Abstract
Acidic pH plays an important role in various pathophysiological states and has been demonstrated to be carcinogenic in animal models. Recent studies have also implicated acidic pH in the development of preneoplastic Barrett's esophagus in human. However, little is known about the molecular mechanism underlying acidic pH-induced carcinogenesis. In the current study, we show that acidic pH, like the topoisomerase II (TOP2) poison VP-16 (demethylepipodophyllotoxin ethylidene-β-d-glucoside), induces tumors in 9,10-dimethyl-1,2-benzanthracene(DMBA)-initiated mice. The following studies in tissue culture models have suggested that acidic pH acts like a TOP2 poison to induce TOP2-mediated DNA damage: (i) acidic pH induces TOP2-dependent DNA damage signals as evidenced by up-regulation of p53 and Ser-139 phosphorylation of H2AX [a substrate for ataxia telangiectasia mutated (ATM)/ATM and Rad3-related (ATR) kinases]; (ii) acidic pH-induced cytotoxicity in tumor cells is reduced in TOP2-deficient cells; (iii) acidic pH increases the mutation frequency of the hypoxanthine phosphoribosyl transferase (HPRT) gene in a TOP2-dependent manner; and (iv) acidic pH induces reversible TOP2-mediated DNA strand breaks in vitro. We discuss the possibility that TOP2-mediated DNA damage may contribute to acidic pH-induced carcinogenesis.
Acidic pH plays an important role in cell death during various pathophysiological states, including ischemia and cancer (1–3). In addition to cell death, acidic pH has been suggested to be involved in carcinogenesis. For example, Barrett's esophagus (BE), which is a preneoplastic disorder of the esophagus (4), has been tightly linked to gastroesophageal acid reflux (5). Studies in rats have also demonstrated that duodenal-content reflux esophagitis induces the development of glandular metaplasia and adenosquamous carcinoma (6). In addition, acid has been shown to promote carcinogenesis in the hamster cheek pouch in combination with the tumor initiator 9,10-dimethyl-1,2-benzanthracene (DMBA) (7).
Despite the importance of acidic pH in carcinogenesis, little is known about its underlying molecular mechanism. Several studies have suggested that acidic pH may induce DNA damage in cells. For example, in cultured Chinese hamster cells, acidic pH induces chromosome aberrations including sister-chromatid exchanges and chromatid-type breaks and gaps (8, 9). Acidic pH, like DNA damaging agents, also up-regulates p53 and induces p53-dependent apoptosis in human adenoma and carcinoma cells (2, 10). These studies suggest that acidic pH may induce DNA damage, which could contribute to its carcinogenic activity.
Human topoisomerase II (TOP2) isozymes are molecular targets for many antitumor drugs (e.g., doxorubicin, etoposide (VP-16), mitoxantrone, and amsacrine; refs. 11–13). These antitumor drugs, referred to as TOP2 poisons, are highly efficient in inducing TOP2-mediated DNA damage in cells. They interfere with the breakage/religation reactions of TOP2 by stabilizing the transient covalent reaction intermediates, the TOP2–DNA covalent complexes, often referred to as TOP2 cleavable or cleavage complexes (14). TOP2-mediated DNA damage is known to trigger tumor cell death (15), and induce extensive gene deletion and rearrangements (16). Patients treated with the TOP2 poison VP-16 exhibit a high frequency of secondary acute myeloid leukemia (AML), primarily due to rearrangement of the MLL gene (17, 18).
Previous studies have demonstrated that both calf thymus and Drosophila TOP2s induce DNA cleavage at pH below 7.0, with an optimum about 5 (19, 20). In the current study, we show that TOP2 is involved in acidic pH-induced DNA damage, mutagenesis, and cytotoxicity. We discuss the possibility that TOP2-mediated DNA damage may contribute to acidic pH-induced carcinogenesis.
Materials and Methods
Chemicals and Cell Lines.
4-Demethylepipodophyllotoxin thenylidene-β-d-glucoside (teniposide; VM-26) was obtained as a gift from the Bristol-Myers Squibb. 4,4-(2,3-Butanediyl)-bis(2,6-piperazinedione) (ICRF-193) was purchased from ICN. All other chemicals were purchased from Sigma. These compounds were dissolved in DMSO. The HL-60 cell line and its mitoxantrone-resistant (TOP2-deficient) variant, HL-60/MX2, were obtained from ATCC (American Type Culture Collection). Human breast cancer cell lines MDA-MB-231, T47D, and their etoposide-resistant cell lines (MDA-MB-231/3000 and T47D/VP1, respectively) were kindly provided by T. Fojo (Medicine Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda). Purification of recombinant human TOP2 isozymes was performed following the published procedure (21). Cells were maintained in RPMI medium 1640 supplemented with 10% FCS. Different pH media (pH 4.0, 4.5, 5.0, 6.0, and 7.5) were buffered by citrate phosphate (15 mM). Antibodies against a synthetic peptide consisting of the last nine amino acids (KATQASQEY) of H2AX with phospho-Serine-139 were obtained from D. Chen (Lawrence Berkeley National Laboratory, Berkeley, CA).
Immunoblotting Analysis.
Cells were subjected to different treatment conditions, and then lysed with 100 μl of 2× SDS sample buffer. Proteins were separated in 10% SDS/PAGE gel and electrophoretically transferred onto a nitrocellulose membrane. All membranes were Ponceau-stained to confirm equal protein loading. The membrane was blocked with 5% milk for 1 h. Immunoblotting was performed by using monoclonal mouse antibody against human p53 (Ab-6) (Oncogene) or antibodies against phosphorylated H2AX. The secondary antibodies were incubated for 1 h at room temperature. Bound secondary antibodies were detected by using the ECL Western procedure (Pierce).
TOP2-Mediated DNA Cleavage Assay.
