2, … , 9) presented sequentially to the center of the visual field. Stimuli had an angular extent of 10–15° in the visual field and duration of 150 ms. The temporal pattern of stimulus presentation is indicated in Fig. 1. Trials began with a visual prompt to blink followed 1.2 s later by a prompt to start. A memory set of 1, 3, 5, or 7 items was presented at interstimulus intervals of 1.2 s. Retention of the memory set after a 3-s delay was interrogated with a probe digit. Subjects were instructed to lift the right index finger after presentation of a probe that was a member of the memory set (in-set) and the left index finger after a probe that was not in the memory set (out-of-set). The probe was distinguished from members of the memory set by the 3-s retention interval. The blink prompt for a subsequent trial followed the probe stimulus by 4 s. The presented integers and the sizes of the memory sets were randomized between trials. In two separate control experiments the stimulus presentation times were identical, but all digits were replaced with identical crosses. Subjects lifted consistently either the right or the left index finger to the “probe” cross. Data were recorded for 20–30 trials per subject for each stimulus set size and condition. The error rate was 0.3%. No feedback was given. Incorrect trials were not included in the analysis.
Figure 1.
Temporal pattern of stimulus presentation for memory set trials. The subject viewed a sequentially presented set of digits (the memory set) followed by a probe integer. Prompts to blink “B” and start “S” preceded each trial. In control trials, every integer was replaced by a cross.
Recordings.
Magnetic field patterns were recorded with a 122-channel whole-scalp MEG array (27). The subject was seated beneath the cryogenic Dewar within a magnetically shielded room. The locations of the MEG sensors were determined from measurements of the magnetic fields produced by three independently energized current loops attached to the subject's scalp. A three-dimensional digitizer (Isotrak 3S1002, Polhemus Navigation Sciences, Colchester, VT) was used to determine the locations of these coils with respect to anatomical features, and thus with respect to the cranial anatomy as defined by magnetic resonance (MR) images (1-T Siemens Magnetom system: recorded at the Helsinki University Central Hospital, Helsinki, Finland). MEG responses were bandpass filtered at 0.03–130 Hz and sampled at 400 Hz. A vertical electro-oculogram was utilized to identify eye movements and blinks. Approval for this study was obtained from the ethical committee of the Helsinki University Hospital. Informed consent was obtained from all subjects after explanation of the nature and possible consequences of the studies.
Data Analysis.
Averaged evoked responses were calculated off-line time-locked with the presentation of the stimuli, the motor responses and the blink prompts. Evoked responses for activity in sensorimotor cortex were characterized as localized neuronal population responses utilizing equivalent current dipoles (ECDs) (28). ECD source locations and orientations were identified from a least-squares fit to the data at 10–40 ms after the lifting of the finger. Distributed neuronal activity that was induced by the visual stimuli at latencies of 140–300 ms was characterized individually for each subject as 1–4 patterns of signals in the MEG array (29). Responses were centered over the occipital and posterior parietal cortex. Blink artifacts recorded by MEG channels near the face were characterized by the pattern of signals recorded by the MEG array at the latency of the peak electro-oculogram response. Activity in anterior cingulate [Brodmann area (BA) 24/32] and left and right prefrontal cortex (BA 44) was approximated by dipolar current flow at the locations determined individually for each subject for Talairach coordinates (1, 20, 32), (−52, 1, 32), and (41, 5, 32), respectively.
Individual subject's MR images were used to locate hippocampal structures. Preliminary visualization of the data with minimum current estimate (MCE) algorithm (30) showed substantial signals in a 4- to 8-Hz bandwidth within ≈1 cm of anterior hippocampus for 6/10 subjects. Activation of hippocampus and associated surrounding cortex was approximated by a dipolar current source located in anterior hippocampus (18). A “realistic” single-compartment boundary-element model for the conducting volume of the brain (31) was determined from the individual subject's MR images and used in the calculation of the pattern of MEG signals recorded by the array for each hippocampal, cingulate, and frontal source.
Signal-space projection (SSP) was used to provide a common framework for a multicomponent analysis of the localized (equivalent current dipole), distributed, and forward-modeled components of the MEG data (29). Waveforms were derived from the raw data by using the pseudoinverse of the SSP source matrix for a single hippocampal component in conjunction with the set of all sensorimotor and distributed occipital, parietal, and blink SSP components. The effective system noise for each SSP component was determined by recording data with no subject under the detector array and subsequently computing noise waveforms for the same SSP source matrix employed for each individual subject. Signal and system noise SSP waveforms were filtered at 4–8 Hz. The onset and offset of stimulus-locked theta was determined by the requirement that the zero-level crossing of the waveform at these instants ceased to be consistent (± rms system noise) with that determined by a waveform with the mean frequency of oscillation of the grand-averaged evoked responses.
