Abstract
Insulin secretion is controlled by the β cell′s metabolic state, and the ability of the secretory granules to undergo exocytosis increases during glucose stimulation in a membrane potential-independent fashion. Here, we demonstrate that exocytosis of insulin-containing secretory granules depends on phosphatidylinositol 4-kinase (PI 4-kinase) activity and that inhibition of this enzyme suppresses glucose-stimulated insulin secretion. Intracellular application of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] stimulated exocytosis by promoting the priming of secretory granules for release and increasing the number of granules residing in a readily releasable pool. Reducing the cytoplasmic ADP concentration in a way mimicking the effects of glucose stimulation activated PI 4-kinase and increased exocytosis whereas changes of the ATP concentration in the physiological range had little effect. The PI(4,5)P2-binding protein Ca2+-dependent activator protein for secretion (CAPS) is present in β cells, and neutralization of the protein abolished both Ca2+- and PI(4,5)P2-induced exocytosis. We conclude that ADP-induced changes in PI 4-kinase activity, via generation of PI(4,5)P2, represents a metabolic sensor in the β cell by virtue of its capacity to regulate the release competence of the secretory granules.
Keywords: Ca2+-dependent activator protein for secretion (CAPS)‖exocytosis‖ insulin‖phosphoinositides
The pancreatic β cell contains 10,000–13,000 insulin-containing secretory granules (1, 2). As in other neuroendocrine cells, the secretory granules in the β cell functionally belong to either a readily releasable pool (RRP) or a reserve pool. In β cells, the size of RRP is limited, and current estimates suggest that as few as 50–100 granules are immediately available for release (3). After the depletion of RRP, this pool is replenished by mobilization of new granules from the reserve pool. Ultrastructural studies indicate that the number of granules residing in the immediate vicinity of the plasma membrane, the docked pool, is sufficient for several hours of glucose-induced insulin secretion (2). This finding has led to the proposal that mobilization does not require physical translocation of the secretory granules and that chemical modification, priming, of granules already in place may be sufficient to account for the refilling of RRP. It has been argued that release of RRP underlies first phase glucose-induced insulin secretion and that second phase release reflects the refilling of this pool (4). A detailed knowledge about distinct subsets of secretory granules and their respective contribution to the different phases of insulin secretion is desirable given that type 2 diabetes associates with abnormalities in the insulin release pattern (5).
After docking of granules to the plasma membrane, phosphatidylinositol (PI) 4-kinases and PI 5-kinases may act in conjunction to modify the two newly juxtaposed membranes, contributing to the acquisition of fusion competence (6, 7). The activities of these kinases, which lead to the sequential synthesis of PI 4-phosphate [PI(4)P] and PI 4,5-bisphosphate [PI(4,5)P2], have been proposed to account for at least part of the requirement for Mg-ATP in the priming process (6, 7). PI(4,5)P2 binds specifically to the Ca2+-dependent activator protein for secretion (CAPS), which is required for Ca2+-triggered exocytosis once ATP-dependent priming has been completed (8–10). This finding indicates that CAPS operates in parallel with the membrane fusion machinery consisting of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins (11).
The pancreatic β cell represents a particularly suitable preparation to study the mechanisms involved in priming because changes in the metabolic state rapidly translate into increased or reduced release competence of the insulin-containing secretory granules. For example, a step elevation of ATP triggers exocytosis within milliseconds (12), whereas ADP exerts a prompt (<5 s) inhibitory action (13). Here, we describe a mechanism by which glucose-induced reciprocal changes in the cytoplasmic ATP and ADP concentrations, via activation of PI 4-kinase, promotes PI(4,5)P2 synthesis, priming of secretory granules, and refilling of RRP. These data provide a framework for the understanding of the amplifying action of glucose on insulin secretion that occurs independently of ATP-sensitive K+-channel activity (14).
Materials and Methods
Preparation and Culture of β Cells.
Mouse pancreatic islets were isolated from NMRI mice (Bomholtgaard, Denmark) as described (15). For electrophysiological experiments, the islets were dispersed into single cells by shaking in Ca2+-free solution. For subcellular fractionation studies, islets were isolated from ob/ob mice (16).
Capacitance Measurements.
Exocytosis was monitored in single β cells as changes in cell membrane capacitance by using the standard whole-cell configuration as described (17, 18). The pipette solution contained (in mM) 125 cesium-glutamate, 10 CsCl, 10 NaCl, 1 MgCl2, 5 Hepes, 0.05 EGTA, 3 Mg-ATP, and 0.01 GTP (pH 7.15 with CsOH) and 0–5 Mg-ADP. The extracellular medium consisted of (in mM) 118 NaCl, 20 tetraethylammonium-Cl, 5.6 KCl, 1.2 MgCl2, 2.6 CaCl2, 5 Hepes (pH 7.40 using NaOH), and 5 d-glucose. The capacitance measurements commenced 2 min after establishment of the whole-cell configuration to allow equilibration between the pipette solution and the cytoplasm. The stimulation protocols consisted of either individual 500-ms depolarizations or trains of fourteen 500-ms depolarizations applied at 1 Hz. In both cases, the pulses went from −70 mV to 0 mV.