TOP2-mediated DNA cleavage assay was performed by using purified recombinant human TOP2 (10 ng each) and 3′-end 32P-labeled YEpG DNA (20 ng each) in a reaction (20 μl each) containing 148 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 10 mM MgCl2, 2 mM CaCl2, and 20 mM citrate phosphate (or Hepes) with different pHs. Incubation was carried out at 37°C for 30 min. In reversal experiments, the incubated reactions were subjected to a second incubation at different temperatures, in excess EDTA (10 mM at 37°C) or with pH adjusted to neutrality (pH 7.5 at 37°C) for another 30 min. The reactions were terminated by the addition of 5 μl of 5% SDS and 1 mg/ml proteinase K, and incubated for an additional 60 min at 37°C. DNA samples were electrophoresed in 1% agarose gel containing 0.5× TPE buffer. Gels were then dried onto Whatman 3MM chromatographic paper and autoradiographed at −80°C by using Kodak XAR-5 films.
Clonogenic Assay.
For attached cells, 250 cells per well were plated in six-well plates and cultured overnight. Cells were treated with different agents for 30 min, washed, and replenished with fresh medium. After 2 wk, cells were stained with 10 mg/ml methylene blue (Sigma) in 50% methanol, and the colony number was counted. For suspension culture (i.e., HL-60 and HL-60/MX2 cells), cells were treated with different agents for 30 min. Subsequently, 250 cells were mixed with 0.3% agar (Sigma, A-9915) and plated on top of 0.5% agar in six-well plates. After 2 wk, colonies were stained with 1% p-iodonitrotetrazolium violet (Sigma) and counted.
Mutation Frequency Measurement.
MDA-MB-231 cells were preselected for 4 days in RPMI medium 1640 supplemented with the HAT medium to remove preexisting hypoxanthine phosphoribosyl transferase (HPRT) mutants. Cells (1 × 106) were then seeded in 100-mm dishes for 24 h before treatment with VM-26 (0.5 μM), camptothecin (CPT; 0.5 μM), or a pH 6.0 medium for 1 h in the presence or absence of ICRF-193 (25 μM). Treated cells were trypsinized and plated in 150-mm dishes. Cells were incubated for 7 days to allow the wild-type HPRT protein to turnover in mutant HPRT cells before 2-amino-6-mercaptopurine (6-thioguanine) (6-TG) selection. 6-TG selection was performed by adding 5 μg/ml 6-TG to cells, followed by plating in 100-mm dishes. After 21 days, cells were stained with 10 mg/ml methylene blue (Sigma) in 50% methanol, and the colony number was counted. For cloning efficiency, 250 cells were plated in medium without 6-TG, and the colony number was counted after 14 days of incubation.
Carcinogenesis Assay Using the Mouse Skin Model.
Female CD-1 mice (3–5 wk old) were used in the carcinogenesis study (22, 23). The back of each mouse was shaved 2 days before different treatments. The tumor initiator DMBA (600 nmol in 100 μl of DMSO) was applied only once during the first week. One hundred microliters of phorbol 12-tetradecanoate 13-acetate (TPA; 10 nmol in 100 μl DMSO), VP-16 (5 μmol in 100 μl DMSO), or acidic pH medium (250 mM citrate phosphate, pH 2.5, in RPMI medium 1640) was applied twice every week. Papillomas appearing on the skin were recorded every week. In the second experiment, a group of five mice were treated with VP-16 (5 μmol in 100 μl of DMSO) twice weekly for 10 wk. At the end of the 10th week, a single application of DMBA (600 nmol in 100 μl of DMSO) was performed. Tumor formation was measured at the end of the 16th week.
Results
Acidic pH Induces Carcinogenesis in DMBA-Initiated Mice.
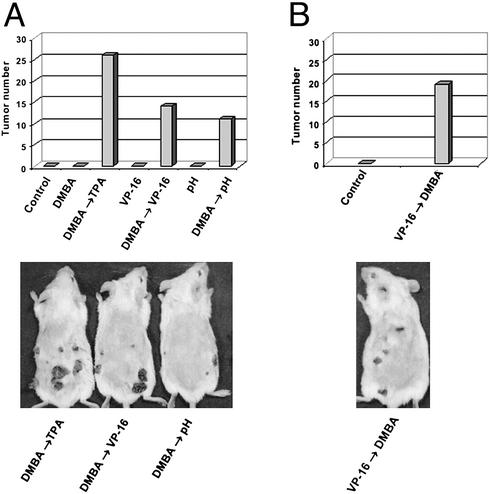
We have tested whether acidic pH can induce tumors in the mouse skin carcinogenesis model. As shown in Fig. 1A, application of acidic pH buffer (100 μl of 250 mM citrate phosphate, pH 2.5) to the mouse skin twice a week for 16 wk resulted in no tumor formation. However, if the mice were initiated with a single application of DMBA followed by repeated application of acidic pH, tumor formation was readily observable (11 papillomas in a group of five mice). As expected, a single application of DMBA by itself resulted in no tumor formation. Interestingly, the prototypic TOP2 poison VP-16 behaved similarly to acidic pH. Repeated application of VP-16 (5 μmol in 100 μl, twice weekly for 16 wk) resulted in no tumor formation. However, a single application of DMBA followed by repeated application of VP-16 resulted in significant tumor formation (14 papillomas in a group of five mice; Fig. 1A). In this case, we have switched the order of application. We found that, whether DMBA was applied before (Fig. 1A) or after (Fig. 1B) VP-16 application, similar numbers of tumors were observed (14 vs. 19 pappilomas). As a positive control, DMBA treatment followed by repeated application of the tumor promoter TPA was shown to result in 26 papillomas in a group of five mice (Fig. 1A). These studies suggest that, like VP-16, acidic pH is potentially carcinogenic.
