Abstract
Angiotensin II (AII) is a major determinant of arterial pressure and volume homeostasis, mainly because of its vascular action via the AII type 1 receptor (AT1R). AII has also been implicated in the development of cardiac hypertrophy because angiotensin I-converting enzyme inhibitors and AT1R antagonists prevent or regress ventricular hypertrophy in animal models and in human. However, because these treatments impede the action of AII at cardiac as well as vascular levels, and reduce blood pressure, it has been difficult to determine whether AII action on the heart is direct or a consequence of pressure-overload. To determine whether AII can induce cardiac hypertrophy directly via myocardial AT1R in the absence of vascular changes, transgenic mice overexpressing the human AT1R under the control of the mouse α-myosin heavy chain promoter were generated. Cardiomyocyte-specific overexpression of AT1R induced, in basal conditions, morphologic changes of myocytes and nonmyocytes that mimic those observed during the development of cardiac hypertrophy in human and in other mammals. These mice displayed significant cardiac hypertrophy and remodeling with increased expression of ventricular atrial natriuretic factor and interstitial collagen deposition and died prematurely of heart failure. Neither the systolic blood pressure nor the heart rate were changed. The data demonstrate a direct myocardial role for AII in the development of cardiac hypertrophy and failure and provide a useful model to elucidate the mechanisms of action of AII in the pathogenesis of cardiac diseases.
The growth response of the adult heart to mechanical overload is enlargement of terminally differentiated cardiomyocytes resulting in heart hypertrophy. This phenotypic change is associated with reprogramming of cardiac gene expression, including reinduction of a set of fetal genes for which atrial natriuretic factor (ANF) is a hallmark (1, 2). Cardiac hypertrophy is often accompanied by cardiac remodeling characterized by cardiomyocyte loss, proliferation of interstitial fibroblasts, and collagen deposition, leading to decreased compliance and increased risk for heart failure (3–6). Although the exact mechanisms involved in initiating and/or maintaining cardiac hypertrophy remain unknown, many neurohumoral systems, particularly the renin–angiotensin system (RAS), have been implicated in the hypertrophic process (reviewed in ref. 7).
RAS is a major determinant of arterial pressure and volume homeostasis in mammals through the action of the vasoactive peptide angiotensin II (AII) on vascular AII type 1 receptor (AT1R) (8). The activity of RAS is increased in several cardiovascular diseases, such as myocardial infarction, myocarditis, cardiomyopathy, and hypertension. It is now well established that angiotensin converting enzyme inhibitors prevent the development of pressure-overload cardiac hypertrophy in animal models and in hypertensive human patients; more recently, AT1R antagonists were found to be effective at repressing cardiac hypertrophy in hypertensive patients (9) and are currently undergoing larger clinical trials in patients with cardiovascular diseases. However, because these treatments impede the action of AII at cardiac as well as vascular levels, reducing blood pressure and thus preventing pressure-overload-induced cardiac hypertrophy, it has been difficult to determine whether AII can act directly on cardiomyocytes, independently of vascular changes. Indeed, although enhanced AII levels may in some instances be compensatory for the decrease in cardiac output and help to normalize blood pressure, the increase in AII may have a parallel effect on the heart leading to hypertrophy. Several studies support a role for AII on the heart: in vivo, the infusion of subpressor doses of AII induced cardiac hypertrophy in rats and mice (10–12), and, in vitro, addition of AII to cultured neonatal cardiomyocytes increased protein synthesis and induced expression of genes that are hallmark of cardiac hypertrophy such as the immediate early and cardiac fetal genes c-fos and ANF (13, 14).
Interestingly, many RAS components, namely angiotensinogen, angiotensin converting enzyme, and both type 1 and 2 AII receptors, are expressed in cardiomyocytes and are up-regulated in cardiac hypertrophy, raising the possibility for an autocrine or paracrine role of AII in the heart (15–17). Moreover, AII receptors are also present on both cardiac fibroblasts (18) and endothelial cells (19)—which normally contaminate primary cardiomyocyte cultures—and AII was shown to induce secretion of growth factors from these cells (19, 20). Interestingly, fibroblasts were shown to be required for maximal cardiomyocytes response to AII- and stretch-induced myocyte hypertrophy (21, 22). Thus, even at the level of the heart, it remains unclear whether AII-induced hypertrophy occurs via a direct action on cardiomyocytes or by a paracrine mechanism involving noncardiomyocytes.
To determine whether AII can act directly on cardiomyocytes to cause cardiac hypertrophy, transgenic (Tg) mice expressing the human AT1R specifically in cardiomyocytes under the control of the mouse α-myosin heavy chain (αMHC) promoter were generated. These Tg mice developed significant cardiomyocyte hypertrophy and cardiac fibrosis leading to congestive heart failure in the absence of blood pressure change. These data demonstrate that myocardial action of AII is sufficient to trigger hypertrophy and remodeling of the heart and support a role for AII in the pathogenesis of cardiac hypertrophy and failure.