Results
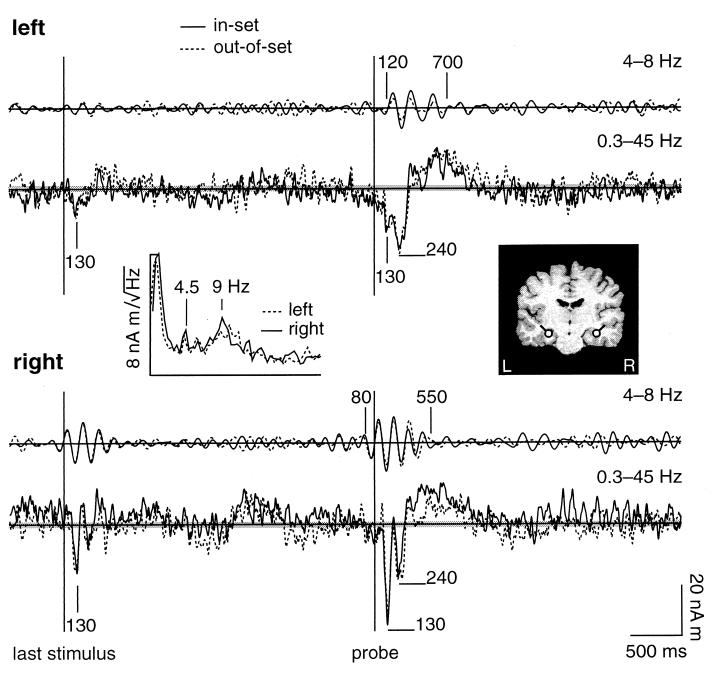
Neuromagnetic responses were recorded throughout the performance of the WM task. Fig. 2 shows waveforms derived from the MEG data that characterized neuronal population activity in and near left and right anterior hippocampus. Peak responses were observed in data filtered from 0.3 to 45 Hz at 130 ms after both memory-set and probe stimuli (see Fig. 2). Reproducible bilateral activity occurred also at 240 ms after the probes. Differences between responses to probes that were included (in-set) or missing (out-of-set) within the memory set were particularly prominent at 300–800 ms in right hippocampal waveforms. Although the evoked response features were reproducible in the grand-averaged data, substantial ongoing activity also appeared both during the retention period and 1–4 s after probe stimuli. In particular, the spectra of stimulus-locked activity after the probe stimuli showed clear peaks at theta (4–8 Hz) and alpha (8–12 Hz) frequencies. Evoked response peaks at 1–4 s after stimulus presentation were not reproducible between blocks of trials.
Figure 2.
Waveforms for left (Upper) and right (Lower) hippocampus for memory set trials. The coronal MR image (Right Inset) shows the locations (dots) and orientations (tails) of the dipolar current generators in hippocampus. The Left Inset shows the amplitude spectra of the activity following the probes for in-set trials. Evoked responses were averaged time-locked with the probe stimulus. Grand-averaged responses for 10 subjects are shown for in-set (solid lines) and out-of-set (dashed lines) trials. The in-set and out-of-set trials were indistinguishable to the subjects before the presentation of the probe stimuli. Waveforms were filtered from 4 to 8 Hz (upper traces) and from 0.3 to 45 Hz (lower traces). The horizontal shaded areas indicate the rms system noise for each average (in-set 887 trials, out-of-set 839 trials).
