Abstract
bir-1, a Caenorhabditis elegans inhibitor-of-apoptosis gene homologous to Survivin is organized in an operon with the transcription cofactor C. elegans SKIP (skp-1). Because genes arranged in operons are frequently linked functionally, we have asked whether BIR-1 also functions in transcription. bir-1 inhibition resulted in multiple developmental defects that overlapped with C. elegans SKIP loss-of-function phenotypes: retention of eggs, dumpy, movement defects, and lethality. bir-1 RNA-mediated interference decreased expression of several gfp transgenes and the endogenous genes dpy-7 and hlh-1. Immunoblot analysis revealed decreased phosphoacetylated histones in bir-1 RNA-mediated interference-treated worms. In a heterologous transfection system, BIR-1 augments thyroid hormone-regulated transcription and has an additive effect with SKIP. These results show that BIR-1 functions in the regulation of transcription and development.
Modulation of specific gene transcription depends on orchestrated events involving the modification of chromatin and cooperation between RNA polymerase II and supporting factors. A large number of events participate in this process including the assembly of RNA polymerase II active complex on particular promoters, cooperation and modification of TFII transcription factors, and interactions with numerous cofactors (for review see ref. 1). Modification of chromatin proteins such as acetylation, methylation, and phosphorylation further influences transcription activation or repression and represents another level of regulation (2–6). Regulation of gene transcription includes complex chromatin reorganization. During this process, histones in promoter regions as well as proteins involved in transcription complex are modified covalently. Modification of histones H3 and H4 includes methylation, acetylation, and phosphorylation of N-terminal residues. A growing number of cofactors and specific enzymes with methyltransferase, acetyltransferase, and aminotransferase activity were shown recently also to precede acetylation and contribute to the activation of transcription (3, 7, 8). Phosphorylation of histones is part of mitotic chromosome condensation but was shown recently also to precede acetylation and contribute to the activation of transcription (9–13).
The Caenorhabditis elegans baculoviral inhibitor-of-apoptosis repeat protein 1 (bir-1) gene in C. elegans encodes a homolog of the human gene Survivin (14, 15). Survivin in mammals functions as an inhibitor of apoptosis (16). In C. elegans, bir-1 has only been linked thus far to spindle midzone formation and cytokinesis (14, 15). Our previous work noted that bir-1 is expressed from an operon with the transcription cofactor SKI-binding protein (SKIP; skp-1) (17). In C. elegans, genes expressed from an operon are often functionally linked (18, 19), suggesting that bir-1 may also have a transcriptional function related to C. elegans SKIP (CeSKIP). CeSKIP is indispensable for C. elegans development, and its loss-of-function phenotype partially overlaps with that of nuclear hormone receptor CHR3 (nhr-23) (17). CeSKIP and bir-1 are expressed strongly during embryonic development and continue to be expressed in certain postmitotic cells to adulthood (17). Furthermore, bir-1 can mediate phosphorylation of histone H3 on serine 10 (15), a phosphorylation that is involved in promoter activation (6, 9, 10, 13). Together, these data suggested that BIR-1 may have a transcriptional function unrelated to cell division.
In this study, we report evidence that BIR-1 is a transcriptional regulator for numerous target genes. We show that the loss of function of bir-1 results in phenotypes that partially overlap with CeSKIP and CHR3 (nhr-23) loss of function. We also show that several transgenes (elt-2∷gfp, hlh-1∷gfp, and hlh-2∷gfp) and two endogenous genes (dpy-7 and hlh-1) are inhibited as a consequence of bir-1 loss of function. Western blot analysis using antibodies against phosphoacetylated histone H3 (S10-P K14-Ac and K9-Ac S10-P) shows that bir-1 inhibition in worms negatively affects phosphoacetylation of histone H3. Finally, we demonstrate that BIR-1 acts as coactivator in a heterologous transfection system, and bir-1-transfected cells have increased phosphoacetylated histones H3. Thus our results show that BIR-1, a member of the inhibitor-of-apoptosis gene family, functions in transcription regulation and is involved in regulation of development.
Methods
Strains.
The following strains expressing GFP fusion proteins were used: JM 63 (elt-2∷gfp) integrated line (20, 21); PD7963 (hlh-1∷gfp) integrated line; PD8097 (hlh-2 ∷gfp) (22, 23); egl-15∷gfp (24), Nde∷gfp (25), and hlh-8∷gfp (26); his2B∷gfp (27), a gift from G. Seydoux (Johns Hopkins University, Baltimore); and PD 6904 (let-858∷gfp), a gift from W. Kelly (Emory University, Atlanta). As a wild type, C. elegans Bristol strain was used and maintained as described (28).
bir-1 RNA-Mediated Interference (RNAi).
The RNAi was introduced by feeding bacteria producing double-stranded (ds)RNA to worms (feeding method) and microinjection of dsRNA into the gonad of young adult hermaphrodites (microinjection method) (29, 30).
The full-length bir-1 genomic sequence was amplified by PCR and cloned into the L4440 vector. The plasmid (4851) was transformed into HT115 Escherichia coli and induced by isopropyl-β-D-galactoside as described (30). The same construct was used for preparation of dsRNA and used for microinjections as described (29, 31). The injected larvae were transferred into new plates at 12-h intervals. The embryos were scored after 12 h of incubation, and the larvae were followed for the next 3–7 days.