Methods
Generation of Tg Mice.
The αMHC-AT1R transgene was constructed by using a 1.23-kbp fragment of the fifth exon of the human AT1R gene (23) containing the entire coding sequences, and minimal amount of 5′ and 3′ untranslated sequences (kindly provided by S. Meloche, Université de Montréal, Montréal) cloned between the 5.4-kbp mouse αMHC promoter (described in ref. 24; a kind gift of J. Robbins, University of Cincinnati) and the Simian virus 40 (SV40) polyadenylation sequence (Fig. 1A). The transgene was microinjected into the pronucleus of fertilized eggs from C57BL/6 × C3H mice. Genomic DNA was isolated from tail biopsies taken from 3-week-old pups and was digested with EcoRI. Pups were screened for the transgene by using either Southern blot analysis or PCR. Southern blots were hybridized with the 32P-labeled full length αMHC-AT1R transgene. Blots were washed at 65°C in 0.1 × standard saline citrate (SSC). For PCR detection, forward (5′- ACC CTT ACC CCA CAT AGA C) and reverse (5′- ACC ATC TTC AGT AGA AGA GTT G) primers from the second intron of the mouse αMHC gene and the 5′ coding region of the human AT1R gene were used. Tg founders were mated with wild-type (Wt) C3H mice. Animals were handled in accordance with institutional guidelines.
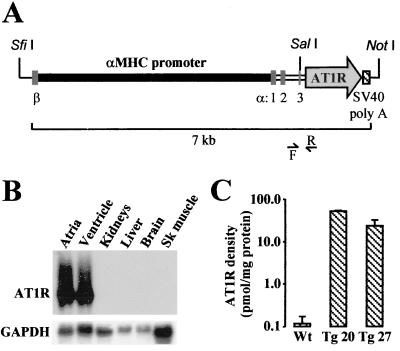
Figure 1.
(A) Schematic representation of the αMHC-AT1R transgene. β and α:1, 2, and 3 denote the position of the last exon of βMHC gene and of the three first exons of αMHC gene. F and R indicate the forward and reverse primers used for screening. (B) Cardiac-specific expression of the AT1R transgene. The tissue specificity of AT1R transgene expression was determined in total RNA by Northern blot analysis as described in Methods. Blots were rehybridized with rat glyceraldehyde-3-phosphate dehydrogenase cDNA probe to control for RNA loading. Sk, skeletal. (C) Expression of human AT1R protein. Density of the AT1R was determined in membranes isolated from ventricles of 62- to 140-day-old Wt (n = 6), 62-day-old Tg 20 (n = 3), and 140-day-old Tg 27 (n = 3) mice as described in Methods, using losartan as competitor (data are means ± SEM).
Northern Blot Analysis.
Total RNA (20 μg) was denatured with formaldehyde and formamide and was size-fractionated on a 1.2% agarose gel as described (25), was transferred to nylon membranes (Micron Separations, Westborough, MA), and was UV-crosslinked by using the Stratalinker 2400 (Stratagene). Blots were first stained with methylene blue to control for transfer and loading and then were hybridized with 32P-labeled human exon 5 AT1R probe and rat cDNA probes for ANF, GATA-4, and glyceraldehyde-3-phosphate dehydrogenase as described (26).
Radioligand Binding Assays.
The density of AT1R was determined by radioligand binding assay as described (27). In brief, crude plasma membranes were prepared from Wt and Tg mice ventricles, and membrane protein concentration was determined by the Bio-Rad protein assay. The competitive binding assays were carried out by using increasing concentration (10−12 to 10−5 mol/liter) of [Sar1, Ile8]AII, losartan (an AT1R selective antagonist), or PD 123319 (an AT2 receptor-specific ligand), and 100–120 pmol/liter of 125I-[Sar1, Ile8]AII (2200 Ci/mmol). Binding data were analyzed by using ebda-ligand software of G. A. McPherson (Biosoft, Milltown, NJ). Receptor densities are expressed as picomoles of sites per milligram of protein.
Tissue Weights and Gross Morphometric Analysis.
Age-matched Wt and Tg mice were killed, and their body weights were determined. The heart, liver, kidneys, and spleen were isolated. Hearts were further dissected to isolate the left and right ventricular free walls and the left and right atria. Tissue weights and femur length were measured with an analytical scale and a micrometer, respectively. Hearts isolated from Wt and Tg 27 mice were fixed with neutral formaldehyde for determination of morphometric changes. After fixation, hearts were cleaned up of connective tissue under a dissection microscope and were photographed, sectioned in half, and photographed again at the same magnification.
Histology.