All phospholipids (Calbiochem) were dissolved in the pipette-filling solution by sonication for 30 min on ice. Monoclonal antibodies to PI(4)P and PI(4,5)P2 were from PerSeptive Biosystems (Framingham, MA). Monoclonal antibody to phosphatidylserine was obtained from ARUP Laboratories (Salt Lake City, UT). Monoclonal anti-PI 4-kinase type II-specific antibody 4C5G was from Upstate Biotechnology (Waltham, MA). Polyclonal anti-p145 CAPS antibody was from T. F. J. Martin (University of Wisconsin, Madison). Phenylarsine oxide was from Calbiochem. All other chemicals were from Sigma. The capacitance measurements were performed at 33°C.
Insulin Release Measurements.
Insulin release from intact mouse pancreatic islets exposed for 1 h to 30 mM K+, 0.2 mM diazoxide (to clamp the membrane potential and study insulin secretion independently of ATP-sensitive K+-channel activity), and 5 or 20 mM glucose were performed as outlined (19).
Sucrose Density Gradient.
Isolated islets (≈500) from ob/ob mice were homogenized in 300 μl of buffer containing 20 mM Hepes, 1 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, and 0.5 μg/ml leupeptin, 0.5 μg/ml aprotinin, 0.5 μg/ml pepstatin, and 0.5 μg/ml antipain. Subcellular fractions were prepared as reported (20). Samples were analyzed on SDS/PAGE by using the Laemmli buffer system. The presence of CAPS and Na+, K+-ATPase was determined by Western blot analysis using polyclonal antibodies. Blots were developed by using ECL (Amersham Biosciences, Piscataway, NJ) as recommended by the manufacturer. Protein content was determined according to ref. 21. Insulin content in each fraction was determined as described (22).
Phosphoinositide Kinase Assay.
Groups of 500 islets were collected in 400 μl of 25 mM TES buffer (pH 6.90) containing (in mM) 5 MgCl2, 1 EGTA, 0.1 DTT, and 10 benzamidine. A homogenate was prepared by sonication (10 s, 40 W) and centrifuged at 100,000 × g (60 min at 4°C). The particulate fraction was solubilized in 400 μl of the same buffer containing 0.125% (vol/vol) Triton X-100 by incubation for 60 min at 4°C. Samples (corresponding to 25 islets) were incubated for 10 min at 30°C in a reaction medium (50 μl) containing 35 mM TES buffer, pH 6.90, 6 mM MgCl2, 1.2 mM EGTA, 0.12 mM DTT, 1 mg/ml phosphoinositide mixture (Sigma) and 50 μM [γ-32P]ATP (2,000–3,000 cpm/pmol). The reaction was terminated by addition of 400 μl of 1M HCl. Lipids were then extracted by addition of 800 μl CHCl3/CH3OH (1:1). The CHCl3 phase was extracted twice with 320 μl CH3OH/1 M HCl (1:1), evaporated, and redissolved in 100 μl CHCl3. For separation of phosphoinositides by TLC, the samples were spotted on heat-activated oxalate-impregnated silica-gel 60 plates together with phosphoinositide standards and developed in CHCl3/CH3OH/H2O/saturated NH3 (50:39:9:5, vol/vol). PI(4)P and PI(4,5)P2 were detected by iodine vapor, and areas corresponding to the standards were scraped into scintillation vials and counted for radioactivity.
Immunohistochemistry.
Rat pancreatic sections were prepared and processed for immunofluorescence histochemistry as described (23). Sections were double-labeled by combining rabbit antiserum to CAPS (diluted 1:300) with mouse monoclonal antibody to insulin (diluted 1:50,000; Sigma) and visualized by using a mixture of Cy5-conjugated donkey anti-rabbit (diluted 1:250) and FITC-conjugated donkey anti-mouse (diluted 1:80) secondary antibodies (Jackson Immuno-Research). Specimens were examined in a Bio-Rad Radiance Plus confocal laser scanning system.
Statistical Analysis.
Results are presented as mean values ± SEM for indicated number of experiments. Statistical significances were evaluated by using Student's t test for paired data or Dunnett's test for multiple comparisons.