The reporter genes elt-2∷gfp, hlh-1∷gfp, hlh-2∷gfp, hlh-8∷gfp, his2B∷gfp, and let-858∷gfp were used to assay the expression of transgenes. Animals were fed bacteria producing bir-1 dsRNA or transformed with empty vector. For transcription induction, the plates contained 4 mM isopropyl-β-D-galactoside, and both cultures were induced with 4 mM isopropyl-β-D-galactoside for 4 h. Approximately 50 animals from each transgenic line were screened for expression of particular transgene with an Olympus (Tokyo) BX60 microscope.
Gene-Expression Assays.
The animals were fed with bacteria producing bir-1 dsRNA or empty vector. Approximately 10,000 worms were used for each experiment. The worms were grown from synchronized L1 stage for one or two generations on 2% agarose plates and collected as young adults just when some started to lay embryos. RT-PCR was performed from total RNA as described (32, 33). The dpy-7, elt-2, and hlh-1 genes were assayed. These experiments were done independently three times in duplicate, and each was assayed by PCR several times. For Western blot analysis, synchronized L1 stage animals were grown for two generations on nematode growth medium plates and collected as young adults. Protein extracts were prepared by using standard protocol with Laemmli buffer. Protein content was estimated by using the BCA protein assay kit (Pierce), and 20 μg of protein were loaded per lane. For immunodetection of phosphoacetylated histones, antibodies specific for S10-P K14-Ac (07-081) (Upstate Biotechnology, Charlottesville, VA) and K9-Ac S10-P (9711S) (Cell Signaling Technology, Beverly, MA) were used at a 1:1,000 dilution. Anti-rabbit secondary antibodies labeled by peroxidase (Sigma) were used at a 1:5,000 dilution. The ECL plus chemiluminescent system (Amersham Pharmacia) was used for detection. Films were scanned and analyzed by using the program NIH IMAGE (www.nih.gov).
Immunohistochemistry.
Worms were kept on plates seeded with bacteria producing bir-1 dsRNA or empty plasmid. Larvae and young adults were transferred onto glass slides coated with polylysine, frozen on dry ice, and fixed shortly by methanol and acetone. Antibodies against phosphoacetylated histones were used at a 1:100 dilution and secondary antibody labeled by fluorescein at a 1:200 dilution. Worms were photographed by using an Olympus BX60 microscope.
Bir-1 Overexpression and Ectopic Expression.
bir-1 genomic sequence was amplified by PCR and cloned into heat-shock promoter vector pPD 49.83 (a kind gift from Andrew Fire, Carnegie Institution of Washington, Baltimore). Transgenic lines were prepared by microinjection, and overexpression was induced by incubating animals at 34°C for 2 h.
Cell Cultures and Transient Transfections.
bir-1 cDNA was cloned into pCDNA3 expression vector (Invitrogen). Flag-SKIP was a gift from Diane Hayward (Johns Hopkins School of Medicine, Baltimore) (34). Human telomerase RNA β1 and murine retinoid X receptor α (mRXRα) were gifts from Paul Yen (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health). MCF-7 (human breast cancer cell line) and cos-7 (green monkey kidney cells) cells were obtained from the American Tissue Culture Collection and maintained as recommended. Briefly, minimum essential medium was supplemented with 10% FCS (Life Technologies, Grand Island, NY) and gentamycin (100 μg/ml) and incubated in 5% CO2 atmosphere at 37°C. For transfections, medium was stripped from thyroid hormone receptor (THR) by using a Bio-Rad AG 1-X8 (200–400 mesh) anion-exchange resin similarly as described (35). Transfections were performed by using FuGENE (Roche) as recommended: the proportions were calculated as for three 24-well plate wells (one 35-mm petri dish) with ratios: 400 ng of reporter plasmid, 150 ng of human THRβ1, mRXRα, flag-SKIP, and bir-1, and 15 ng of control cytomegalovirus-Renilla luciferase vector. Empty vectors and plasmid pBluescript SK(−) were used to substitute for receptor, bir-1, or flag-SKIP. Plasmid pBluescript was also used to maintain the total amount of DNA constant (1 μg or 1,115 ng in some experiments) and 3 μl of FuGENE. Where possible, all plasmids were mixed first as a master mix and split after mixing to minimize inequalities. All experiments were done at least twice and in triplicate or quadruplicate. After preparation of transfection suspensions and appropriate incubations, the medium with hormone-depleted serum and with gentamycin was added, mixed, and 1 ml of transfection medium was overlaid over cells. After a 24-h incubation, the medium was removed and exchanged for fresh medium supplemented with 10−6 M triiodothyronine (T3, Sigma) dissolved in ethanol as 10−3 M stock solution or with ethanol only. Trichostatine A (TSA) was dissolved also in ethanol and used at final concentration 10 nM. Cells were harvested 24 h later and used for the dual luciferase method (Promega) as recommended by the manufacturer. The human telomerase RNA β1, mRXRα, F2 thyroid-responsive construct, and DR4 construct were kind gifts from Paul Yen, and ME-TRE was a kind gift from Keiko Ozato (National Institute of Child Health and Human Development, National Institutes of Health, Bethesda). bir-1 was amplified from total RNA prepared from mixed stages of C. elegans and cloned into pCDNA3 expression vector.
For immunodetection of phosphoacetylated histones in bir-1-transfected cells, MCF-7 cells were transfected with bir-1 pCDNA3 or empty pCDNA3 vector. Ratios were as for transcription assay [150 ng of bir-1 pCDNA3 or empty vector, pBluescript SK(−] to fill the DNA content to 1 μg per 35-mm petri dish). Cells were incubated for 24 h and processed for Western blot analysis.