Hearts were first washed and stopped in diastole by perfusion with PBS containing 50 mM KCl and then were fixed by perfusion with Bouin solution followed by an overnight incubation at 4°C with agitation in the same fixative. Hearts were cut longitudinally and were embedded in paraffin, and serial cuts of 5 μm were performed. Sections were stained with Mason's trichrome. For immunohistochemical analysis, rehydrated sections were blocked with 10% normal goat serum in Tris-buffered saline (TBS) containing 0.3% Tween 20 (TBT) for 10 min and then were exposed for 24–48 h to a rabbit polyclonal anti-ANF (99–126) antibody diluted 1:1,000 in the blocking solution at 4°C. Sections were washed in TBT, blocked for 1 h and then incubated with a goat anti-rabbit IgG coupled to biotin diluted 1:250 in the blocking solution for 1 h. Sections were washed, were blocked for 1 h, and were exposed to streptavidin-coupled to peroxidase diluted 1:500 in the blocking solution for 45 min. Exposure of heart sections to 0.03% diaminobenzidine and 0.03% H2O2 in TBS for 3 min resulted in a visible immunoreaction. Sections were counterstained with methyl green.
Physiologic Measurements.
Systolic blood pressure and heart rate were determined by tail-cuff technique using the Visitech systems BP-2000 (Apex, NC) after 6 days of training sessions (unrecorded measurements) from 9 to 11 a.m. daily.
Statistics.
Results are expressed as mean ± SEM. Homogeneity of variance was tested by using an F test, and then statistical significance was estimated by using the appropriate Student's t test for unpaired observations using the analysis toolpak of Microsoft Excel. A P < 0.05 was considered significant.
Results
To determine whether AII can induce cardiac hypertrophy through a direct action on cardiomyocytes, Tg mice overexpressing the human AT1R specifically in cardiomyocytes using the mouse αMHC promoter were generated (Fig. 1A). This promoter has been shown to confer cardiac-specific expression to many separate transgene lines (28–32), and two independent groups have shown that transgene expression is restricted to cardiomyocytes (28, 33). Ten of thirty-seven mice screened were found positive (Table 1). Eight founders showed the expected 50% transmission, typical of a Mendelian inheritance.
Table 1.
Transmission rate of αMHC-AT1R Tg mice
| Tg founders | Tg offsprings
|
|
|---|---|---|
| Fraction of the offsprings | Percent of the offsprings | |
| 3 | 0/13 | 0 |
| 17 | 51/86 | 59 |
| 20 | 65/106 | 61 |
| 21 | 11/33 | 33 |
| 25 | 15/20 | 75 |
| 26 | 10/18 | 56 |
| 27 | 52/100 | 52 |
| 30 | 9/26 | 34 |
| 32 | 4/9 | 44 |
| 35 | 0/10 | 0 |
Tg founder mice were mated with wild-type C3H, and transmission frequency of the αMHC-AT1R transgene to their offsprings was determined by Southern blot analysis or PCR as described in Methods.
Tissue-specific transgene expression was assessed at the mRNA level by Northern blot analysis using 80-day-old mice. As expected, expression of the transgene was restricted to the atria and ventricles. No expression was detected in the kidneys, liver, brain, or skeletal muscle (Fig. 1B). Next, the density of AT1R was determined in membranes isolated from the ventricles of Wt and Tg mice by using 125I-[Sar1, Ile8]AII as ligand and [Sar1, Ile8]AII, losartan, and PD 123319 as competitors. In Wt mice, the ligand was completely displaced by [Sar1, Ile8]AII and losartan but not by PD 123319, indicating that, in postnatal hearts, AII receptors were exclusively type 1 (data not shown). The density of AT1R was increased by > 200-fold in four of five Tg lines analyzed (data not shown) and were respectively 450- and 200-fold higher in the ventricles of Tg 20 and Tg 27, the two lines that were further characterized, as compared with Wt mice (Fig. 1C).
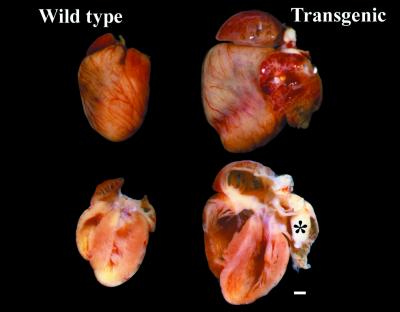
One of the first phenotypes noted in Tg 20 and Tg 27 was spontaneous death starting at 80 days of age. The spontaneous death rate was ≈40× higher in Tg mice compared with age-matched control (20 vs. 0.5%). The average age of death was 140 ± 1 days (n = 30) for Tg 27 and 238 ± 4 days (n = 17) for Tg 20; most Tg 27 mice did not survive past 1 year. Necropsy of these mice revealed dilatation of the heart, fibrotic tissue, especially in the left atrium, and pleural effusion and/or ascites, suggesting that they died of congestive heart failure. Animals were also killed at various ages to assess cardiac changes before heart failure. Gross examination of 120-day- and older Tg hearts revealed significant hypertrophy (Fig. 2). The ventricles and atria were extremely enlarged, and dissection of the hearts revealed significant remodeling of Tg as compared with Wt hearts. Consistently, the left ventricle was elongated and the free wall was thinner toward the apex. The size of the right ventricle cavity was significantly increased. Fibrotic tissue was also observed in the lumen of the left atrium appendix that could have resulted from an accumulation of blood and subsequent formation of clot in the left atrium because of left ventricular failure.