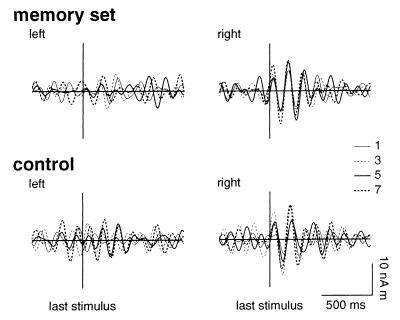
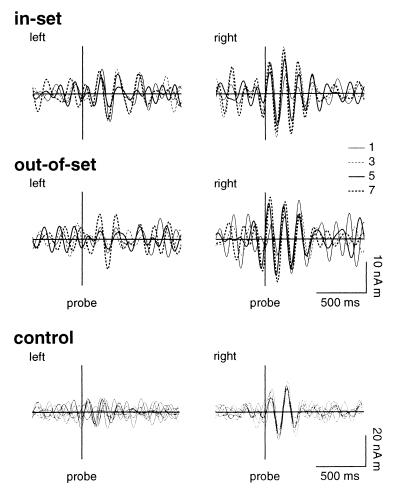
Filtering the averaged evoked responses at 4–8 Hz revealed that periods of reproducible theta-band activity were associated with stimulus presentation in data for 8/10 subjects. Reset of the phase of theta was observed in the right hippocampal waveforms both for memory-set (Fig. 3) and probe (Fig. 4) stimuli. The onset of phase-locked activity preceded the stimuli by ≈80 ms. The right hemisphere anticipatory phase reset persisted even when all digits were replaced by crosses. In contrast, stimulus-locked activity in left hippocampal waveforms was associated only with the probe stimuli and commenced ≈120 ms after stimulus presentation.
Figure 3.
Grand averaged evoked responses to the last stimulus before the retention period for left (Left) and right (Right) hippocampus. Responses are shown for memory-set and control trials and for each set size of 1, 3, 5, and 7 items. Waveforms were filtered from 4 to 8 Hz.
Figure 4.
Grand averaged evoked responses to the probe stimulus for left (Left) and right (Right) hippocampus. Responses are shown for in-set and out-of-set memory trials and for control trials for each set size of 1, 3, 5, and 7 items. Waveforms were filtered from 4 to 8 Hz.
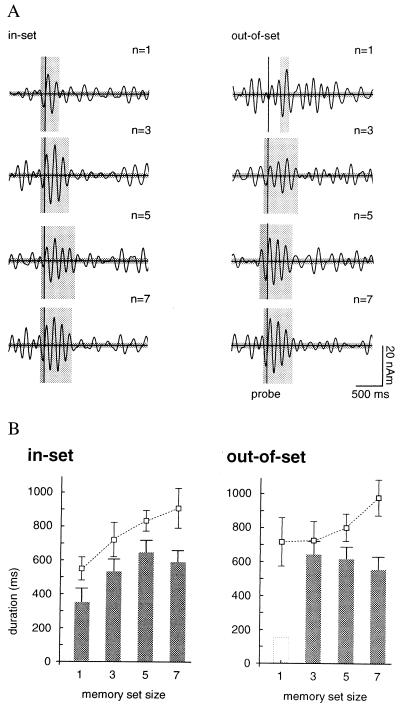
The duration of ≈7-Hz stimulus-locked theta was a function of both the stimulus type (memory set item or probe) and the size of the memory set, even though all stimuli were of identical physical character and familiarity to the subject. Right hippocampal theta associated with the last stimulus in the memory set persisted for 380–500 ms. Theta-band activity associated with in-set probes for a memory set of one item terminated much earlier, at ≈340 ms after the presented stimuli. The duration of theta after in-set probe stimuli increased linearly with set size to a maximum of 650 ms for five items (n = 5), with a decreased value of 570 ms for set size n = 7 (see Fig. 5A). Activity after out-of-set probes also was of significantly longer duration than for responses to the memory set stimuli, with an average duration ranging from 530 to 625 ms. Hippocampal responses to “probe” stimuli during the control trials failed to show sustained theta oscillations.
Figure 5.
(A) Dependence of the duration of stimulus-locked 4- to 8-Hz theta on memory set size is indicated by the vertical shaded areas for in-set and out-of-set trials. The system noise is shown by the horizontal shaded area. (B) Bar graphs show the duration of theta in right hippocampus after the probe stimulus for in-set and out-of-set trials. The mean reaction time as a function of memory set size is indicated by the dashed lines.
Psychophysical results for Sternberg memory tasks show a well-known linear dependence of the mean reaction time on memory set size for both in-set and out-of-set probes (26). Fig. 5B shows a similar linear increase of the reaction time with memory load for the WM task used here. The reaction times do not differ significantly for in-set and out-of-set items (responses for out-of-set probes of n = 1 are of exceptional character). We compared this behavior to the dependence of the duration of theta on memory load. The offset of stimulus-locked theta occurred on average ≈320 ms before the mean reaction time, but only for in-set probes. Thus a linear dependence of duration of theta on set size was observed for in-set, but not for out-of-set probes.