Results and Discussion
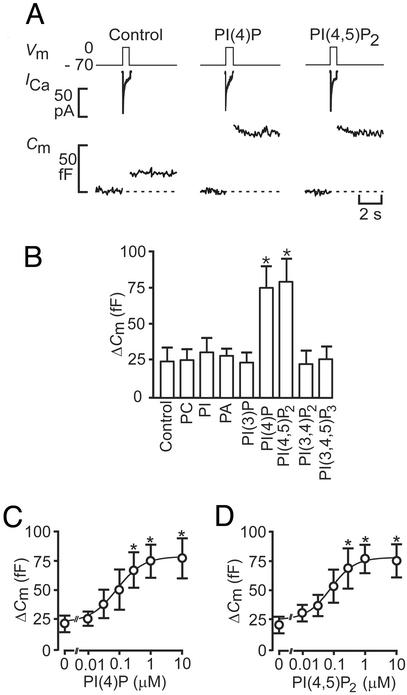
Fig. 1A shows Ca2+ currents and associated changes in cell capacitance elicited by individual 500-ms voltage-clamp depolarizations going from −70 mV to 0 mV. Under control conditions, the depolarization evoked a capacitance increase of 25 fF, corresponding to the release of approximately eight secretory granules using a conversion factor of 3 fF per granule (as expected for granules with a diameter of 350 nm and a specific capacitance of 0.9 μF/cm2; ref. 2). When the same experiment was performed after inclusion of either 1 μM PI(4)P or 1 μM PI(4,5)P2 in the pipette solution, the exocytotic responses amounted to 75 fF. On average, PI(4)P and PI(4,5)P2 (1 μM) produced a 3-fold stimulation of exocytosis (Fig. 1B). The stimulation occurred without any additional increase in the Ca2+ currents; the integrated Ca2+ current averaged 6.9 ± 0.8 pC under control conditions and 7.1 ± 0.7 pC and 7.0 ± 0.8 pC in the presence of PI(4)P and PI(4,5)P2, respectively. Thus, the stimulation must be accounted for by mechanisms other than mere acceleration of Ca2+ influx. The stimulatory action was specific for PI(4)P and PI(4,5)P2 and was not shared by PI 3-phosphate, PI 3,4-bisphosphate, PI 3,4,5-trisphosphate, phosphatidylcholine, PI, or phosphatidic acid, irrespective of whether they were applied at 1 μM (Fig. 1B) or at a 10-fold higher concentration (not shown). The ability of PI(4)P and PI(4,5)P2 to stimulate exocytosis was concentration-dependent (Fig. 1 C and D). The data points (mean values) were approximated to the Hill equation, yielding EC50 values of 81 nM and 75 nM for PI(4)P and PI(4,5)P2, respectively. The cooperativity factors were 1.0 and 1.1 for the mono- and diphosphoinositides.
Figure 1.
PI(4)P and PI(4,5)P2 stimulate exocytosis in β cells. (A) Individual voltage-clamped mouse β cells were stimulated with single 500-ms depolarizations (Vm, Top). The resultant Ca2+ currents (ICa, Middle) and increases in cell capacitance (Cm, reflecting fusion of insulin-containing secretory granules with the plasma membrane) recorded under control conditions and in the presence of 1 μM PI(4)P and 1 μM PI(4,5)P2 are shown. (B) Histogram summarizing the capacitance increases elicited by single voltage-clamp depolarizations from −70 mV to 0 mV under control conditions and after inclusion of 1 μM of phosphatidylcholine (PC), PI, phosphatic acid (PA), PI(4)P, PI(4,5)P2, PI 3,4-bisphosphate [PI(3,4)P2], and PI 3,4,5-trisphosphate [PI(3,4,5)P3]. (C) Capacitance increases (ΔCm) elicited by single voltage-clamp depolarizations recorded in the presence of increasing intracellular concentrations (0–10 μM) of PI(4)P. (D) As in C but using PI(4,5)P2 instead. The curves in C and D represent least-squares fit of the mean values to the Hill equation. Data are quoted as mean values ± SEM of five to seven individual experiments. *, P < 0.05.