Figure 2.
Human AT1R overexpression in cardiomyocytes induced massive remodeling of the heart. Hearts from age-matched Wt and Tg 27 mice were washed with PBS and fixed in neutral formaldehyde. Hearts were cleaned up of connective tissue, were photographed, were sectioned in two, were cleaned up of blood clots, and were rephotographed at the same magnification. *, the fibrotic tissue in the left atrium. (Bar = 1 mm.)
The tissue weights of the different cardiac compartments, liver, and kidneys were determined in Wt, Tg 20, and Tg 27 mice (Table 2). The weights of the left and right ventricular free walls and of left and right atria isolated from Tg 27 mice were increased 1.3-, 1.7-, 3.9-, and 3-fold, respectively, as compared with age-matched Wt mice. The same changes were obtained whether the data was expressed as heart weight relative to body weight or relative to femur length. No difference in body and kidney weights was noted. In contrast, the liver weight was increased 33%, suggesting the presence of congestive heart failure. Similar changes were observed in Tg 20 mice with cardiac hypertrophy increasing with time. Left ventricular/body weights were 2.5-fold higher in 130-day-old Tg 20 relative to Wt mice, and 3.7-fold higher in older 400-day-old animals compared with age-matched Wt. It is noteworthy that cardiac hypertrophy was induced in a hemodynamic independent fashion in Tg mice as systolic blood pressure and heart rate were not altered by AT1R overexpression (Table 2).
Table 2.
Systolic blood pressure, heart rate, and body, cardiac tissues, liver, kidneys and spleen weights of Wt and Tg 27 mice
| Wt (9) | Tg 27 (11) | |
|---|---|---|
| Age, days | 125.0 ± 0.3 | 129.5 ± 8.8 |
| Body weight (BW), g | 29.96 ± 0.30 | 29.11 ± 1.17 |
| Femur length (FL), mm) | 16.23 ± 0.16 | 15.77 ± 0.27 |
| Left ventricle free wall (LV) | ||
| LV weight, mg | 62.03 ± 2.59 | 81.97 ± 5.82* |
| LV/BW, mg/g | 2.07 ± 0.07 | 2.81 ± 0.18† |
| LV/FL, mg/mm | 3.83 ± 0.18 | 5.17 ± 0.33§ |
| Right ventricle free wall (RV) | ||
| RV weight, mg | 25.90 ± 1.17 | 44.16 ± 3.09§ |
| RV/BW, mg/g | 0.86 ± 0.03 | 1.51 ± 0.09¶ |
| RV/FL, mg/mm | 1.60 ± 0.07 | 2.79 ± 0.17§ |
| Left atrium (LA) | ||
| LA weight, mg | 7.62 ± 1.63 | 29.53 ± 4.89† |
| LA/BW, mg/g | 0.25 ± 0.05 | 1.01 ± 0.16† |
| LA/FL, mg/mm | 0.47 ± 0.10 | 1.89 ± 0.34† |
| Right atrium (RA) | ||
| RA weight, mg | 7.66 ± 0.45 | 23.18 ± 2.63‡ |
| RA/BW, mg/g | 0.26 ± 0.02 | 0.81 ± 0.10‡ |
| RA/FL, mg/mm | 0.47 ± 0.03 | 1.47 ± 0.16‡ |
| Liver (L) | ||
| L weight, g | 1.69 ± 0.06 | 2.24 ± 0.16* |
| L/BW, g/g | 56.67 ± 1.57 | 77.15 ± 5.16† |
| L/FL, g/mm | 104.55 ± 3.83 | 141.18 ± 8.77† |
| Kidneys (K) | ||
| K weight, mg | 517.11 ± 32.07 | 458.70 ± 37.44 |
| K/BW, mg/g | 17.22 ± 0.83 | 15.66 ± 0.96 |
| K/FL, mg/mm | 31.93 ± 2.07 | 28.87 ± 1.99 |
| Systolic blood pressure, mmHg | 112.9 ± 4.3 | 108.9 ± 3.1 |
| Heart rate, beats/min | 578.8 ± 14.7 | 590.3 ± 11.7 |
Systolic blood pressure and heart rate were determined in age-matched Wt and Tg 27 mice. Then the animals were killed, the body weight was determined, and the heart, liver, kidneys, and spleen, were isolated. The heart was further dissected as described in Methods. The data are mean ± SEM for the number of mice shown between parentheses. *, P < 0.01. †, P < 0.001. ‡, P < 0.0001. §, P < 0.00001. ¶, P < 0.000001.