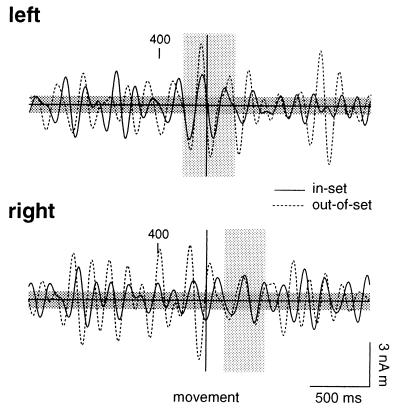
We investigated the possibility that a fixed temporal relationship existed between the offset of hippocampal theta and the motor response by averaging the MEG signals time-locked with movement. As shown in Fig. 6, averaged evoked responses for both in-set and out-of-set trials showed 4- to 8-Hz activity in left and right hippocampal waveforms. However, this activity did not terminate at a stereotypic instant before movement. In contrast, theta was observed from ≈350 ms before to ≈350 ms after the motor response in the left hippocampus, whereas activity on the right showed movement-locked 4- to 8-Hz activity only after the motor response.
Figure 6.
Grand averaged evoked responses time-locked with the motor response for activity in left (Upper) and right (Lower) hippocampus for control trials. Responses are shown for in-set and out-of-set trials. Waveforms were filtered from 4 to 8 Hz.
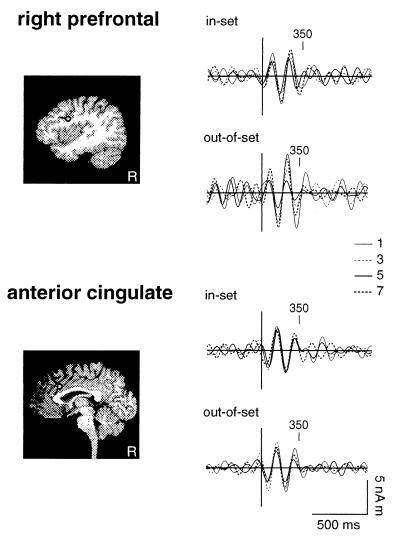
We compared hippocampal responses to stimulus-locked activity generated in cortical regions known to be involved in working memory. Waveforms for anterior cingulate (BA 24/32; Fig. 7 Lower) and right prefrontal cortex (BA 44; Fig. 7 Upper) showed an onset and duration of 4- to 8-Hz activity for memory-set trials which was dissimilar to that observed in the hippocampal waveforms. No phase reset was observed before stimulus presentation. Responses showed neither sustained oscillations nor set-size dependence of the duration of theta-band activity.
Figure 7.
Grand averaged evoked responses to the probe stimulus for activity in right prefrontal (Upper) and anterior cingulate (Lower) cortex for memory-set trials. Responses are shown for in-set and out-of-set trials and for set sizes of 1, 3, 5, and 7 items. Waveforms were filtered from 4 to 8 Hz.
Discussion
Functional imaging studies demonstrate that WM is mediated by a distributed network including frontal and posterior cortices (32, 33), hippocampus (34, 35), and cerebellum (36). Hippocampal structures are believed to be essential for orientation to perturbations in the sensory scene (37, 38) and are implicated in the utilization of a context established by a constellation of stimuli for integration with incoming input (39, 40).
Theta oscillations are strongly associated with memory function. Electroencephalographic (EEG) scalp recordings have revealed a correlation at 4–7 Hz between signals recorded over prefrontal and parietal cortex during the retention period of a WM task (41). Tasks in which the memory load is systematically increased show a corresponding increase of theta in frontal electrodes (42). Theta-band oscillations in EEG recordings are present also during tasks that involve episodic memory: the successful encoding and retrieval of remembered items is correlated with an increased power of 4- to 7-Hz theta (43). Subdural recordings on epileptic patients identify theta in signals recorded over many cortical areas during a spatial navigation task (44). Although EEG results indicate that memory function in humans may be indexed by theta-band oscillatory activity, this method has not been extended to the identification of hippocampal responses per se.