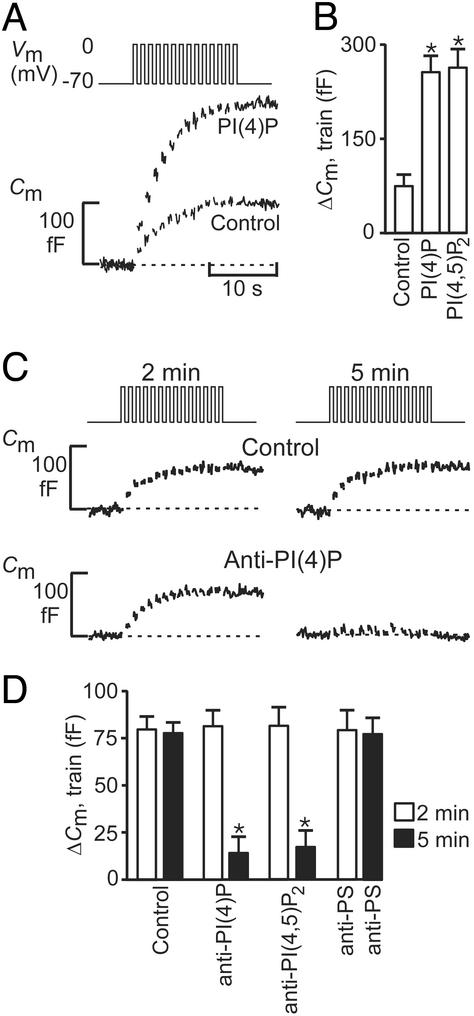
As remarked above, secretory vesicles in a variety of endocrine cell types including the β cell can functionally be divided into a RRP and a reserve pool (24). To determine at which stage PI(4)P and PI(4,5)P2 modulate exocytosis, we next recorded the secretory response elicited by trains of voltage-clamp depolarizations (Fig. 2A). Under control conditions, there was a progressive decline in the amplitude of the exocytotic responses during the train until eventually (after the eighth pulse), depolarization failed to produce any further increase in cell capacitance. The decline in exocytotic capacity we attribute to the depletion of RRP, and the capacitance ceases to increase once this pool is empty. In a series of five experiments, the total capacitance increase evoked by the train amounted to 70 ± 12 fF (Fig. 2B). Using a conversion factor of 3 fF per granule, RRP can be estimated to contain 20–25 granules at the onset of the train. The cessation of exocytosis was not attributable to inactivation of the Ca2+ current because the peak and integrated Ca2+ currents measured at the end of the train were only marginally reduced relative the initial amplitude (<25%; not shown, but see ref. 18). In the presence of 1 μM PI(4)P or 1 μM PI(4,5)P2, the magnitude of the exocytotic responses evoked by the train increased ≈3.5-fold over the control level (Fig. 2B). Again, repetitive stimulation was associated with a progressive decrease in the amplitude of the exocytotic responses, and eventually exocytosis ceased despite continued stimulation. These data suggest that the lipids increase the number of granules belonging to RRP.
Figure 2.
PI(4)P and PI(4,5)P2 are required for refilling of RRP. (A) Individual β cells were stimulated by using a train of fourteen 500-ms depolarizations (Vm, Upper). The associated increases in cell capacitance (Cm, Lower) were measured under control conditions and after inclusion of 1 μM PI(4)P in the pipette solution dialyzing the cell interior. (B) Histogram summarizing the magnitude of the exocytotic responses elicited by trains of depolarizations (Cm, train) under control conditions and in the presence of 1 μM PI(4)P and 1 μM PI(4,5)P2. (C) Exocytosis (Cm) elicited by two trains of depolarizations 2 min (Left) and 5 min (Right) after establishment of standard whole-cell configuration under control conditions (control) and in a different cell after infusion of an antibody directed against PI(4)P [25 μg/ml, anti-PI(4)P]. (D) Histogram summarizing exocytosis elicited by 1st (2 min, open bars) and 2nd train of depolarizations (5 min after reaching whole-cell configuration, filled bars) under control conditions, in the presence of 25 μg/ml anti-PI(4)P, 25 μg/ml of an antibody directed against PI(4,5)P2 [anti-PI(4,5)P2] or 25 μg/ml of an antibody against phosphatidylserine (anti-PS). In B and D, data are quoted as mean values ± SEM of five experiments in each group. *, P < 0.01.
We next studied the effects of PI(4)P on the refilling of RRP. Fig. 2C illustrates an experiment in which two trains of voltage-clamp depolarizations were applied to the same β cell 2 and 5 min after establishment of the whole-cell configuration. It is clear that the capacitance increase evoked by the second train (5 min; 78 ± 11 fF; n = 5) was of the same magnitude as that elicited by the first train (2 min; 82 ± 13 fF; n = 5). Thus, under control conditions, RRP has been fully replenished during the 3-min interval (Fig. 2D). When the same type of experiment was repeated in the presence of a monoclonal antibody directed against PI(4)P (25 μg/ml), the response to the first train (2 min infusion) was close to that observed under control conditions (79 ± 14 fF; n = 5; Fig. 2 C and D), indicating that the antibody did not interfere with exocytosis per se. However, exocytosis during the second train (Fig. 2C) was almost abolished, and the total capacitance increase averaged only 17 ± 9 fF (P < 0.001 vs. the response to the second train in the absence of the antibody; n = 5). Similar data were obtained in the presence of a monoclonal antibody to PI(4,5)P2 (25 μg/ml; Fig. 2D). By contrast, no inhibition was obtained with a monoclonal antibody against phosphatidylserine, demonstrating the specificity of the action to the effects of PI(4)P and PI(4,5)P2. The inhibition by the PI(4)P and PI(4,5)2 antibodies could be overcome by the addition of exogenous PI(4)P and PI(4,5)P2 to the pipette solution (10 μM, data not shown). We conclude that PI(4)P and PI(4,5)P2 act at the level of RRP refilling.