Figure 1.
Acidic pH and VP-16 induce tumors in the mouse skin carcinogenesis model. (A) Acidic pH and VP-16 induce papillomas in DMBA-initiated mice. Groups of five mice were treated with different drugs (in DMSO) or pH 2.5 medium as described in Materials and Methods. DMBA (600 nmol in 100 μl of DMSO) was applied only once during the first week. TPA (10 nmol in 100 μl of DMSO), VP-16 (5 μmol in 100 μl of DMSO), and pH 2.5 medium (250 mM citrate phosphate-buffered RPMI medium 1640) were applied twice every week for 16 wk. The total number of papillomas appearing on the mouse skin in each group was measured. (B) DMBA induces papillomas in VP-16-initated mice. A group of five mice were treated with VP-16 (5 μmol in 100 μl of DMSO) twice weekly for 10 wk. At the end of the 10th week, a single application of DMBA (600 nmol in 100 μl of DMSO) was performed. Tumor formation was measured at the end of the 16th week.
Acidic pH Induces p53 Up-Regulation and Ser-139 Phosphorylation of H2AX.
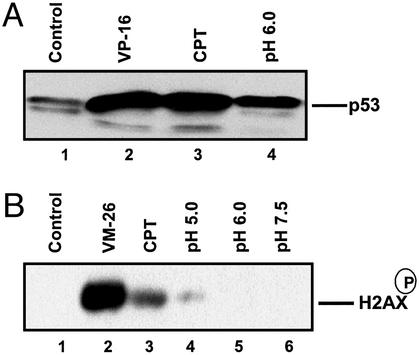
Previous studies have demonstrated that acidic pH induces up-regulation of p53 in human glioblastoma cells (10). In the current study, we show that acidic pH also induces up-regulation of p53 in breast cancer ZR75-1 cells. As shown in Fig. 2A, incubation of ZR75-1 cells in pH 6.0 medium for 1 h induced up-regulation of p53. As positive controls, both VP-16 (a TOP2 poison) and CPT (a TOP1 poison) were shown to induce more pronounced up-regulation of p53. In addition to p53, ataxia telangiectasia mutated (ATM)/ATM and Rad3-related (ATR) family kinases are also important DNA damage signaling molecules (24). ATM/ATR kinases are known to phosphorylate a large number of substrates, including H2AX, which is phosphorylated at serine 139 (25, 26). As shown in Fig. 2B, incubation of mouse embryo fibroblast (MEF) cells in RPMI medium 1640 (pH 5.0) for 1 h resulted in detectable phosphorylated H2AX. A more pronounced increase of phosphorylated H2AX was observed in MEF cells treated with VM-26 (10 μM) and CPT (10 μM). These results suggest that acidic pH induces DNA damage signals.
Figure 2.
Acidic pH induces up-regulation of p53 and phosphorylation of H2AX. (A) Acidic pH induces up-regulation of p53. Breast cancer ZR75-1 cells were treated with VP-16 (25 μM), CPT (25 μM), or RPMI medium 1640 (pH 6.0) for 1 h. Treated cells were lysed directly with SDS sample buffer and prepared for immunoblotting by using anti-p53. (B) Acidic pH induces phosphorylation of H2AX. Mouse embryo fibroblast (MEF) cells were treated with VM-26 (10 μM), CPT (10 μM), and different pH media for 1 h. Cells were then lysed and processed for detection of the phosphorylated H2AX by immunoblotting using antibodies against the phosphorylated H2AX epitope.
Acidic pH-Induced DNA Damage Signaling and Cytotoxicity Is Reduced in TOP2-Deficient Cells.
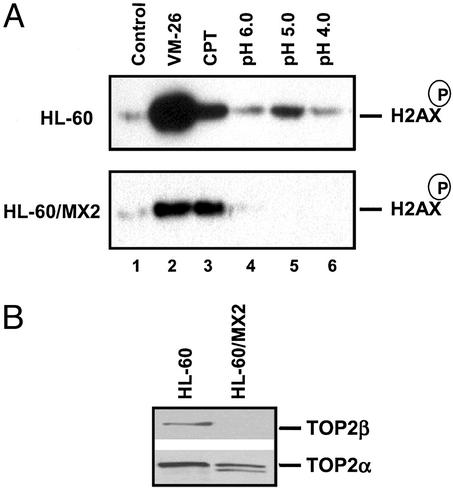
To determine whether acidic pH-induced DNA damage signaling involves TOP2, acidic pH-induced phosphorylation of H2AX in a pair of TOP2 wild-type (HL-60) and TOP2-deficient cells (HL-60/MX2) was investigated (see Fig. 3B for TOP2 isozyme levels in HL-60 and HL-60/MX2; refs. 27 and 28). As shown in Fig. 3A, incubation of HL-60 cells in RPMI medium 1640 (pH 5.0) for 1 h resulted in increased phosphorylation of Ser-139 of H2AX. RPMI medium 1640 (pH 4.0 or pH 6.0) was not as effective as RPMI medium 1640 (pH 5.0). Importantly, acidic pH (pH 5.0)-induced phosphorylation of H2AX was significantly diminished in TOP2-deficient HL-60/MX2 cells, suggesting that acidic pH-induced DNA damage signaling is TOP2-mediated. As a positive control, VM-26-induced phosphorylation of H2AX was shown to be similarly reduced in HL-60/MX2 cells as compared with HL-60 cells. By contrast, CPT (a TOP1 poison)-induced phosphorylation of H2AX was little changed in HL-60/MX2 cells as compared with HL-60 cells, suggesting that the reduced DNA damage signaling was specific for TOP2-mediated DNA damage.
Figure 3.