Results
C. elegans bir-1 Loss of Function Leads to Developmental Defects Independent of Its Known Cell-Division Functions.
Loss of bir-1 activity in C. elegans by RNAi leads to early embryonic arrest due to cell-division defects (14, 15). To bypass this lethality and assay BIR-1 functions postembyronically, we treated larvae with bir-1 RNAi by using a bacterial feeding method. Several developmental defects were observed in treated animals (Fig. 1). bir-1 RNAi resulted in an egg-laying (Egl) phenotype in 80% of young adult animals (n = 3,408) that became nearly fully penetrant in older adults. bir-1 RNAi-treated hermaphrodites laid 50% the normal number of embryos compared with control animals (n = 20 for each group). The vulva and egg-laying muscles were formed normally suggesting a neuronal defect as the cause of the Egl phenotype in these bir-1 RNAi adults (Fig. 2 B–D).
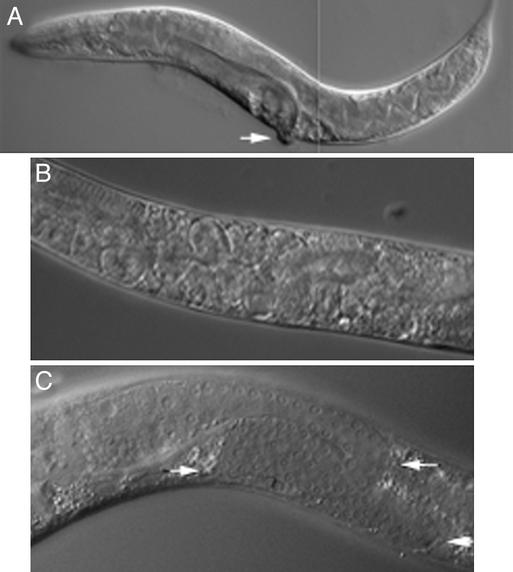
Figure 1.
Developmental defects caused by bir-1 inhibition. (A) An affected animal with dumpy (short and thick) phenotype after treatment with bir-1 RNAi. Protrusion of the egg-laying organ is indicated by an arrow. (B) Retention of embryos in the uterus of an adult hermaphrodite treated by bir-1 RNAi. Note the multinucleate embryos. (C) Defective growth of germ line in bir-1 RNAi-treated animals: The germ line makes two turns (arrows), and its growth is elongated. The position of the DTC is marked (arrowhead).
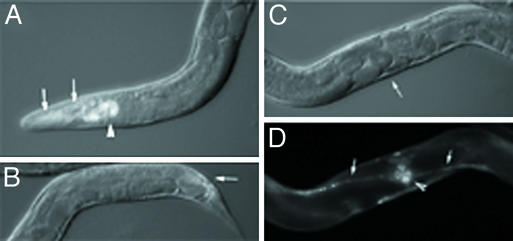
Figure 2.
bir-1 RNAi does not influence the expression of CeSKIP∷gfp. (A) An animal expressing the CeSKIP∷gfp transgene after treatment with bir-1 RNAi. The expression of CeSKIP is not decreased by bir-1 RNAi. The strong expression is in pharynx (arrowhead), neurons, epidermal cells (arrows) in head region, and in ventral neuronal cord. (B) Distal part of the same animal showing expression of CeSKIP∷gfp in neurons, epidermal cells, and intestinal muscles. Note the properly developed intestinal muscles expressing CeSKIP∷gfp (arrow). (C) The presence of Egl phenotype and multinucleate embryos in the same animal as shown in A documenting the developmental defects caused by bir-1 RNAi (Nomarski optics). The arrow indicates the position of the egg-laying organ shown in D. (D) The strong expression of CeSKIP∷gfp in ventral neuronal cord (arrows) and in egg-laying muscles (arrowhead), which are also normally developed.
A short and fat dumpy (Dpy) phenotype was also observed in a fraction (n = 992, 29%) of larvae at L3 and L4 stage and young adult animals when L1 larvae were treated with bir-1 RNAi (Fig. 1A). If larvae L2 were treated with bir-1 RNAi, the effect was less pronounced and visible later during adulthood (n = 208, 26%). In animals showing the pronounced Dpy phenotype the movement was also affected. The worms showed slow uncoordinated (Unc) movement. Non-Dpy animals also had movement defects after bir-1 RNAi that consisted of larger-than-normal amplitudes of body curvature.
There was also a germ-line defect in 20% of bir-1 RNAi-treated animals (n = 680). Wild-type adult hermaphrodites have two germ-line-filled gonad arms arranged symmetrically above the vulva. During the L2–L4 larval stages, the gonads form by elongation of the germ-line and somatic gonad primordium. At the leading edge of each elongating gonad arm is a distal tip cell (DTC), the migration path of which dictates the shape of the gonad. After the L3 molt, the DTCs U-turn and continue to grow in opposite directions, resulting in two symmetrically reflexed gonads. The gonads in bir-1 RNAi-treated animals often made multiple turns (usually three) during elongation (Fig. 1C). The elongation defect appeared to be stochastic, and the growth of only one gonad arm was affected in any given animal. Cells composing egg laying and anal muscles were normally developed and observed on transgenic lines expressing CeSKIP∷gfp (Fig. 2).
Interestingly, the DTCs did not have defects in cell division as might be expected from the known BIR-1 function in cytokinesis, suggesting that only a DTC migration defect was responsible for the abnormal gonads.
There were two additional defects observed in bir-1 RNAi-treated animals that were present less frequently. In 10% (n = 344) of bir-1 RNAi-treated animals, there was an intestinal defect consisting of gut distention. A similar percentage of animals had a protruding vulva (Pvul) phenotype (Fig. 1A).
bir-1 Inhibition Negatively Affects Gene Expression.