We observed resetting of the phase of ≈7-Hz theta associated both with the memory-set and probe stimuli in hippocampal MEG waveforms. A striking feature of the theta reset in the present data was the onset of stimulus-locked theta-band activity in right hippocampus before the presentation of the stimuli. The anticipatory right hippocampal responses observed in the present study occurred under the condition of a predictable temporal relationship between the stimuli. Both right hemisphere prefrontal and inferior parietal cortices and cerebellum have been implicated in the processing of the temporal features of sensory input during WM tasks (36). We suggest that hippocampal involvement with these timing networks may facilitate preparation of hippocampal networks crucial for the processing of the anticipated input.
A characteristic feature of the Sternberg paradigm is the linear dependence of mean reaction time on set size for both in-set and out-of-set probes, with the interesting exception of an increased reaction time to out-of-set probes for memory-set size n = 1. One interpretation of the psychophysical results is that the mean reaction time may index a search of the memory set. Sternberg originally proposed a serial-exhaustive-scanning (SES) model in which each memory set is scanned completely before the decision to execute a motor response is initiated (45). This search is executed regardless of the emergence of congruence between the memory set and probe, and thus is presumably similar in duration for both in-set and out-of-set probes. Serial position effects (46) and observed differences in the processing of in-set and out-of-set probes (47) challenge the SES model. Recently, models for an oscillatory short-term memory buffer have been developed for cortical and/or hippocampal networks that account for both the linear dependence of reaction time on set-size and serial position effects (48).
The MEG data show an approximately linear increase of the duration of stimulus-locked theta for in-set probes for set sizes n = 1, 3, and 5. However, the duration of phase-locked theta for out-of-set probes is not linear with set size. Thus the determinant of mean reaction time in this WM task cannot be the duration of 4- to 8-Hz hippocampal theta. Moreover, we see no evidence of an offset of 4- to 8-Hz theta that is phase-locked with and prior to movement. Thus, although a hippocampal process may be reflected in the duration of the stimulus-locked theta, there is no evidence that this activity is linked to movement per se. In the single trial data for rat, the stimulus-locked theta observed during a WM task was dominated by ongoing oscillations in the theta band (22). The functional significance of these two phenomena may differ. We suggest that stimulus-locked theta oscillations observed here may index involvement of hippocampal networks in the cognitive processing of stimulus-linked information.
Hippocampal networks are implicated in the detection of stimulus novelty and/or salience in the context of an ongoing input or task. Determination of the novelty of a stimulus presumes two functions: on-line monitoring of sensory input and comparison with a body of preexistent information. The classic example is an oddball stimulus embedded in a train of identical stimuli. Both depth electrode recordings on patients (49) and MEG recordings from normal adults (18–20) and patients (50) have revealed evoked neuronal population responses in hippocampus to oddball stimuli.
Novelty in the oddball paradigm is defined with reference to stimuli presented in the recent past. The retention of information over a time interval for the performance of a cognitive or motor task presumably would engage WM networks. Thus there may be a very delicate interplay between the processes of novelty detection, WM, and the consolidation of new experiences into long-term memory. We observed sustained ongoing hippocampal theta for the out-of-set probes that was of greater duration than that observed for the memory-set items. We suggest that these stimuli were processed as novel items with respect to a context established by the contents of working memory.
There is a clear association between the phase of hippocampal theta and effective modulation of synaptic strength by input (4, 5). We speculate that the sustained theta following out-of-set probes may enhance processing of stimuli that are novel. These items may be salient candidates for consolidation into long-term memory in association with the contents of WM. A mandatory termination of this processing stage may occur over the time frame that characterized the processing of the out-of-set probes, i.e., ≈530–625 ms.
A limited duration is characteristic of hippocampal theta activity that has been observed in several species. Stimulus-locked theta observed in rats during a WM task persisted over a similar time frame of ≈700 ms (about 6 oscillations) (22). The learning of a visual discrimination task in cats was accompanied by stimulus-locked theta, with activity commencing on the onset of a tone signifying the beginning of a trial. Maximum responses occurred during the period of maximum learning (50–80% accuracy). After successful learning (100% accuracy) phase-locking commenced immediately on the onset of the tone and continued for about 600 ms (24).
Finally, the existence of a finite time-frame for processing of a stimulus that probes the contents of WM may have an interesting consequence for the capacity limit of WM. The classic Sternberg WM task for digits has a well-known capacity limit of 7 ± 2 memory set items that can be interrogated successfully by a probe stimulus. Although hippocampal processes may not be essential in the determination of this limit, it is intriguing that the duration of stimulus-locked theta for in-set probes increases linearly with set size in our data. A limit was achieved at 5–7 items for a duration of theta of 570–650 ms. We suggest that this kind of temporal constraint in corticohippocampal networks may provide a mechanism that limits the capacity of WM.