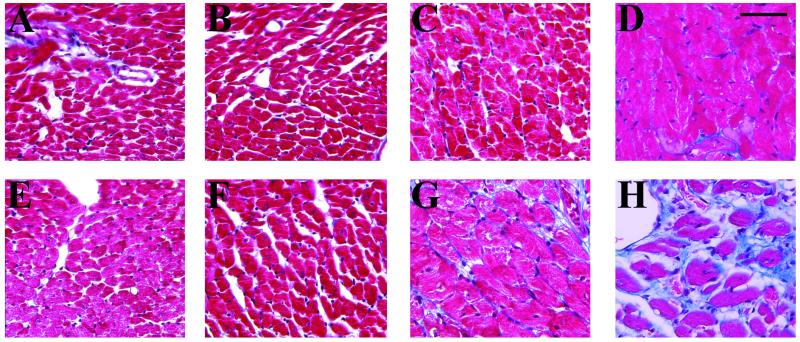
Age-dependent morphologic changes were examined in Mason's trichrome-stained left ventricular sections of age-matched Wt and Tg 27 mice (Fig. 3). No gross changes were detected before 41 days postnatal; at 65 days, the interstitial space was more prominent in Tg 27 hearts, suggesting the loss of some cardiomyocytes, and cardiomyocytes began to be enlarged in Tg 27 when compared with Wt mice (Fig. 3 F vs. B). At 111 days, the cardiomyocytes were significantly hypertrophied, and interstitial fibrosis started to be evident in Tg mice (Fig. 3 G vs. C). Interstitial fibrosis was also prominent in both atria (data not shown; Fig. 5 C vs. A). At later stages (158 days old), several empty spaces filled with collagen were observed in the Tg ventricles, suggesting further cardiomyocyte loss (Fig. 3 H vs. D). Similar findings were observed in the ventricles of Tg 20 mice (data not shown).
Figure 3.
Age-dependent morphological changes in the left ventricle of Tg 27 mice. Five-micrometer heart sections from age-matched Wt (A–D) and Tg (E–F) mice were stained with Masson's. Left ventricular sections of 41- (A), 65- (B), 111- (C), and 182- (D) day-old Wt mice were compared with sections of 41- (E), 65- (F), 111- (G), and 152- (H) day-old Tg mice. (Bar = 50 μm.) The blue staining reveals collagen. Note the increase in interstitial space and in myocyte size starting at day 65 and the massive reduction in myocyte number and the enhanced collagen deposition at days 111 and 158.
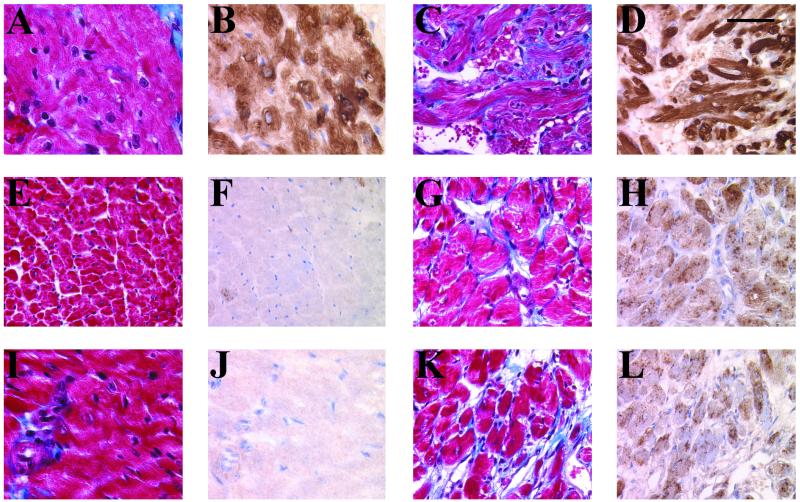
Figure 5.
Increased expression of ANF and collagen deposition in the ventricles of Tg mice. Five-micrometer heart sections from 111-day-old right atria (A and B) and left (E and F) and right (I and J) ventricles of Wt and right atria (C and D) and left (G and H) and right (K and L) ventricles of Tg 27 were immunostained with a rabbit polyclonal anti-ANF antibody and were counterstained with methylgreen (B, D, F, H, J, and L), and collagen deposition (blue) was determined by Masson's trichrome staining (A, C, E, G, I, and K). Note the homogeneously increased ANF immunoreactivity in both Tg ventricles. Interstitial fibrosis is detected in both ventricles and atria of Tg as compared with Wt mice. Significant cardiomyocyte hypertrophy is also evident in Tg ventricles. (Bar = 50 μm.)