If bir-1 is required for gene expression, we reasoned that it might be possible to see changes in transgene expression after bir-1 RNAi. To test this, we used strains expressing GFP fusion proteins from specific promoters and assayed expression after feeding animals bacteria producing bir-1 dsRNA. As reported previously, bir-1 RNAi of adults results in 100% embryonic lethality at the 30-cell (or less) stage. To assay transgene expression in these arrested embryos, we used hlh-2∷gfp, which would be expected to be expressed in all cells at this stage. The effect of bir-1 RNAi on embryos was observed on progeny of hermaphrodites fed by bacteria producing dsRNA. In contrast to the ubiquitous expression observed in untreated embryos, hlh-2∷gfp was observed in only four to six cells of bir-1 RNAi-arrested embryos. It should be noted that the cells of arrested embryos were not normal, and the nucleus was irregular in morphology, suggesting that loss of hlh-2∷gfp expression was secondary to early cytokinesis defects reported previously (14, 15).
Because of problems with interpretation of gene expression in arrested embryos, we focused on postembryonic expression in bir-1 RNAi-treated animals. We used elt-2∷gfp and hlh-1∷gfp transgenes to assay larval expression. elt-2∷gfp is an intestinal-specific reporter gene that we used as a marker for transcription in developed larvae. In controls the transgene is expressed in nuclei of all 20 intestinal cells. In bir-1 RNAi experiments, the fluorescence was visible in four to six nuclei in the proximal part of the intestine and in one or two nuclei in the distal part of the intestine. In the remaining cells the fluorescence was decreased strongly or disappeared, which is consistent with the inhibition of transcription (Fig. 3 A and B). The hlh-1∷gfp is expressed in all body wall muscles during larval development and in adults (Fig. 3C). The expression of this transgene decreased in bir-1 RNAi-treated animals (Fig. 3D), although not as dramatically as seen with elt-2∷gfp.
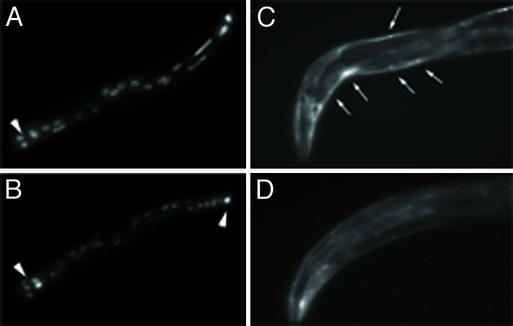
Figure 3.
The effect of bir-1 inhibition on gene expression. (A) A part of the body of L4 larva showing the expression of elt-2∷gfp in intestinal cells. The bulbus of pharynx (arrowhead) and normal expression of intestinal cells are marked. (B) Decreased fluorescence of the elt-2∷gfp transgene is shown in most intestinal cells of a similar larva L4 treated by bir-1 RNAi. The expression of elt-2∷gfp is not changed in six nuclei in proximal part of the intestine and in one cell in distal part (arrowheads). (C) The normal expression of hlh-1∷gfp transgene in body wall muscles (arrows) in head and proximal part of the body of a young adult hermaphrodite. (D) Expression of the hlh-1∷gfp transgene in an animal treated with bir-1 RNAi is decreased markedly.
bir-1 Inhibition Affects the Expression of Endogenous Genes.
To determine whether the expression of endogenous genes could be affected by bir-1 RNAi we treated ≈10,000 larvae with bacteria expressing bir-1 RNAi and tested the expression of the endogenous genes hlh-1 and dpy-7. The expression of dpy-7 decreased >50% compared with controls, and hlh-1 expression was also lowered in bir-1 RNA-treated animals (approximately one-fifth of control animals).
As a complementary approach to bir-1 inhibition by RNAi, we wanted to know whether overexpression of bir-1 would have any effect in C. elegans. We prepared transgenic lines carrying bir-1 regulated by a heat-shock promoter and examined animals after heat shock for any visible phenotypes. There were no visible phenotypic changes in worms overexpressing bir-1.
BIR-1 Augments Transcription from Thyroid Hormone-Regulated Promoters.
Our results in C. elegans suggested that bir-1 activity was required for normal gene expression and development. To provide additional evidence that BIR-1 could indeed affect transcription we used a cell culture system in which gene expression could be assayed more easily. Because no immortal cell line exists for C. elegans, we opted for a mammalian cell-culture assay with THRs regulating the expression of a luciferase reporter gene. THRs are powerful transcription factors that both activate or repress the responsive promoters. Both activation (in the presence of the ligand T3) and repression are easily detectable in transfection experiments (36, 37). MCF-7 (human breast cancer cell line) and cos-7 (green monkey kidney cells) cells have very low levels of endogenous functional THRs in Western blot analysis (data not shown). For transfection experiments, we amplified bir-1 from C. elegans total RNA by PCR and cloned it into a pCDNA3 expression vector.
We assayed thyroid hormone responsiveness in this cell line in the absence or presence of cotransfected C. elegans bir-1. In the MCF-7 cancer cell line, cotransfections of bir-1 increased both basal as well as T3/THR-induced transcription from thyroid-responsive promoters (Fig. 4A). The basal transcription increase was ≈20–50%. The positive effect of BIR-1 was similar in T3/THR-induced transcription. BIR-1 enhanced also TSA-induced and TSA/thyroid hormone-induced transcription (Fig. 4B). TSA is a strong histone deacetylase inhibitor that was shown to increase transcription from viral as well as thyroid hormone-regulated promoters (38). To test whether SKIP cooperates with BIR-1 in transfection experiments, we used a human homologue cloned in expression vector with flag sequence (a kind gift from Diane Hayward) that was shown to function in transfection experiments (34) and was more likely to interact with mammalian cofactors in transcription assay. SKIP had an additional positive effect on T3-induced transcription (Fig. 4C). The transcription stimulation effect of BIR-1 and SKIP and their additive effect was observed also in cos-7 cells (Fig. 4 D and E).