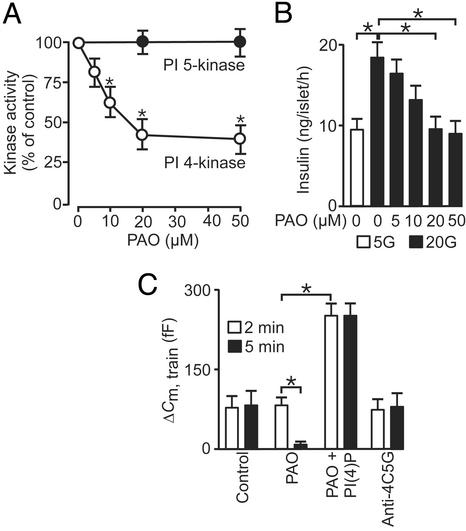
PI 4-kinase has been identified on isolated chromaffin granules and presynaptic vesicles and is specifically inhibited by phenylarsine oxide (PAO; refs. 7 and 25). PAO produced a dose-dependent inhibition of PI 4-kinase activity in β cells with an IC50 of ≈10 μM; 40% of the kinase activity was resistant to PAO, possibly reflecting the presence of additional types of PI 4-kinases. This result is consistent with the identification of two classes of PI 4-kinases (types II and III), which can be distinguished by their sensitivity to PAO (only type III being affected; ref. 26). PAO (20–50 μM) did not affect PI 5-kinase activity (Fig. 3A), suggesting that its action is selective for the PI 4-kinase. The actions of PAO on insulin secretion echoed the effects on PI 4-kinase activity (Fig. 3B). Increasing glucose from 5 to 20 mM in depolarized islets, doubled insulin secretion. This effect was dose-dependently inhibited by PAO, and, at concentrations of ≥20 μM, insulin secretion in the presence of 20 mM glucose was the same as that observed at 5 mM of the sugar (Fig. 3B).
Figure 3.
PI 4-kinase activity is required for RRP refilling and insulin secretion in mouse β cells. (A) PI 4-kinase (open circles) and PI 5-kinase activities (filled circles) measured in islet homogenates in the presence of 0–50 μM PAO. (B) Insulin secretion measured during 1 h in the presence of 30 mM extracellular K+ and 0.2 mM diazoxide at 5 mM (open bar) and 20 mM glucose plus 0–50 μM PAO (filled bars). (C) Histogram summarizing exocytosis elicited by first (2 min, open bars) and second train of depolarizations (5 min after reaching whole-cell configuration, filled bars) under control conditions and in the presence of 20 μM PAO with or without 1 μM of PI(4)P and after inclusion of antibody directed against type II PI 4-kinase (anti-4C5G). Data are presented as mean values ± SEM of four (A), five (B), and five (C) experiments. *, P < 0.01.
We determined the role of the PI 4-kinase in the recruitment of granules for release by using trains of depolarizations applied 2 and 5 min after establishment of the whole-cell configuration (Fig. 3C). Importantly, PAO had no effect on the response to the first train of depolarizations (open bars) but almost fully suppressed exocytosis elicited by the second train (filled bars). The inhibitory action of PAO on exocytosis could be superseded by addition of 1 μM PI(4)P in the pipette solution (Fig. 3C). In fact, in the simultaneous presence of PAO and PI(4)P, exocytosis elicited by the train was the same as that obtained in the presence of PI(4)P alone (compare Fig. 2B). PAO has been reported to inhibit phosphotyrosine phosphatase activity (27). However, it is unlikely that such an effect underlies the suppression on exocytosis because the general phosphotyrosine phosphatase inhibitor vanadyl hydroperoxide (0.5 mM) lacked effect on exocytosis (data not shown). The ability of PAO to suppress exocytosis elicited by repetitive stimulation therefore argues that type III PI 4-kinase activity is required for priming of secretory granules in the β cell. This notion is reinforced by the finding that the monoclonal antibody (4C5G; 50–150 μg/ml), which selectively inhibits type II PI 4-kinase activity (28), was without effect on exocytosis (Fig. 3C).
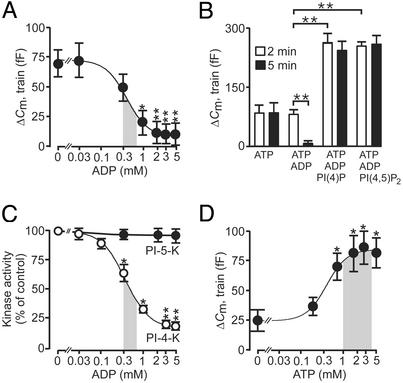
It has previously been reported that elevation of cytoplasmic ADP inhibits exocytosis in β cells (13, 29). We now show that the inhibitory effect of ADP on exocytosis elicited by two trains of 14 voltage-clamp depolarizations is concentration-dependent, with an IC50 of 0.37 mM (Fig. 4A); note that responses to the first train are not affected (Fig. 4B, open bars). These experiments were conducted in the continuous presence of 3 mM ATP. Interestingly, the action of ADP on exocytosis can be antagonized by inclusion of either PI(4)P or PI(4,5)P2 in the intracellular solution (Fig. 4B). We correlated the inhibition of exocytosis produced by ADP to changes in PI 4-kinase activity. ADP inhibited PI 4-kinase activity concentration dependently with an IC50 of 0.58 mM, whereas no effect on PI 5-kinase activity was observed at concentrations up to 5 mM of the nucleotide (Fig. 4C). These data provide strong evidence that ADP interferes with the refilling of RRP, but not exocytosis of granules that have already progressed into this pool, by inhibiting PI 4-kinase activity. Intracellular ADP levels have been shown to decrease from 0.6 mM at 1 mM glucose to 0.3 mM at ≥10 mM glucose (ref. 30; derived by taking the intracellular volume of the β cell to be ≈1 pl). It is pertinent to note that this range of concentrations is in fair agreement with that where the greatest changes of PI 4-kinase activity and exocytosis occur (shaded areas in Fig. 4 A and C). ATP stimulated exocytosis with an EC50 value of 0.56 mM (Fig. 4D). However, it is notable that exocytosis is little affected by variations of the intracellular ATP concentration in the physiological range (1–4 mM, shaded area; ref. 30). These observations raise the interesting possibility that glucose exerts it amplifying action on insulin secretion primarily by lowering cytoplasmic ADP rather than increasing ATP.