Acidic pH-induced H2AX phosphorylation is defective in a TOP2 mutant cell line. (A) HL-60 and HL-60/MX2 cells were treated with VM-26 (10 μM), CPT (10 μM), and different pH media for 1 h. Cells were then lysed and processed for detection of the phosphorylated H2AX. (B) HL-60 and HL-60/MX2 cells were lysed and processed for detection of TOP2α and TOP2β.
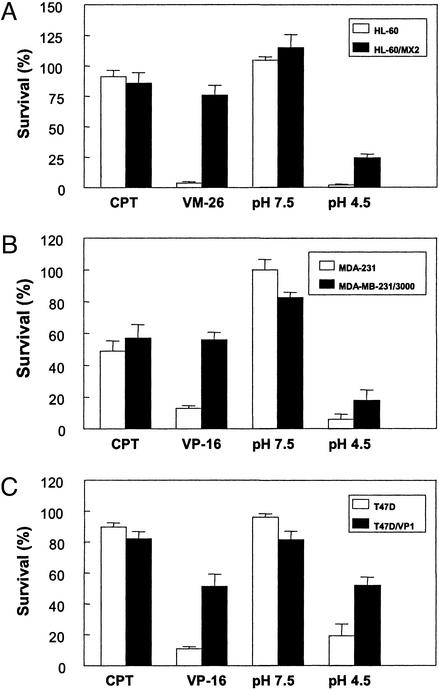
To determine whether acidic pH-induced cytotoxicity is also TOP2-mediated, acidic pH-induced cytotoxicity was measured in several pairs of wild-type and TOP2-deficient cells, HL60 vs. HL-60/MX2, MDA-MB-231 vs. MDA-MB-231/3000, and T47D vs. T47D/VP1. If TOP2-DNA covalent complexes are responsible for acidic pH-induced cytotoxicity, these TOP2-deficient cells are expected to be cross-resistant to acidic pH. As shown in Fig. 4A, TOP2-deficient MDA-MB-231/3000 cells were more resistant to a brief 30-min treatment of an acidic pH medium (pH 4.5) as measured by a clonogenic assay. VM-26, a TOP2 poison, and CPT, a TOP1 poison, served as positive and negative controls, respectively. VM-26-induced cytotoxicity was similarly reduced in TOP2-deficient MDA-MB-231/3000 cells whereas CPT-induced cytotoxicity was little affected (Fig. 4A). Similarly, TOP2-deficient T47D/VP1 and HL-60/MX2 cells were shown to be more resistant to a brief 30-min treatment of an acidic medium (pH 4.5) than their respective parental cells (T47D and HL-60 cells) as determined by clonogenic assays (Fig. 4 B and C). These results suggest that acidic pH-induced cytotoxicity, like acidic pH-induced DNA damage signaling, involves TOP2.
Figure 4.
Acidic pH cytotoxicity is reduced in TOP2-deficient cells. Cytotoxicity was measured by using the clonogenic assay as described in Materials and Methods. (A) Acidic pH cytotoxicity is reduced in TOP2-deficient MDA-MB-231/3000 cells. MDA-MB-231 and TOP2-deficient MDA-MB-231/3000 (attached cells) were treated with 50 μM VP-16, 1 μM CPT, pH 4.5 medium, or pH 7.5 medium for 30 min. Cells were then replenished with drug-free neutral medium and incubated for ≈2 wk. Clonogenic survival was measured by counting colonies on plates. (B) Acidic pH cytotoxicity is reduced in TOP2-deficient T47D/VP1 cells. T47D and TOP2-deficient T47D/VP1 cells (attached cells) were treated with 50 μM VP-16 or pH 4.5 medium for 30 min. Cells were then incubated in drug-free neutral medium for 2 wk. Clonogenic survival was also measured as described in A. (C) Acidic pH cytotoxicity is reduced in TOP2-deficient HL-60/MX2 cells. HL-60 and TOP2-deficient HL-60/MX2 cells (suspension cells) were treated with 2 μM VM-26 or pH 4.5 medium for 30 min. Cells were then resuspended in soft agar (in drug-free neutral medium), and clonogenic survival in soft agar was measured after ≈2 wk as described in Materials and Methods.
Acidic pH-Induced Mutagenesis Is Antagonized by a TOP2 Catalytic Inhibitor ICRF-193.
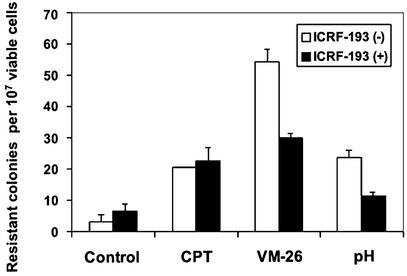
Resistance to 6-TG, which is primarily due to mutations in the HPRT gene, has been used extensively for measuring the mutation frequency in mammalian cells (29). As shown in Fig. 5, a brief exposure (1 h) of MDA-MB-231 breast cancer cells to VM-26 (0.5 μM), CPT (0.5 μM), or acidic pH (pH 6.0) medium resulted in an increase in 6-TG resistance. To test whether acidic pH (and VM-26)-stimulated mutagenesis is TOP2-mediated, the TOP2 catalytic inhibitor ICRF-193 (25 μM) was used to antagonize the action of TOP2. As shown in Fig. 5, ICRF-193 was able to antagonize the mutagenic activity of both VM-26 and acidic pH, but not CPT. In the case of VM-26 and acidic pH, the number of 6-TG resistant colonies was reduced by ≈50% in the presence of ICRF-193 (Fig. 5). These results suggest that acidic pH-induced mutagenesis also involves TOP2.
Figure 5.
Acidic pH increases 6-TG resistance. MDA-MB-231 cells were treated with 0.5 μM VM-26, 0.5 μM CPT, pH 6.0 medium, or pH 7.4 medium (control) for 1 h in the presence or absence of ICRF-193 (25 μM), followed by recovery in RPMI medium 1640 (pH 7.4) for 7 days. Selection with 6-TG was performed as described in Materials and Methods.