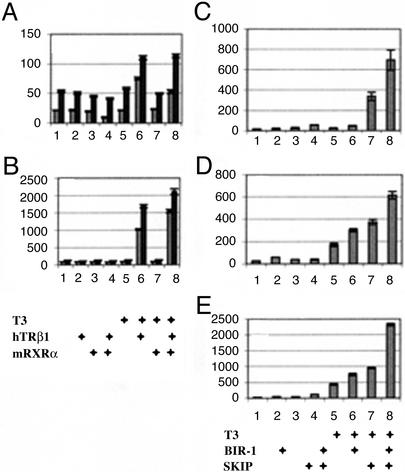
Figure 4.
bir-1 augments thyroid hormone-dependent transcription in a heterologous transfection system. (A) Cotransfection of bir-1 enhances basal as well as thyroid hormone-induced transcription in MCF-7 cells. Experiments cotransfected with empty vector are indicated by light columns, and experiments cotransfected with bir-pCDNA3 are indicated by dark columns. Experiments 1–8 are transfected with a reporter (ME-TRE-luc) and control reporter cytomegalovirus-Renilla luciferase: 1–4 are treated with vehicle, 5–8 are treated with thyroid hormone, 1 and 5 are cotransfected with empty vectors pCDNA3, 2 and 6 are cotransfected with human telomerase RNA β1 (hTRβ1), 3 and 7 are cotransfected with mRARα, and 4 and 8 are cotransfected with human telomerase RNA β1 and mRXRα. Results are in relative luciferase units related to control Renilla luciferase expression. BIR-1 (dark columns) augments basal as well as thyroid hormone-induced transcription. (B) BIR-1 has a stimulatory effect on thyroid hormone-induced transcription in MCF-7 cells treated with the histone deacetylase inhibitor TSA. Cells are transfected as described for A. Note that thyroid hormone stimulation of transcription is conserved on TSA background, but the values (related to the same amount of transfected Renilla vector) are ≈20 times higher. (C) The effect of bir-1 and flag-human SKIP are additive in THR-dependent transcription. Experiments 1–8: MCF-7 cells are transfected with a reporter (ME-TRE-luc), control reporter cytomegalovirus-Renilla luciferase, human THRβ1, and mRXRα. Cells are treated with vehicle in the experiments shown in lines 1–4 and with thyroid hormone in 5–8; 1 and 5 are cotransfected with empty vector (pCDNA3), and 2 and 6 are cotransfected with Bir-1 pCDNA3; 3 and 7 are cotransfected with flag-SKIP, and 4 and 8 are cotransfected with Bir-1 pCDNA3 and flag-SKIP. (D and E) The positive effect of BIR-1 and SKIP and their additive effect on thyroid hormone-induced transcription in cos-7 cells. Experiments are as described for C. Cells are treated with vehicle (D) or 10 nM TSA (E). The additive effect of BIR-1 and flag-SKIP is apparent in basal and T3- and T3/TSA-dependent transcription.
BIR-1 Affects Phosphoacetylation of Histones H3.
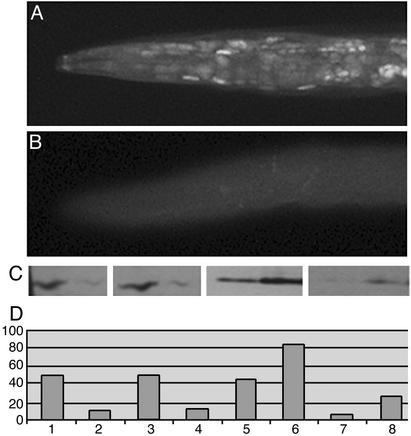
Because BIR-1 was shown to increase mitotic phosphorylation of histone H3 on serine 10 and phosphorylation of this residue was linked recently to promoter activation, we assayed whether bir-1 expression affects phosphoacetylation of histone H3 by using specific antibodies. To assay histone H3 phosphoacetylation we used commercially available antibodies (directed against S10-P K14-Ac H3 and K9-Ac S10-P H3). Histones H3 are conserved between C. elegans and mammals. The sequence covering the peptide used for generation of purchased antibodies is identical with the C. elegans sequence of histone H3. Western blot analysis of C. elegans protein extracts revealed one major band with a mass of 15 kDa recognized by both antibodies at reducing conditions. Staining of fixed specimens showed strong nuclear staining of dividing cells of embryos and larvae. bir-1 RNAi animals caused a decrease of phosphoacetylated histones recognized by both antibodies. The decrease in immunohistochemical staining of fixed animals was mimicked by Western blot analysis of protein extracts from worms treated by bir-1 RNAi (Fig. 5C, lanes 2 and 4).
Figure 5.