Figure 4.
ADP inhibits exocytosis by suppression of PI 4-kinase activity and RRP replenishment. (A) Exocytosis (ΔCm) elicited by the second of two trains of fourteen 500-ms depolarizations applied 2 and 5 min after establishment of standard whole-cell configuration in the presence 0–5 mM Mg-ADP and 3 mM ATP. The responses to the first train were unaffected by the nucleotide. The curve was drawn by approximating the Hill equation to the mean values. The shaded area highlights the physiological range of ADP concentrations observed at low and high glucose (data taken from 30). (B) Histogram summarizing exocytosis elicited by first (2 min, open bars) and second (5 min after establishing whole-cell configuration, filled bars) train of depolarizations under control conditions (3 mM ATP alone), in the presence of 3 mM ATP and 3 mM ADP, in the presence of 3 mM ATP, 3 mM ADP, and 1 μM of either PI(4)P or PI(4,5)P2. (C) PI 4-kinase (open circles) and PI 5-kinase (filled circles) activities measured in islet homogenates in the presence of 0–5 mM ADP. The curve for PI 4-kinase activity was drawn by approximating the Hill equation to the mean values. The shaded area highlights the physiological range of ADP concentrations observed at low and high glucose (data taken from ref. 30). (D) As in A but the experiment was conducted in the presence of 0–5 mM Mg-ATP. The shaded area indicates the range of ATP concentrations measured in β cells at low and high glucose concentrations (data taken from ref. 30). Data are presented as mean values ± SEM of five experiments. *, P < 0.05; **, P < 0.01.
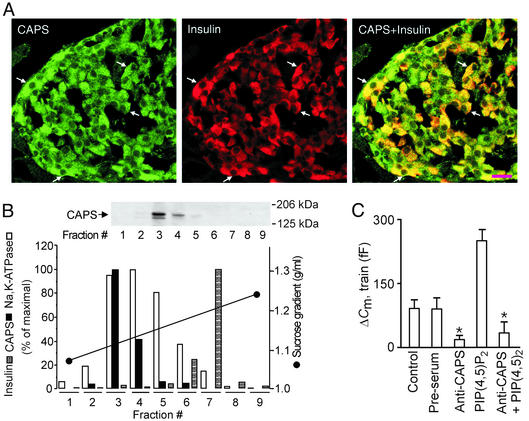
We finally considered the possible molecular mechanisms mediating the effects of ADP on exocytosis in the β cell. In this context, we believe that the ability of ADP to regulate PI 4-kinase activity and thus PI(4)P and PI(4,5)P2 synthesis plays an important role. The protein CAPS, which is required for Ca2+-dependent exocytosis in a variety of neuroendocrine cells (10), has been shown to bind PI(4,5)P2 (8) and may therefore mediate the effects of the lipid on exocytosis in β cells. Double-labeling immunofluorescence histochemistry of pancreatic islets demonstrated CAPS immunoreactivity in all pancreatic islet cells, including insulin-secreting β cells (Fig. 5A). Subcellular fractionation studies of pancreatic islets further revealed that CAPS primarily localized to the plasma membrane-enriched fractions (indicated by accumulation of Na+-K+ ATPase), whereas little immunoreactivity was observed in the secretory granules (insulin; Fig. 5B). These observations are in keeping with previous data from rat brain homogenates indicating that CAPS associates with the plasma membrane (31). Inclusion of an antibody against CAPS (1.5 mg/ml) in the pipette-filling solution strongly inhibited exocytosis elicited by a train of depolarizations applied 2 min after establishment of the whole-cell configuration (Fig. 5C), while not influencing the magnitude of the whole-cell Ca2+ current (data not shown). No effect was observed with preimmune serum (Fig. 5C). The inhibitory action of the CAPS antibody could not be overcome by the inclusion of PI(4,5)P2 (10 μM) in the pipette solution (Fig. 5C). The same was true if the cell was dialysed for 5 min to allow complete wash-in of PI(4,5)P2 before initiation of the experiment (data not shown). These data are consistent with previous studies (9, 32) and support the view that CAPS plays an essential role in Ca2+-dependent exocytosis of primed granules and that PI(4,5)P2 acts at an earlier step. We acknowledge that the exact mechanism(s) by which PI(4,5)P2 and CAPS promote exocytosis remain(s) to be elucidated.