Acidic pH Induces Reversible TOP2-Mediated DNA Strand Breaks in Vitro.
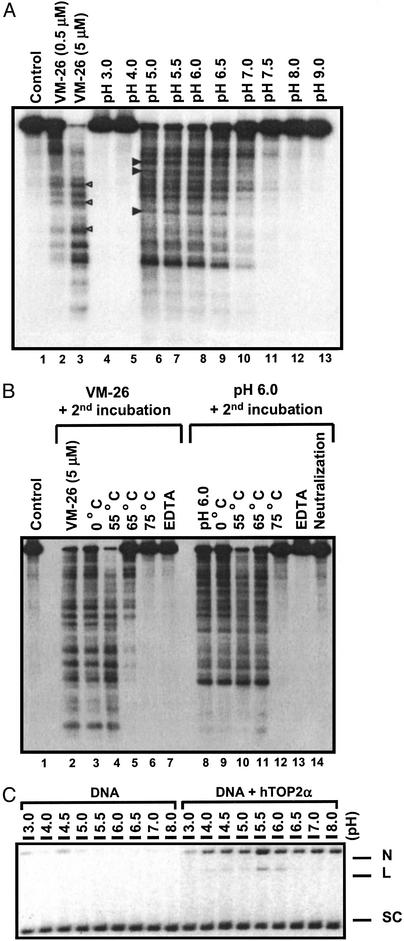
To test whether acidic pH induces TOP2-mediated DNA damage, we have performed DNA cleavage assay using purified human TOP2 isozymes and 32P-end-labeled linearized plasmid DNA. As shown in Fig. 6A, extensive cleavage of DNA into small DNA fragments occurred between pH 5.0 and 7.0 in the presence of human TOP2α. Above pH 7.5 or below pH 4.0, little DNA cleavage was detectable. The strongest cleavage was observed at pH 5.0, consistent with the results obtained with both calf thymus and Drosophila TOP2s (19, 20). Similar results were obtained with human TOP2β (data not shown). Unlike clinically useful TOP2 poisons such as VP-16 and doxorubicin, which are stimulated 30- to 100-fold by 1 mM ATP (30), acidic pH-induced DNA cleavage was insensitive to ATP (data not shown). The DNA cleavage pattern, which is reflective of DNA cleavage specificity, was also slightly different between acidic pH- and VM-26-induced DNA cleavages (the differences were marked by arrow heads on the side of the cleavage lanes in Fig. 6A).
Figure 6.
Acidic pH induces TOP2-mediated DNA breaks in vitro. (A) Acidic pH induces TOP2-mediated DNA cleavage in vitro. 3′-end 32P-labeled linearized YEpG DNA and hTOP2α were incubated in reactions with different pHs (lanes 4–13) for 30 min at 37°C as described in Materials and Methods. Reactions were terminated with SDS/proteinase K. VM-26 (0, 0.5, and 5 μM) was used as control in a standard cleavage reaction at pH 7.5 (lanes 1–3). (B) Acidic pH-induced DNA cleavage is reversible. DNA cleavage in the presence of hTOP2α was performed as described in A at pH 6.0 (lanes 8–14), pH 7.5 (control), or pH 7.5 with 5 μM VM-26 (lanes 2–7). Before termination, the reactions were subjected to a second incubation under different reversal conditions (indicated on top of each lane) for 30 min. The reversal conditions for the second incubation include different temperatures (lanes 3–6 and 9–12), neutralization of the reactions to pH 7.5 (lane 14), and excess EDTA (10 mM; lanes 7 and 13). (C) Acidic pH induces both single- and double-stranded DNA breaks. Supercoiled pBluescript SK(+) DNA was used to react with hTOP2α in various pH conditions as described in A. The reactions were terminated by SDS/proteinase K and subjected to electrophoresis in 1.0% agarose gel containing 0.5 μg/ml ethidium bromide. N, nicked DNA; L, linear DNA; SC, supercoiled DNA.
Like DNA cleavages induced by TOP2 poisons, acidic pH-induced DNA cleavage was reversible. As shown in Fig. 6B, neutralization of the reaction (compare lane 8 with lane 14), addition of excess EDTA (10 mM EDTA final concentration; compare lane 8 with lane 13), or shifting the temperature from 37°C to 75°C (compare lane 8 with lane 12) in a second incubation (30 min) before termination of the reaction with 1% SDS resulted in essentially no cleavage. As a control, VM-26-induced DNA cleavage was shown to be reversed in a second incubation with either excess EDTA (compare lane 2 with lane 7) or at 75°C (compare lane 2 with lane 6). The reversibility of the DNA cleavage is the hallmark of topoisomerase cleavage complexes (31). These results thus suggest that acidic pH stabilizes reversible TOP2 cleavage complexes in vitro. Previous studies have demonstrated that acidic pH-induced DNA breaks are primarily single-strand breaks (20). As shown in Fig. 6C, acidic pH induced both single-strand breaks as evidenced by the nicked DNA (marked N) and double-strand breaks as evidenced by the linear DNA (marked L). At pH 5.5, TOP2-mediated DNA cleavage generated more nicked DNA than linear DNA (Fig. 6C), suggesting that single-strand breaks are the predominant product of acidic pH-induced DNA damage.
Discussion
Acidic pH is known to cause genomic instability and is causally linked to carcinogenesis (8, 9, 32). In the current study, we have shown that acidic pH can induce tumors in DMBA-initiated mice.