BIR-1 affects phosphoacetylation of histones H3. (A) Immunodetection of S10-P K14-Ac histone H3 in L4 larva. Most nuclei are labeled. (B) Decrease of labeling for S10-P K14-Ac histone H3 in bir-1 RNAi-treated larva L4. (C) Western blot analysis of phosphoacetylated histones H3. Larvae L1–L4 were treated by bir-1 RNAi (lanes 2 and 4) by feeding with bacteria producing dsRNA or carrying empty vector (lanes 1 and 3). Protein extracts were separated by PAGE, blotted on membrane, and immunoanalyzed by using antibody against phosphoacetylated histones H3 [lanes 1 and 2 for S10-P K14-Ac H3 (07-081) and lanes 3 and 4 for K9-Ac S10-P H3 (9711S)]. There is a decrease in phosphoacetylated histone in bir-1 RNAi-treated worms. Lanes 5–8, Western blot analysis of protein extracts from transfected MCF-7 cells. Cells were transfected with bir-1 pCDNA3 (lanes 6 and 8) or empty vector (lanes 5 and 7), harvested 24 h later, and processed for Western blot by using antibodies against S10-P K14-Ac H3 (lanes 5 and 6) and K9-Ac S10-P H3 (lanes 7 and 8). BIR-1 leads to an increase in phosphoacetylated histones H3 (lanes 2 and 4). (D) Densitometric analysis of results.
To determine whether bir-1 overexpression affects the level of phosphoacetylated histones positively, we transfected MCF-7 cells with bir-1 and assayed the level of phosphoacetylated histones H3 by Western blot. Cells transfected with bir-1 had an increased level of immunoreactive phosphoacetylated histones H3 recognized by both antibodies (Fig. 5C, lanes 6 and 8). The positive effect was observed also on cos-7 and NIH 3T3 cells transfected with BIR-1 (data not shown).
Discussion
bir-1 Loss of Function Triggers Developmental Changes Not Related to Cell Division.
Previous studies have shown that bir-1 is required in early development for proper cytokinesis (14, 15). Loss of BIR-1 activity in the embryo leads to early lethality resulting from the effects of abnormal cell divisions. In this study, we show that bir-1 is also required for functions not related to cell division, most likely transcriptional regulation of some genes in C. elegans. Loss of bir-1 activity postembryonically results in multiple developmental defects and a decrease of histone H3 phosphoacetylation. RNAi delivered by feeding bacteria producing dsRNA specific for bir-1 leads to strong phenotypes (Egl, dpy, and larval lethality) that overlaps with CeSKIP loss of function (17). However, some characteristic phenotypic changes of bir-1 and CeSKIP loss of function differ. bir-1 RNAi does not result in molting defects that are characteristic for CeSKIP loss of function, and CeSKIP RNAi does not lead to defects in cytokinesis that are observed on embryos developing in bir-1 RNAi-treated animals.
Moreover, CeSKIP RNAi caused embryonic arrest at the time of gastrulation (at the 50- to 100-cell stage) and strong molting defects in larvae but no defects in cell division. In contrast, bir-1 RNAi caused early embryonic arrest at the two-cell stage, and the arrested embryos were multinucleated. In RNAi experiments using bacteria expressing ds bir-1 RNAi, the embryonic arrest occurred later at the 16- or 30-cell stage. Keeping with this, bir-1 RNAi did not alter the pattern of expression of CeSKIP∷gfp from the transgene containing complete bir-1 genomic sequence (Fig. 2 A, B, and D). This indicates that phenotypic changes caused by bir-1 and CeSKIP RNAi are specific for both of these genes.
The developmental defects resulting from bir-1 RNAi cannot be attributed to defects in cell division, which does not occur in the affected cells during later larval stages. The Egl phenotype was observed in animals that had properly developed egg-laying organs, egg-laying and uterine muscles, and neurons. The defect is most likely of neuronal origin as that for the defective movement. Both defects are characteristic for both bir-1 and CeSKIP loss of function.
The developmental changes are highly penetrant but the growth of germ line, an organ with intensive cell divisions, M lineage development, oocytes, sperm development, and spermatheca are not reduced. One possibility is that chromatin proteins are different, as is the case for sperm chromatin (39, 40). Another possibility is that the transcription function of BIR-1 is more sensitive to bir-1 RNAi at the level used in our experiments.
Nevertheless, the germ lines (the proximal and distal parts of gonads) are developing normally. The proper development of DTCs was also observed in Nomarski optics. This is in contrast to embryos developing in bir-1 RNAi-affected animals defective in cell division as described elsewhere (14, 15) and observed in our experiments.
BIR-1 Is Involved in Regulation of Transcription by RNA Polymerase II.
We have found that CHR3 (nhr-23) (31, 33), CeSKIP (17), and bir-1 loss of function have partially overlapping phenotypes.
CeSKIP loss of function leads to marked decrease of RNA polymerase II-dependent transcription of specific genes (17). dpy-7, a collagen gene, was found to be inhibited in CHR3 (nhr-23) loss of function (33) by RNAi. We show here that bir-1 RNAi leads to decreased transcription of some (but not all) transgenes (elt-2∷gfp and hlh-1∷gfp) that were found to be decreased also by CeSKIP RNAi.
These results indicate that bir-1 may be required for activities of the same promoters that depend on CeSKIP and CHR3 (nhr-23) and suggest that not all promoters are equally sensitive to BIR-1.
Moreover, BIR-1 expression led to stimulation of T3-dependent as well as basal transcription from thyroid hormone-responsive promoters. The effect was further additive with transcription stimulation by the histone deacetylase inhibitor TSA. These data suggest that BIR-1 effect and histone acetylation are cooperative.
BIR-1 Affects Phosphoacetylation of Histones H3.