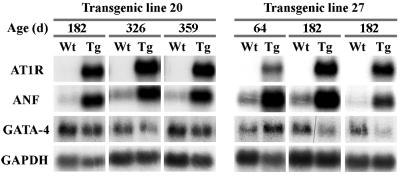
Classic biochemical features that accompany cardiac hypertrophy were also observed in Tg mice. Northern blot analysis revealed that ANF expression was increased in the ventricles of Tg 20 and Tg 27 mice as compared with age-matched Wt mice (Fig. 4) whereas the expression of transcription factor GATA-4 was not significantly altered. Increased expression of ANF was also observed at the protein level, as evidenced by immunohistochemical studies. ANF was increased throughout the ventricles of Tg mice reaching nearly atrial levels whereas in Wt ventricles, it is detected only in a few cardiomyocytes (Fig. 5).
Figure 4.
RNA analysis of changes in gene expression in the ventricles of Tg mice. Total RNA was extracted from ventricles of age-matched Wt, Tg 20, and Tg 27 mice. The expression of AT1R, ANF, GATA-4, and glyceraldehyde-3-phosphate dehydrogenase were determined by Northern blot as described in Methods.
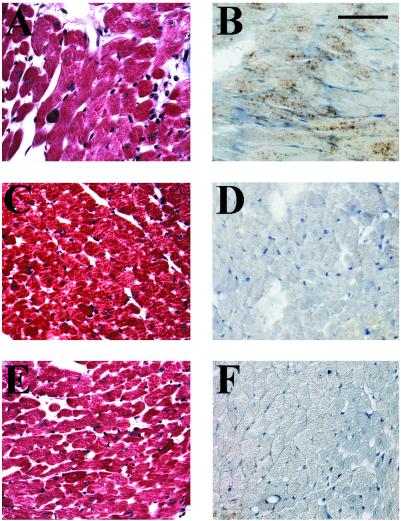
To ascertain AT1R-specific dependence of the cardiac phenotype, 60-day-old transgenic mice (n = 4) were administered 30 mg/kg/day of the AT1R antagonist losartan in drinking water (a generous gift of Merck Frosst Canada, Kirkland, QC Canada) for 2 months. As shown in Fig. 6, losartan but not vehicle treatment prevented myocyte hypertrophy, collagen deposition, and ANF induction. Thus, overexpression of AT1R in cardiomyocytes induced, under steady state conditions, morphologic changes over a 4- to 5-month period that mimic those observed during the development of cardiac hypertrophy and congestive heart failure in classical animal models of pressure-overload hypertrophy and in humans.
Figure 6.
Prevention of the hypertrophic phenotype by the AT1R antagonist losartan. Five-micrometer heart sections from 120-day-old Tg mice treated with vehicle (A and B) or losartan (C and D) were stained for collagen with Masson's trichrome (A and C) or were immunostained with the anti-ANF antibody (B and D). Control Wt littermates are similarly presented (E and F). (Bar = 50 μm.)
Discussion
The vasoactive peptide hormone angiotensin II (AII) has been implicated in the development of cardiac hypertrophy. Moreover, AII has been postulated to be the humoral mediator of mechanical stretch-induced cardiac hypertrophy. However, whether the cardioregulatory function of AII is attributable to activation of myocardial AII receptors (ATRs) or is a consequence of peripheral vascular changes remains uncertain.
In the present work, we provide evidence for a role of myocardial AT1R receptors in cardiac hypertrophy and remodeling independently of hemodynamic changes. Indeed, myocardial-specific overexpression of the human AT1R produced initially cardiomyocyte hypertrophy followed by interstitial collagen deposition, myolysis, and cardiac remodeling. This in turn was accompanied by increased incidence of premature death caused by congestive heart failure. The pronounced cardiac changes observed in this study contrast with the absence of morphological changes in the heart of Tg mice overexpressing the type 2 angiotensin receptor under the control of the same αMHC promoter (29); thus, AII can directly induce classical features of cardiac hypertrophy, remodeling, and failure via its myocardial type 1 receptor.
AT1 receptors were shown to be up-regulated in several models of cardiac hypertrophy (17, 34, 35), although the functional significance of this change was not clear. The cardiac changes described in this study in mice with targeted overexpression of the AT1R show that increased myocyte receptor number is sufficient to initiate and maintain cardiac hypertrophy and remodeling. Interestingly, despite the increased receptor density, cell signaling could be further stimulated by exogenous AII; in preliminary experiments, infusion of a subpressor dose of AII (100 ng/kg/day) for 2 days led to sudden death and further increases in cardiac mass in AT1R Tg but not in Wt mice. Hoffmann et al. (36) also observed enhanced pressure-overload-induced hypertrophy in Tg rats overexpressing the human AT1R, although cardiac changes were minimal in basal conditions, a result, possibly, of a lower level of transgene expression. In contrast, Hein et al. (37) reported prenatal lethality in Tg mice overexpressing the mouse AT1R from the αMHC promoter, although postnatal survival up to 7 days could be achieved by administration of angiotensin converting enzyme inhibitor or AT1R antagonist during pregnancy. The strain of mice used by Hein et al. (37) was an outbred of Swiss strain, which has two renin genes—as opposed to the strain used in the present study, which has only one renin gene; because mice with two renin genes have enhanced RAS activity and are more sensitive to cardiac manipulation of the angiotensin system (38), the more severe phenotype observed by Hein et al. (37) may reflect hyperstimulation of the already increased AT1R levels in transgenic animals. Importantly, and irrespective of the qualitative changes noted in these different Tg models, myocardial overexpression of the AT1R consistently leads to cardiac hypertrophy and enhanced mortality, be it under basal or stress conditions. It is therefore tempting to speculate that enhanced expression of AT1R may contribute to the progression of cardiac hypertrophy and the onset of heart failure in other experimental animal models and in humans.