To study the molecular basis for acidic pH-induced carcinogenesis, we have investigated the possibility that acidic pH may induce DNA damage in tissue culture models. Indeed, acidic pH, like DNA damaging agents, up-regulates p53 in breast cancer ZR75-1 cells. This result is consistent with a previous study in which acidic pH (pH 6.5) has been shown to up-regulate p53 in human glioblastoma cells (10). The possibility that acidic pH induces DNA damage is further supported by our studies on ATM/ATR family kinases. Acidic pH was shown to activate ATM/ATR family kinases as evidenced by increased phosphorylation on Serine 139 of histone H2AX (26). It is particularly interesting to note that the optimal pH for activation of ATM/ATR kinases was pH 5.0. These studies were performed by adjusting the extracellular pH (pHe) in the tissue culture medium. Intracellular pH (pHi) is known to be regulated by multiple mechanisms when cells are exposed to a nonphysiological pHe environment (33–35). The influence of pHe on pHi has been well studied. For example, when HL-60 cells are exposed to acidic pH medium in the range of 6.1 to 6.8 (pHe), the pHi in HL-60 cells has been determined to range from 6.5 to 7.2 (about 0.4 unit higher than pHe) within 1 h (36). Although the pHi was not determined in our studies, it seems likely that the observed DNA damaging signals were due to intracellular acidification.
Acidic pH-induced DNA damage signaling seems to involve TOP2 because acidic pH-induced activation of ATM/ATR kinases (as evidenced by phosphorylation of H2AX at Serine 139) was significantly reduced in TOP2-deficient HL-60/MX2 cells. Studies on acidic pH-induced cytotoxicity have also provided indirect support for a role of TOP2 in acidic pH-induced DNA damage. In three independent TOP2-deficient cell lines (HL-60/MX2, MDA-MB-231/3000, and T47D/VP1), acidic pH was shown to induce significantly greater cytotoxicity in their corresponding wild-type cells. All these studies have pointed to a role for TOP2 in mediating acidic pH-induced DNA damage.
Our studies have also suggested that TOP2-mediated DNA damage contributes to acidic pH-induced genomic instability. We have demonstrated in the current study that both acidic pH and VM-26 stimulated mutagenesis as measured by 6-TG resistance. Most strikingly, acidic pH- and VM-26-stimulated mutagenesis was antagonized by ICRF-193, a TOP2 catalytic inhibitor known to antagonize TOP2 cleavage complex formation (37). By contrast, CPT-stimulated mutagenesis was not affected by ICRF-193. These results are consistent with the notion that acidic pH-stimulated mutagenesis is in part due to the formation of TOP2 cleavage complexes.
All these results can be explained by the formation of TOP2 cleavage complexes in acidotic cells. We have made numerous attempts using different techniques [e.g., the in vivo complex of enzyme (ICE) and band depletion methods; refs. 38 and 39] to directly demonstrate the presence of TOP2 cleavage complexes in acidic pH-treated cells. However, we have been unable to demonstrate reproducibly and convincingly the presence of TOP2 cleavage complexes in cells possibly due to the low level of TOP2 cleavage complexes induced by acidic pH. As an alternative, we have studied TOP2 cleavage complex formation in vitro by using purified TOP2. Our studies using purified hTOP2α have demonstrated that acidic pH in the range of 5.0 to 7.0 can induce TOP2-mediated DNA cleavage. The possibility that DNA depurination may contribute to acidic pH-induced DNA cleavage (40, 41) was ruled out because acidic pH-treated DNA (under our assay conditions) did not induce DNA cleavage in a subsequent incubation with TOP2 at neutral pH (data not shown). The result from our reversal experiment in which pH-induced DNA cleavage was completely abolished on neutralization also supports this conclusion. The reversibility of DNA cleavage suggests that the acidic pH-induced DNA cleavage most certainly reflects the formation of TOP2 cleavage complexes. The simplest explanation for acidic pH-induced DNA cleavage is that the religation reaction of TOP2 is inhibited by acidic pH. The pH profile of TOP2-mediated DNA cleavage could suggest the involvement of a histidine residue. We have mutated the nonessential histidine, His-795 (42), which is located near the active site tyrosine (Tyr-805) of TOP2α, into alanine, and found the pH profile of TOP2-mediated DNA cleavage to be unchanged. Clearly, further studies are necessary to establish the molecular basis for acidic pH-induced formation of TOP2 cleavage complexes.
Based on our in vitro studies, we can estimate the efficiency of acidic pH in trapping TOP2 cleavage complexes as compared with VM-26. The amount of TOP2 cleavage complexes trapped in a pH 5.0 reaction buffer is about the same as that trapped by 1 μM VM-26 in the absence of ATP (Fig. 6A). In the presence of ATP (which is a closer approximation to the in vivo situation), the amount of TOP2 cleavage complexes trapped by VM-26 is stimulated 30- to 100-fold (30) whereas that trapped by acidic pH is minimally affected (this study). Consequently, acidic pH may trap the same amount of TOP2 cleavage complexes as 10–30 nM VM-26. Neither the band depletion assay nor the ICE method has the sensitivity to detect TOP2 cleavage complexes induced by 10–30 nM VM-26.
Our carcinogenesis studies have shown that both VP-16 and acidic pH induce tumors in DMBA-initiated mice. Considering their differential potency in inducing TOP2 cleavage complexes, their comparable potency in inducing tumors is surprising. This result could suggest that acidic pH may induce tumors by a different mechanism (e.g., increased reactive oxygen species production and/or altered calcium homeostasis) than VP-16. On the other hand, the dose-response of VP-16 in inducing tumors has not been determined. Being both an anticancer drug and a carcinogen, the dose-response of VP-16 in tumor formation may be complex. The concentration of VP-16 used in our current study could have exceeded the optimal concentration for stimulation of carcinogenesis. Consequently, the comparison between the potency of VP-16 and acidic pH might not be relevant. The acidic pH used in our carcinogenesis studies is quite low (pH 2.5). However, the exposure of the susceptible cells in the mouse skin to this low pH solution is likely to be quite transient because the solution dries out within a few minutes. The exact pHi in the susceptible cells on the mouse skin cannot be properly estimated. Consequently, the potential role of TOP2 in carcinogenesis needs to be rigorously investigated. The conditional TOP2 knockout mice could be useful for establishing the role of TOP2 in acidic pH-induced carcinogenesis.