Complex covalent modification of histones is part of the transcription activation process. Histone modifications include methylation, acetylation, and phosphorylation. Although histone acetylation is a well established component of transcription activation, phosphorylation and specific methylation of histones have been shown recently to be part of specific transcription activation (refs. 6, 9, and 11; for review see refs. 2, 4, and 7). Phosphorylation of serine 10 of histone H3 is linked to acetylation of lysine 14 in Gcn5-regulated promoters (10) and even facilitates the lysine 14 acetylation (10, 11). Interestingly, BIR-1 was shown to phosphorylate serine 10 of histone H3 in connection with aurora kinase AIR-2 (15). Several H3 serine 10-specific kinases have been identified including Ip11/aurora family kinases and Snf1 (12, 41). Snf1 was shown to activate transcription of INO1 by phosphorylation of serine 10 of histone H3, which in turn facilitates acetylation of H3 on lysine 14 (12). Snf1 also regulates Adr1-dependent transcription by directly enhancing Adr1 binding to chromatin (42). Phosphoacetylation of histone H3 on serine 10 and lysine 14 is part of protein kinase A-mediated gene activation by follicle-stimulating hormone in granulosa cells of the ovary (43).
In the present study, bir-1 RNAi led to a decrease of both S10-P K14-Ac and K9-Ac S10-P phosphoacetylated histones H3 in worms. By immunohistochemistry we observed that the decrease of the level of phosphoacetylated histones H3 caused by bir-1 RNAi was not only linked to cell division but also was visible in nondividing cells. In addition, MCF-7, cos-7, and NIH 3T3 cells transfected with bir-1 had increased levels of phosphoacetylated histones. Thus, phosphoacetylation of histones H3 on serine 10 is likely to be directly involved in the effect of bir-1 on transcription.
Members of the inhibitor-of-apoptosis family usually contain several repeats of BIR domain and a RING domain. The general function of the proteins with the RING domain is probably E3 ubiquitin ligase activity. The caspase recruitment domain is another domain frequently found in inhibitor-of-apoptosis proteins. The simplest members of the family such as BIR-1 or Survivin are composed of just one single BIR domain. The C. elegans genome contains two Bir genes; the second one, bir-2, codes for a protein with a tandem of two BIR domains. In contrast to pronounced phenotypes obtained by bir-1 RNAi, BIR-2 cannot substitute the function of BIR-1. This differs from the finding that BIR-1 function can be rescued by the human homologue Survivin (14).
In conclusion, we show that BIR-1 functions in transcription regulation. It is also possible that BIR-1 contributes to specific histone phosphorylation during the transcription activation process.
Acknowledgments
We thank Dr. Michael Krause (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health) for support, advice, and critical reading of the manuscript and constructs hlh-1, hlh-2, and hlh-8; Dr. Paul Yen for constructs DR-4-luc and F2-luc; Dr. Andrew Fire for vector L4440 and pPD 49.83; Dr. Diane Hayward for flag-SKIP; Dr. Keiko Ozato for ME-TRE-luc; and Dr. Jacob Robbins (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health) for critical reading of the manuscript. Z.K. is supported by Grant Agency of the Czech Republic Grant 304-00-1128 and Ministry of Education of the Czech Republic Grant 11110000-3, and M.K. is supported by Grant Agency of the Czech Republic Grant 304-02-1347. Z.K. and M.K. are associates at the Diabetes Branch, National Institute of Diabetes Digestive and Kidney Diseases, National Institutes of Health.
Abbreviations
- BIR-1
Caenorhabditis elegans baculoviral inhibitor-of-apoptosis repeat protein 1
- SKIP
SKI-binding protein
- CeSKIP
C. elegans SKIP
- RNAi
RNA-mediated interference
- ds
double-stranded
- mRXRα
murine retinoid X receptor α
- THR
thyroid hormone receptor
- DTC
distal tip cell
- T3
triiodothyronine
- TSA
Trichostatine A
References
- 1.Ito M, Roeder R G. Trends Endocrinol Metab. 2001;12:127–134. doi: 10.1016/s1043-2760(00)00355-6. [DOI] [PubMed] [Google Scholar]
- 2.Felsenfeld G, Clark D, Studitsky V. Biophys Chem. 2000;86:231–237. doi: 10.1016/s0301-4622(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 3.Jenuwein T, Allis C D. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Berger S L, Felsenfeld G. Mol Cell. 2001;8:263–268. doi: 10.1016/s1097-2765(01)00330-6. [DOI] [PubMed] [Google Scholar]
- 5.Crosio C, Fimia G M, Loury R, Kimura M, Okano Y, Zhou H, Sen S, Allis C D, Sassone-Corsi P. Mol Cell Biol. 2002;22:874–885. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Lin Q, Yoon H G, Huang Z Q, Strahl B D, Allis C D, Wong J. Mol Cell Biol. 2002;22:5688–5697. doi: 10.1128/MCB.22.16.5688-5697.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger S L. Curr Opin Genet Dev. 2002;12:142–148. doi: 10.1016/s0959-437x(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 8.Hasan S, Hottiger M O. J Mol Med. 2002;80:463–474. doi: 10.1007/s00109-002-0341-7. [DOI] [PubMed] [Google Scholar]