Figure 5.
CAPS is involved in Ca2+- and PI(4,5)P2-dependent exocytosis in β cells. (A) Images of sections of rat endocrine pancreas stained for CAPS (Left) and insulin (Center) and after overlay (Right). CAPS immunoreactivity in single β cells is indicated by arrows. (Scale bar = 20 μm.) (B Upper) Western blot illustrating the presence of CAPS in different subcellular fractions 1–9. (B Lower) Quantitation of the abundance of CAPS (filled bars) and Na+, K+-ATPase α-subunit (plasma membrane fractions, open bars), insulin content (granule-enriched fractions, hatched bars), and the sucrose gradient (black line). The experiment was repeated twice with identical results. (C) Histogram summarizing exocytosis elicited by trains of depolarizations applied 2 min after establishment of the whole-cell configuration under control conditions, in the presence of preimmune serum (preserum; 1.5 mg/ml), after inclusion of an antibody against CAPS (anti-CAPS, 1.5 mg/ml), and the CAPS antibody plus 1 μM of PI(4,5)P2. Data are mean values ± SEM of five experiments. *, P < 0.01.
In β cells, glucose dose-dependently increases ATP and decreases ADP levels. The resulting rise in the ATP at the expense of ADP is an important regulator of the two major signaling pathways involved in glucose-induced insulin secretion. The first one of these pathways uses ATP-sensitive K+-channels to couple glucose metabolism to electrical activity, Ca2+ influx, and initiation of insulin secretion. The second pathway is exerted at the level of granule priming and regulates the β cell secretory capacity by modulation of the granules' release competence (compare ref. 14). Our data raise the interesting possibility that PI 4-kinase plays an important role for glucose-dependent priming in β cells and that the sugar modulates PI(4)P and PI(4,5)P2 synthesis primarily via ADP-dependent regulation of PI 4-kinase activity. The mechanism we outline provides a possible explanation to the defective insulin secretion observed in patients with type 2 diabetes. This form of the disease has been shown to correlate with impaired glucose metabolism leading to reduced production of ATP and a corresponding increase in ADP (33). It is pertinent that cellular ATP content and PI(4)P and PI(4,5)P2 levels, as well as PI 4-kinase activity, have been reported to be reduced in islets from diabetic rats (34). Given the present observations, such an effect can be expected to impair the refilling of RRP, resulting in a reduction in the number of release-competent granules. It is tempting to speculate that such impairment may account for both the complete loss of first-phase insulin secretion and reduction of second-phase insulin secretion, the salient characteristics of the secretion defect seen in patients with type 2 diabetes (5).
Acknowledgments
We thank Dr. T. F. J. Martin (University of Wisconsin) for providing us with CAPS antiserum. This study was supported by the Swedish Research Council, the Royal Swedish Academy of Sciences, the Swedish Diabetes Association, the Novo Nordisk Foundation, the Juvenile Diabetes Foundation International, Åhlén-stiftelsen, Knut and Alice Wallenbergs stiftelse, the National Institutes of Health (Grant DK-5850), the Danish Diabetes Association, and funds of Karolinska Institutet.
Abbreviations
- CAPS
Ca2+-dependent activator protein for secretion
- PI
phosphatidylinositol
- PI(4)P
PI 4-phosphate
- PI(4,5)P2
PI 4,5-bisphosphate
- PAO
phenylarsine oxide
- RRP
readily releasable pool
References
- 1.Dean P M. Diabetologia. 1973;9:115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- 2.Olofsson C S, Gopel S O, Barg S, Galvanovskis J, Ma X, Salehi A, Rorsman P, Eliasson L. Pflügers Arch. 2002;444:43–51. doi: 10.1007/s00424-002-0781-5. [DOI] [PubMed] [Google Scholar]
- 3.Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S. News Physiol Sci. 2000;15:72–77. doi: 10.1152/physiologyonline.2000.15.2.72. [DOI] [PubMed] [Google Scholar]
- 4.Easom R A. Semin Cell Dev Biol. 2000;11:253–266. doi: 10.1006/scdb.2000.0174. [DOI] [PubMed] [Google Scholar]
- 5.Hosker J P, Rudenski A S, Burnett M A, Matthews D R, Turner R C. Metabolism. 1989;38:767–772. doi: 10.1016/0026-0495(89)90064-4. [DOI] [PubMed] [Google Scholar]