We have shown that neither VP-16 (also acidic pH) nor DMBA alone can induce tumors in mice. However, VP-16 (also acidic pH) induces large numbers of tumors in DMBA-initiated mice. It seems that VP-16 and acidic pH behave as tumor promoters in the mouse skin carcinogenesis model. In Fig. 1B, we have also shown that the treatment sequence of VP-16 and DMBA is not important in stimulating the formation of tumors, a result distinctly different from that of tumor promoters. However, tumor promotion has been subdivided into stage-I and stage-II (43). In contrast to the stage-II tumor promotion, which is associated with increased cell proliferation, the stage-I tumor promotion is irreversible and may be due to permanent alteration of the genetic materials (44). In addition, tumor formation is not affected by the reversal of the sequence of treatment with the initiator and the stage-I tumor promoter (45). It seems possible that VP-16 and acidic pH may act as stage-I tumor promoters.
DMBA, a tumor initiator, is known to be a strong point mutagen and is associated with oncogenic ras mutations (46). On the other hand, VP-16 is known to induce deletions and DNA rearrangements (47). Based on our current results, acidic pH, being a TOP2 poison, is likely to be a rearrangement mutagen. It will be interesting to test whether DNA sequence rearrangements could be the underlying mechanism for stage-I tumor promotion. Clearly, further studies are necessary to establish the molecular mechanism of TOP2-mediated carcinogenesis.
Our results obtained in mice could be relevant to the etiology of BE. BE is tightly associated with gastroesophageal acid reflux (5). Based on our results, acid reflux could introduce mutations to cells in the esophagus due to acidic pH-induced DNA damage. However, acid reflux also causes inflammatory responses that are known to contribute to carcinogenesis. It will be interesting to determine whether acidic pH-induced DNA damage plays an important role in the development of BE.
Acknowledgments
We are grateful to Dr. David Chen for providing us with anti-H2AX antibodies, and Dr. Tito Fojo for some of the TOP2-deficient cell lines. This work was supported by National Institutes of Health Grants GM27731 and CA39662.
Abbreviations
- TOP2
topoisomerase II
- VM-26
4-demethylepipodophyllotoxin thenylidene-β-d-glucoside (teniposide)
- VP-16
demethylepipodophyllotoxin ethylidene-β-d-glucoside (etoposide)
- CPT
camptothecin
- DMSO
dimethyl sulfoxide
- ICRF-193
4,4-(2,3-butanediyl)-bis(2,6-piperazinedione)
- 6-TG
2-amino-6-mercaptopurine (6-thioguanine)
- DMBA
9,10-dimethyl-1,2-benzanthracene
- TPA
phorbol 12-tetradecanoate 13-acetate
- HPRT
hypoxanthine phosphoribosyl transferase
- pHi
intracellular pH
- pHe
extracellular pH
- BE
Barrett's esophagus
- ATM
ataxia telangiectasia mutated
- ATR
ATM and Rad3-related
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kubasiak L A, Hernandez O M, Bishopric N H, Webster K A. Proc Natl Acad Sci USA. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams A C, Collard T J, Paraskeva C. Oncogene. 1999;18:3199–3204. doi: 10.1038/sj.onc.1202660. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto D, Kiyozuka Y, Uemura Y, Yamamoto C, Takemoto H, Hirata H, Tanaka K, Hioki K, Tsubura A. J Cancer Res Clin Oncol. 2000;126:191–197. doi: 10.1007/s004320050032. [DOI] [PubMed] [Google Scholar]
- 4.Chevfec G, Schnell T, Sontag S. Am J Clin Pathol. 1992;98:5–7. doi: 10.1093/ajcp/98.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen N, Ransohoff D F. J Am Med Assoc. 2002;287:1972–1981. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 6.Pera M, Brito M J, Poulsom R, Riera E, Grande L, Hanby A, Wright N A. Carcinogenesis. 2000;21:1587–1591. [PubMed] [Google Scholar]
- 7.Adams J, Heintz P, Gross N, Andersen P, Everts E, Wax M, Cohen J. Arch Otolaryngol Head Neck Surg. 2000;126:405–409. doi: 10.1001/archotol.126.3.405. [DOI] [PubMed] [Google Scholar]
- 8.Morita T, Nagaki T, Fukuda I, Okumura K. Mutat Res. 1992;268:297–305. doi: 10.1016/0027-5107(92)90235-t. [DOI] [PubMed] [Google Scholar]
- 9.Morita T. Mutat Res. 1995;334:301–308. doi: 10.1016/0165-1161(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsubo T, Wang X, Takahashi A, Ohnishi K, Saito H, Song C W, Ohnishi T. Cancer Res. 1997;57:3910–3913. [PubMed] [Google Scholar]
- 11.Wang J C. Nat Rev Mol Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 12.Li T K, Liu L F. Annu Rev Pharmacol Toxicol. 2001;41:53–77. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Liu L F. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- 14.D'Arpa P, Liu L F. Biochim Biophys Acta. 1989;989:163–177. doi: 10.1016/0304-419x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- 15.Ritke M K, Rusnak J M, Lazo J S, Allan W P, Dive C, Heer S, Yalowich J C. Mol Pharmacol. 1994;46:605–611. [PubMed] [Google Scholar]