The mechanisms by which myocardial AT1R causes biochemical and morphologic alterations in myocytes and nonmyocytes remain uncertain. In isolated cardiomyocytes, AT1Rs have been shown to be coupled to the Gαq protein (39); in this respect, it is interesting to note that Tg mice overexpressing Gαq from the same αMHC promoter develop a similar phenotype as the one described in this study (32). In cultured cardiomyocytes, Gαq overexpression also leads to cardiac hypertrophy (40); this is in contrast to the absence of hypertrophy in cardiomyocyte cultures deprived of fibroblasts and stimulated with AII, which had led to the hypothesis that AT1R-dependent alterations of myocytes require interactions with other cardiac cell types (21, 22). The similar cardiac phenotype that results from either Gαq or AT1R overexpression in vivo suggests that other G-coupled myocardial receptors may cooperate with AT1R to activate Gαq-dependent cell signaling pathways required for initiating and/or maintaining cardiac hypertrophy and remodeling. Endothelin type A receptors are also coupled to Gαq protein (41) and can apparently act synergistically with AT1R in myocyte signaling, at least in vitro (42). Moreover, the vasoactive peptide hormone endothelin-1, which is secreted from both myocytes and nonmyocytes, is a potent inducer of cardiomyocyte hypertrophy, and endothelin antagonists can block in vitro stretch-induced myocyte hypertrophy as effectively as AII antagonists (22, 42). Whether in vivo AII action on the heart involves endothelin-1 can now be addressed by using the AT1R Tg mice. Finally, the increased collagen deposition may reflect the action on nonmyocytes of a myocyte-derived growth factor such as AII, endothelin-1, and/or type β transforming growth factor, all of which are up-regulated in hypertrophied cardiomyocytes (43–49) and are known promoters of nonmyoctyte proliferation and/or inducers of collagen synthesis (43–46). The availability of the AT1R Tg model should allow further elucidation of the exact function of these and other growth factors in the cross-talk between the various cardiac cell types at specific stages of cardiac disease progression.
Acknowledgments
The authors are grateful to Lise Laroche for expert secretarial assistance and members of the Nemer laboratory and the Medical Research Council of Canada (MRC) Hypertension Group for helpful discussions. This work was supported by a grant from the Medical Research Council (MT-13056). M.N. is a Medical Research Council Scientist.
Abbreviations
- AII
angiotensin II
- AT1R
angiotensin II type 1 receptor
- Tg
transgenic
- αMHC
α-myosin heavy chain
- ANF
atrial natriuretic factor
- RAS
renin–angiotensin system
- Wt
wild-type
References
- 1.Chien K R, Zhu H, Knowlton K U, Miller-Hance W, van Bilsen M, O'Brien T X, Evans S M. Annu Rev Physiol. 1993;55:77–95. doi: 10.1146/annurev.ph.55.030193.000453. [DOI] [PubMed] [Google Scholar]
- 2.Hefti M A, Harder B A, Eppenberger H M, Schaub M C. J Mol Cell Cardiol. 1997;29:2873–2892. doi: 10.1006/jmcc.1997.0523. [DOI] [PubMed] [Google Scholar]
- 3.Anversa P, Kajstura J, Olivetti G. Curr Opin Cardiol. 1996;11:245–251. doi: 10.1097/00001573-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Swynghedauw B. Bull Acad Natl Med. 1998;182:665–682. [PubMed] [Google Scholar]
- 5.Colucci W S. Am J Cardiol. 1997;80:15L–25L. doi: 10.1016/s0002-9149(97)00845-x. [DOI] [PubMed] [Google Scholar]
- 6.Bishop J E. Mol Med Today. 1998;4:69–75. doi: 10.1016/S1357-4310(97)01193-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y C, Zhu Y Z, Gohlke P, Stauss H M, Unger T. Am J Physiol. 1997;80:110A–117A. doi: 10.1016/s0002-9149(97)00465-7. [DOI] [PubMed] [Google Scholar]
- 8.Hall J E. Am J Physiol. 1986;250:R960–R972. doi: 10.1152/ajpregu.1986.250.6.R960. [DOI] [PubMed] [Google Scholar]