- 6.Hay J C, Fisette P L, Jenkins G H, Fukami K, Takenawa T, Anderson R A, Martin T F J. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- 7.Wiedemann C, Schäfer T, Burger M M. EMBO J. 1996;15:2094–2101. [PMC free article] [PubMed] [Google Scholar]
- 8.Loyet K M, Kowalchyk J A, Chaudhary A, Chen J, Prestwich G D, Martin T F J. J Biol Chem. 1998;273:8337–8343. doi: 10.1074/jbc.273.14.8337. [DOI] [PubMed] [Google Scholar]
- 9.Rupnik M, Kreft M, Sikdar S K, Grilc S, Romih R, Zupancic G, Martin T F J, Zorec R. Proc Natl Acad Sci USA. 2000;97:5627–5632. doi: 10.1073/pnas.090359097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walent J H, Porter B W, Martin T F J. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- 11.Weber T, Zemenman B V, McNew J A, Westermann B, Gmachl M, Parlati F, Sollner T H, Rothman J E. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 12.Eliasson L, Renstrom E, Ding W G, Proks P, Rorsman P. J Physiol (London) 1997;503:399–412. doi: 10.1111/j.1469-7793.1997.399bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barg S, Eliasson L, Renström E, Rorsman P. Diabetes. 2002;51:S74–S82. doi: 10.2337/diabetes.51.2007.s74. [DOI] [PubMed] [Google Scholar]
- 14.Henquin J C. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 15.Høy M, Efanov A M, Bertorello A M, Zaitsev S V, Olsen H L, Bokvist K, Leibiger B, Leibiger I B, Zwiller J, Berggren P O, Gromada J. Proc Natl Acad Sci USA. 2002;99:6773–6777. doi: 10.1073/pnas.102157499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson T, Arkhammar P, Hallberg A, Hellman B, Berggren P O. Biochem J. 1987;248:329–336. doi: 10.1042/bj2480329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ämmälä C, Eliasson L, Bokvist K, Larsson O, Ashcroft F M, Rorsman P. J Physiol (London) 1993;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gromada J, Høy M, Renstrom E, Bokvist K, Eliasson L, Gopel S, Rorsman P. J Physiol (London) 1999;518:745–759. doi: 10.1111/j.1469-7793.1999.0745p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buschard K, Høy M, Bokvist K, Olsen H L, Madsbad S, Fredman P, Gromada J. Diabetes. 2002;51:2514–2521. doi: 10.2337/diabetes.51.8.2514. [DOI] [PubMed] [Google Scholar]
- 20.Chibalin A V, Katz A I, Berggren P O, Bertorello A M. Am J Physiol. 1997;273:C1458–C1465. doi: 10.1152/ajpcell.1997.273.5.C1458. [DOI] [PubMed] [Google Scholar]
- 21.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 22.Herbert V, Lau K S, Gottlieb C W, Bleicher S J. J Clin Endocrinol Metab. 1965;25:1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsson G, Bean A J, Scheller R H, Juntti-Berggren L, Deeney J T, Berggren P O, Meister B. Proc Natl Acad Sci USA. 1994;91:12487–12491. doi: 10.1073/pnas.91.26.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neher E. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 25.Wiedemann C, Schäfer T, Burger M M, Sihra T S. J Neurosci. 1998;18:5594–5602. doi: 10.1523/JNEUROSCI.18-15-05594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barylko B, Gerber S H, Binns D D, Grichine N, Khvotchev M, Südhof T, Albanesi J P. J Biol Chem. 2001;276:7705–7708. doi: 10.1074/jbc.C000861200. [DOI] [PubMed] [Google Scholar]
- 27.Levenson R M, Balckshear P J. J Biol Chem. 1989;264:19984–19993. [PubMed] [Google Scholar]
- 28.Endemann G C, Graziani A, Cantley L C. Eur J Biochem. 1991;273:63–66. doi: 10.1042/bj2730063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barg S, Huang P, Eliasson L, Nelson D J, Obermüller S, Rorsman P, Thévenod F, Renström E. J Cell Sci. 2001;114:2145–2154. doi: 10.1242/jcs.114.11.2145. [DOI] [PubMed] [Google Scholar]
- 30.Detimary P, Dejonghe S, Ling Z, Pipeleers D, Schuit F, Henquin J C. J Biol Chem. 1998;273:33905–33908. doi: 10.1074/jbc.273.51.33905. [DOI] [PubMed] [Google Scholar]
- 31.Berwin B, Floor E, Martin T F J. Neuron. 1998;21:137–145. doi: 10.1016/s0896-6273(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 32.Elhamdani A, Martin T F J, Kowalchyk J A, Artalejo C R. J Neurosci. 1999;19:7375–7383. doi: 10.1523/JNEUROSCI.19-17-07375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan A, Chandramouli V, Östenson C-G, Löw H, Landau B R, Efendic S. Diabetes. 1990;39:456–459. doi: 10.2337/diab.39.4.456. [DOI] [PubMed] [Google Scholar]
- 34.Morin L, Giroix M-H, Portha B. Biochem Biophys Res Commun. 1996;228:573–578. doi: 10.1006/bbrc.1996.1700. [DOI] [PubMed] [Google Scholar]