- 16.Chen C L, Fuscoe J C, Liu Q, Pui C H, Mahmoud H H, Relling M V. J Natl Cancer Inst. 1996;88:1840–1847. doi: 10.1093/jnci/88.24.1840. [DOI] [PubMed] [Google Scholar]
- 17.Felix C A. Med Pediatr Oncol. 2001;36:525–535. doi: 10.1002/mpo.1125. [DOI] [PubMed] [Google Scholar]
- 18.Lovett B D, Strumberg D, Blair I A, Pang S, Burden D A, Megonigal M D, Rappaport E F, Rebbeck T R, Osheroff N, Pommier Y G, et al. Biochemistry. 2001;40:1159–1170. doi: 10.1021/bi002361x. [DOI] [PubMed] [Google Scholar]
- 19.Liu L F, Rowe T C, Yang L, Tewey K M, Chen G L. J Biol Chem. 1983;258:15365–15370. [PubMed] [Google Scholar]
- 20.Zechiedrich E L, Christiansen K, Andersen A H, Westergaard O, Osheroff N. Biochemistry. 1989;28:6229–6236. doi: 10.1021/bi00441a014. [DOI] [PubMed] [Google Scholar]
- 21.Wasserman R A, Austin C A, Fisher L M, Wang J C. Cancer Res. 1993;53:3591–3596. [PubMed] [Google Scholar]
- 22.Brooks G, Evans A T, Aitken A, Evans F J. Carcinogenesis. 1989;10:283–288. doi: 10.1093/carcin/10.2.283. [DOI] [PubMed] [Google Scholar]
- 23.Chouroulinkov I, Lasne C, Lowy R, Wahrendorf J, Becher H, Day N E, Yamasaki H. Cancer Res. 1989;49:1964–1969. [PubMed] [Google Scholar]
- 24.Shiloh Y. Curr Opin Genet Dev. 2001;11:71–77. doi: 10.1016/s0959-437x(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 25.Ward I M, Chen J. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 26.Burma S, Chen B P, Murphy M, Kurimasa A, Chen D J. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 27.Harker W G, Slade D L, Drake F H, Parr R L. Biochemistry. 1991;30:9953–9961. doi: 10.1021/bi00105a020. [DOI] [PubMed] [Google Scholar]
- 28.Harker W G, Slade D L, Parr R L, Holguin M H. Cancer Res. 1995;55:4962–4971. [PubMed] [Google Scholar]
- 29.Kulling S E, Metzler M. Food Chem Toxicol. 1997;35:605–613. doi: 10.1016/s0278-6915(97)00022-7. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Mao Y, Zhou N, Hu T, Hsieh T S, Liu L F. J Biol Chem. 2001;276:15990–15995. doi: 10.1074/jbc.M011143200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, D'Arpa P, Liu L F. Cancer Cells. 1990;2:23–27. [PubMed] [Google Scholar]
- 32.LeBoeuf R A, Kerckaert G A. Carcinogenesis. 1986;7:1431–1440. doi: 10.1093/carcin/7.9.1431. [DOI] [PubMed] [Google Scholar]
- 33.Clausen T. Can J Physiol Pharmacol. 1992;70,Suppl.:S219–S222. doi: 10.1139/y92-265. [DOI] [PubMed] [Google Scholar]
- 34.Deitmer J W, Rose C R. Prog Neurobiol. 1996;48:73–103. doi: 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 35.Hackam D J, Grinstein S, Rotstein O D. Shock. 1996;5:17–21. doi: 10.1097/00024382-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Park H J. Yonsei Med J. 1995;36:473–479. doi: 10.3349/ymj.1995.36.6.473. [DOI] [PubMed] [Google Scholar]
- 37.Ishida R, Miki T, Narita T, Yui R, Sato M, Utsumi K R, Tanabe K, Andoh T. Cancer Res. 1991;51:4909–4916. [PubMed] [Google Scholar]
- 38.Subramanian D, Kraut E, Staubus A, Young D C, Muller M T. Cancer Res. 1995;55:2097–2103. [PubMed] [Google Scholar]
- 39.Desai S D, Liu L F, Vazquez-Abad D, D'Arpa P. J Biol Chem. 1997;272:24159–24164. doi: 10.1074/jbc.272.39.24159. [DOI] [PubMed] [Google Scholar]
- 40.Sabourin M, Osheroff N. Nucleic Acids Res. 2000;28:1947–1954. doi: 10.1093/nar/28.9.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cline S D, Jones W R, Stone M P, Osheroff N. Biochemistry. 1999;38:15500–15507. doi: 10.1021/bi991750s. [DOI] [PubMed] [Google Scholar]
- 42.Okada Y, Ito Y, Kikuchi A, Nimura Y, Yoshida S, Suzuki M. J Biol Chem. 2000;275:24630–24638. doi: 10.1074/jbc.M003243200. [DOI] [PubMed] [Google Scholar]
- 43.Slaga T J, Klein-Szanto A J, Fischer S M, Weeks C E, Nelson K, Major S. Proc Natl Acad Sci USA. 1980;77:2251–2254. doi: 10.1073/pnas.77.4.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lahiri-Chatterjee M, Katiyar S K, Mohan R R, Agarwal R. Cancer Res. 1999;59:622–632. [PubMed] [Google Scholar]
- 45.Kinzel V, Furstenberger G, Loehrke H, Marks F. Carcinogenesis. 1986;7:779–782. doi: 10.1093/carcin/7.5.779. [DOI] [PubMed] [Google Scholar]
- 46.Dandekar S, Sukumar S, Zarbl H, Young L J, Cardiff R D. Mol Cell Biol. 1986;6:4104–4108. doi: 10.1128/mcb.6.11.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchetti F, Bishop J B, Lowe X, Generoso W M, Hozier J, Wyrobek A J. Proc Natl Acad Sci USA. 2001;98:3952–3957. doi: 10.1073/pnas.061404598. [DOI] [PMC free article] [PubMed] [Google Scholar]