Acknowledgments
We thank M. Kajola, M. Hämäläinen, S. Tissari, K. Uutela, and M. Seppä for software development, V. Jousmäki for technical assistance, and O. Jensen for comments on the manuscript. MR scans were obtained at the Department of Radiology, Helsinki University Central Hospital. This research was supported by National Institutes of Health Grant NS34533.
Abbreviations
- MEG
magnetoencephalographic or magnetoencephalography
- WM
working memory
- MR
magnetic resonance
References
- 1.Vanderwolf C H. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe J, Dostrovsky J. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe J, Recce M L. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 4.Stanton P K, Sejnowski T J. Nature (London) 1989;339:215–227. doi: 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- 5.Huerta P T, Lisman J E. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 6.Halgren E, Babb T L, Crandall P H. Electroencephalogr Clin Neurophysiol. 1978;44:778–781. doi: 10.1016/0013-4694(78)90212-2. [DOI] [PubMed] [Google Scholar]
- 7.Arnolds D E, Lopes da Silva F H, Aitink J W, Kamp A, Boeijinga P. Electroencephalogr Clin Neurophysiol. 1980;50:324–328. doi: 10.1016/0013-4694(80)90160-1. [DOI] [PubMed] [Google Scholar]
- 8.Isokawa-Akesson M, Wilson C L, Babb T L. Exp Neurol. 1987;98:137–151. doi: 10.1016/0014-4886(87)90079-3. [DOI] [PubMed] [Google Scholar]
- 9.Huh K, Meador K J, Lee G P, Loring D W, Murro A M, King D W, Gallagher B B, Smith J R, Flanigin H F. Neurology. 1990;40:1177–1181. doi: 10.1212/wnl.40.8.1177. [DOI] [PubMed] [Google Scholar]
- 10.Meador K J, Thompson J L, Loring D W, Murro A M, King D W, Gallagher B B, Lee G P, Smith J R, Flanigin H F. Neurology. 1991;41:869–872. doi: 10.1212/wnl.41.6.869. [DOI] [PubMed] [Google Scholar]
- 11.Halgren E. Hippocampus. 1991;2:153–161. doi: 10.1002/hipo.450010204. [DOI] [PubMed] [Google Scholar]
- 12.Tesche C D, Krusin-Elbaum L, Knowles W D. Brain Res. 1988;462:190–193. doi: 10.1016/0006-8993(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 13.Kyuhou S, Okada Y. J Neurophysiol. 1993;70:2665–2668. doi: 10.1152/jn.1993.70.6.2665. [DOI] [PubMed] [Google Scholar]
- 14.Okada Y, Xu C. Neurosci Lett. 1996;211:155–158. doi: 10.1016/0304-3940(96)12740-3. [DOI] [PubMed] [Google Scholar]
- 15.Okada Y C, Wu J, Kyuhou S. Electroencephalogr Clin Neurophysiol. 1997;103:474–485. doi: 10.1016/s0013-4694(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Okada Y C. Electroencephalogr Clin Neurophysiol. 1998;107:361–373. doi: 10.1016/s0013-4694(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 17.Okada Y C, Kaufman L, Williamson S J. Electroencephalogr Clin Neurophysiol. 1983;55:417–426. doi: 10.1016/0013-4694(83)90130-x. [DOI] [PubMed] [Google Scholar]
- 18.Tesche C D, Karhu J, Tissari S. Brain Res Cogn Brain Res. 1996;4:39–47. doi: 10.1016/0926-6410(95)00044-5. [DOI] [PubMed] [Google Scholar]
- 19.Nishitani N, Nagamine T, Fujiwara N, Yazawa S, Shibasaki S. J Cogn Neurosci. 1998;10:231–247. doi: 10.1162/089892998562672. [DOI] [PubMed] [Google Scholar]
- 20.Tesche C D, Karhu J. J Cogn Neurosci. 1999;11:424–436. doi: 10.1162/089892999563517. [DOI] [PubMed] [Google Scholar]
- 21.Tesche C D. Brain Res. 1997;749:53–60. doi: 10.1016/s0006-8993(96)01286-3. [DOI] [PubMed] [Google Scholar]
- 22.Givens B. NeuroReport. 1996;8:159–163. doi: 10.1097/00001756-199612200-00032. [DOI] [PubMed] [Google Scholar]