- 9.Nowak S J, Corces V G. Genes Dev. 2000;14:3003–3013. doi: 10.1101/gad.848800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo W S, Trievel R C, Rojas J R, Duggan L, Hsu J Y, Allis C D, Marmorstein R, Berger S L. Mol Cell. 2000;5:917–926. doi: 10.1016/s1097-2765(00)80257-9. [DOI] [PubMed] [Google Scholar]
- 11.Cheung P, Tanner K G, Cheung W L, Sassone-Corsi P, Denu J M, Allis C D. Mol Cell. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- 12.Lo W S, Duggan L, Tolga N C, Emre, Belotserkovskya R, Lane W S, Shiekhattar R, Berger S L. Science. 2001;293:1142–1146. doi: 10.1126/science.1062322. [DOI] [PubMed] [Google Scholar]
- 13.Lefebvre B, Ozato K, Lefebvre P. EMBO Rep. 2002;3:335–340. doi: 10.1093/embo-reports/kvf066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser A G, James C, Evan G I, Hengartner M O. Curr Biol. 1999;9:292–301. doi: 10.1016/s0960-9822(99)80137-7. [DOI] [PubMed] [Google Scholar]
- 15.Speliotes E K, Uren A, Vaux D, Horvitz H R. Mol Cell. 2000;6:211–223. doi: 10.1016/s1097-2765(00)00023-x. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosini G, Adida C, Altieri D C. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 17.Kostrouchova M, Housa D, Kostrouch Z, Saudek V, Rall J E. Proc Natl Acad Sci USA. 2002;99:9254–9259. doi: 10.1073/pnas.112213799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumenthal T, Spieth J. Curr Opin Genet Dev. 1996;6:692–698. doi: 10.1016/s0959-437x(96)80022-0. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal T, Evans D, Link C D, Guffanti A, Lawson D, Thierry-Mieg J, Thierry-Mieg D, Chiu W L, Duke K, Kiraly M, Kim S K. Nature. 2002;417:851–854. doi: 10.1038/nature00831. [DOI] [PubMed] [Google Scholar]
- 20.Fukushige T, Hawkins M G, McGhee J D. Dev Biol. 1998;198:286–302. [PubMed] [Google Scholar]
- 21.Fukushige T, Hendzel M J, Bazett-Jones D P, McGhee J D. Proc Natl Acad Sci USA. 1999;96:11883–11888. doi: 10.1073/pnas.96.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krause M, Harrison S W, Xu S Q, Chen L, Fire A. Dev Biol. 1994;166:133–148. doi: 10.1006/dbio.1994.1302. [DOI] [PubMed] [Google Scholar]
- 23.Krause M, Park M, Zhang J M, Yuan J, Harfe B, Xu S Q, Greenwald I, Cole M, Paterson B, Fire A. Development (Cambridge, UK) 1997;124:2179–2189. doi: 10.1242/dev.124.11.2179. [DOI] [PubMed] [Google Scholar]
- 24.DeVore D L, Horvitz H R, Stern M J. Cell. 1995;83:611–620. doi: 10.1016/0092-8674(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 25.Harfe B D, Fire A. Development (Cambridge, UK) 1998;125:421–429. doi: 10.1242/dev.125.3.421. [DOI] [PubMed] [Google Scholar]
- 26.Corsi A K, Kostas S A, Fire A, Krause M. Development (Cambridge, UK) 2000;127:2041–2051. doi: 10.1242/dev.127.10.2041. [DOI] [PubMed] [Google Scholar]
- 27.Praitis V, Casey E, Collar D, Austin J. Genetics. 2001;157:1217–1226. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fire A, Xu S, Montgomery M K, Kostas S A, Driver S E, Mello C C. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 30.Timmons L, Fire A. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- 31.Kostrouchova M, Krause M, Kostrouch Z, Rall J E. Development (Cambridge, UK) 1998;125:1617–1626. doi: 10.1242/dev.125.9.1617. [DOI] [PubMed] [Google Scholar]
- 32.Johnstone I L, Barry J D. EMBO J. 1996;15:3633–3639. [PMC free article] [PubMed] [Google Scholar]
- 33.Kostrouchova M, Krause M, Kostrouch Z, Rall J E. Proc Natl Acad Sci USA. 2001;98:7360–7365. doi: 10.1073/pnas.131171898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou S, Fujimuro M, Hsieh J J, Chen L, Miyamoto A, Weinmaster G, Hayward S D. Mol Cell Biol. 2000;20:2400–2410. doi: 10.1128/mcb.20.7.2400-2410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuels H H, Stanley F, Casanova J. Endocrinology. 1979;105:80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- 36.Petty K J, Desvergne B, Mitsuhashi T, Nikodem V M. J Biol Chem. 1990;265:7395–7400. [PubMed] [Google Scholar]
- 37.Tomura H, Lazar J, Phyillaier M, Nikodem V M. Proc Natl Acad Sci USA. 1995;92:5600–5604. doi: 10.1073/pnas.92.12.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones K E, Brubaker J H, Chin W W. Endocrinology. 1994;134:543–548. doi: 10.1210/endo.134.2.8299553. [DOI] [PubMed] [Google Scholar]
- 39.Lewis J D, Ausio J. Biochem Cell Biol. 2002;80:353–361. doi: 10.1139/o02-083. [DOI] [PubMed] [Google Scholar]
- 40.Sassone-Corsi P. Science. 2002;296:2176–2178. doi: 10.1126/science.1070963. [DOI] [PubMed] [Google Scholar]
- 41.Hsu J Y, Sun Z W, Li X, Reuben M, Tatchell K, Bishop D K, Grushcow J M, Brame C J, Caldwell J A, Hunt D F, et al. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 42.Young E T, Kacherovsky N, Riper K V. J Biol Chem. 2002;277:38095–38103. doi: 10.1074/jbc.M206158200. [DOI] [PubMed] [Google Scholar]
- 43.Salvador L M, Park Y, Cottom J, Maizels E T, Jones J C, Schillace R V, Carr D W, Cheung P, Allis C D, Jameson J L, Hunzicker-Dunn M. J Biol Chem. 2001;276:40146–40155. doi: 10.1074/jbc.M106710200. [DOI] [PubMed] [Google Scholar]