- 23.Adey W R, Walter D O. Exp Neurol. 1963;7:186–209. doi: 10.1016/0014-4886(63)90054-2. [DOI] [PubMed] [Google Scholar]
- 24.Porter R, Adey W R, Brown T S. Exp Neurol. 1964;10:216–235. doi: 10.1016/0014-4886(64)90064-0. [DOI] [PubMed] [Google Scholar]
- 25.Buzsáki G, Grastyan E, Tveritskaya I N. Electroencephalogr Clin Neurophysiol. 1979;47:64–74. doi: 10.1016/0013-4694(79)90033-6. [DOI] [PubMed] [Google Scholar]
- 26.Sternberg S. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 27.Ahonen A I, Hämäläinen M, Kajola M, Knuutila J, Laine P, Lounasmaa O, Parkkonen L, Simola J, Tesche C D. Phys Scr T. 1993;49:198–205. [Google Scholar]
- 28.Brenner D, Lipton J, Kaufman L, Williamson S J. Science. 1978;189:81–83. doi: 10.1126/science.199.4324.81. [DOI] [PubMed] [Google Scholar]
- 29.Tesche C D, Uusitalo M, Ilmoniemi R, Huotilainen M, Kajola M, Salonen O. Electroencephalogr Clin Neurophysiol. 1995;95:189–200. doi: 10.1016/0013-4694(95)00064-6. [DOI] [PubMed] [Google Scholar]
- 30.Uutela K, Hämäläinen M, Somersalo E. NeuroImage. 1999;10:173–180. doi: 10.1006/nimg.1999.0454. [DOI] [PubMed] [Google Scholar]
- 31.Hämäläinen M, Sarvas J. IEEE Trans Biomed Eng. 1989;36:165–171. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- 32.Baddeley A. Working Memory. Oxford: Oxford Univ. Press; 1986. [Google Scholar]
- 33.Rypma B, D'Esposito M. Proc Natl Acad Sci USA. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldman-Rakic P S. In: Brain Organization and Memory: Cells, Systems and Circuits. McGaugh J L, Weinberger N M, Lynch G, editors. New York: Oxford Univ. Press; 1989. pp. 1–29. [Google Scholar]
- 35.Baddeley A. J Cogn Neurosci. 1992;4:281–288. doi: 10.1162/jocn.1992.4.3.281. [DOI] [PubMed] [Google Scholar]
- 36.Harrington D L, Haaland K Y, Knight R T. J Neurosci. 1998;18:1085–1095. doi: 10.1523/JNEUROSCI.18-03-01085.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tulving E, Markowitsch H J, Kapur S, Habib R, Houle S. NeuroReport. 1994;5:2525–2528. doi: 10.1097/00001756-199412000-00030. [DOI] [PubMed] [Google Scholar]
- 38.Knight R. Nature (London) 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- 39.Squire L R, Zola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 40.Wallenstein G V, Eichenbaum H, Hasselmo M E. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 41.Sarnthein J, Petsche H, Rappelsberger P, von Stein A. Proc Natl Acad Sci USA. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gevins A, Smith M E, McEvoy L, Yu D. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- 43.Klimesch W, Doppelmayr M, Schimke H, Ripper B. Psychophysiology. 1997;34:169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- 44.Kahana M J, Sekuler R, Caplan J B, Kirschen M, Madsen J R. Nature (London) 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- 45.Sternberg S. Acta Psychol (Amsterdam) 1969;30:276–315. [Google Scholar]
- 46.Clifton C, Birenbaum S. J Exp Psychol. 1970;86:69–76. [Google Scholar]
- 47.McElree B, Dosher B A. J Exp Psychol Gen. 1989;118:346–373. [Google Scholar]
- 48.Jensen O, Lisman J E. J Neurosci. 1998;18:10688–10699. doi: 10.1523/JNEUROSCI.18-24-10688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halgren E, Squires N K, Wilson C L. Science. 1980;210:803–805. doi: 10.1126/science.7434000. [DOI] [PubMed] [Google Scholar]
- 50.Nishitani N, Ikeda A, Nagamine T, Honda M, Mikuni N, Taki W, Kimura J, Shibasaki H. Brain. 1999;122:687–707. doi: 10.1093/brain/122.4.687. [DOI] [PubMed] [Google Scholar]