- 9.Thurmann P A, Kenedi P, Schmidt A, Harder S, Rietbrock N. Circulation. 1998;98:2037–2042. doi: 10.1161/01.cir.98.19.2037. [DOI] [PubMed] [Google Scholar]
- 10.Harada K, Komuro I, Shiojima I, Hayashi D, Kudoh S, Mizuno T, Kijima, Matsubara H, Sugaya T, Murakami K, Yazaki Y. Circulation. 1998;97:1952–1959. doi: 10.1161/01.cir.97.19.1952. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Ohta K, Hamaguchi A, Yukimura T, Miura K, Iwao H. Hypertension. 1995;25:1252–1259. doi: 10.1161/01.hyp.25.6.1252. [DOI] [PubMed] [Google Scholar]
- 12.Susic D, Nunez E, Frohlich E D, Prakash O. Hypertension. 1996;28:265–268. doi: 10.1161/01.hyp.28.2.265. [DOI] [PubMed] [Google Scholar]
- 13.Booz G W, Baker K M. Hypertension. 1996;28:635–640. doi: 10.1161/01.hyp.28.4.635. [DOI] [PubMed] [Google Scholar]
- 14.Sadoshima J, Xu Y, Slayter H S, Izumo S. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 15.Lindpaintner K, Jin M W, Niedermaier N, Wilhelm M J, Ganten D. Circ Res. 1990;67:564–573. doi: 10.1161/01.res.67.3.564. [DOI] [PubMed] [Google Scholar]
- 16.Ogiku N, Ishida R, Saeki K, Sugiura M. Hypertens Res. 1996;19:179–187. doi: 10.1291/hypres.19.179. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki J, Matsubara H, Urakami M, Inada M. Circ Res. 1993;73:439–447. doi: 10.1161/01.res.73.3.439. [DOI] [PubMed] [Google Scholar]
- 18.Crabos M, Roth M, Hahn A W, Erne P. J Clin Invest. 1994;93:2372–2378. doi: 10.1172/JCI117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer T A, Ungureanu-Longrois D, Singh K, de Zengotita J, DeUgarte D, Alali A, Gadbut A P, Lee M A, Balligand J L, Kifor I, et al. Am J Physiol. 1997;272:H958–H968. doi: 10.1152/ajpheart.1997.272.2.H958. [DOI] [PubMed] [Google Scholar]
- 20.Booz G W, Baker K M. Cardiovasc Res. 1995;30:537–543. [PubMed] [Google Scholar]
- 21.Sil P, Sen S. Hypertension. 1997;30:209–216. doi: 10.1161/01.hyp.30.2.209. [DOI] [PubMed] [Google Scholar]
- 22.Harada M, Saito Y, Nakagawa O, Miyamoto Y, Ishikawa M, Kuwahara K, Ogawa E, Nakayama M, Kamitani S, Hamanaka I, et al. Heart Vessels. 1997. , Suppl. 12, 198–200. [PubMed] [Google Scholar]
- 23.Curnow K M, Pascoe L, Davies E, White P C, Corvol P, Clauser E. Mol Endocrinol. 1995;9:1250–1262. doi: 10.1210/mend.9.9.7491117. [DOI] [PubMed] [Google Scholar]
- 24.Palermo J, Gulick J, Colbert M, Fewell J, Robbins J. Circ Res. 1996;78:504–509. doi: 10.1161/01.res.78.3.504. [DOI] [PubMed] [Google Scholar]
- 25.Paradis P, Dumont M, Belichard P, Rouleau J L, Lemaire S, Brakier-Gingras L. Biochem Cell Biol. 1992;70:593–598. doi: 10.1139/o92-090. [DOI] [PubMed] [Google Scholar]
- 26.Charron F, Paradis P, Bronchain O, Nemer G, Nemer M. Mol Biol Cell. 1999;19:4355–4365. doi: 10.1128/mcb.19.6.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fareh J, Touyz R M, Schiffrin E L, Thibault G. Circ Res. 1996;78:302–311. doi: 10.1161/01.res.78.2.302. [DOI] [PubMed] [Google Scholar]
- 28.Agah R, Frenkel P A, French B A, Michael L H, Overbeek P A, Schneider M D. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, Murasawa S, Kizima K, Maruyama K, Horiuchi M, et al. J Clin Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akhter S A, Milano C A, Shotwell K F, Cho M C, Rockman H A, Lefkowitz R J, Koch W J. J Biol Chem. 1997;272:21253–21259. doi: 10.1074/jbc.272.34.21253. [DOI] [PubMed] [Google Scholar]
- 31.Robbins J. Trends Cardiovasc Med. 1997;7:185–191. doi: 10.1016/S1050-1738(97)00048-0. [DOI] [PubMed] [Google Scholar]
- 32.D'Angelo D D, Sakata Y, Lorenz J N, Boivin G P, Walsh R A, Liggett S B, Dorn G W. Proc Natl Acad Sci USA. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soonpaa M H, Field L J. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 34.Fujii N, Tanaka M, Ohnishi J, Yukawa K, Takimoto E, Shimada S, Naruse M, Sugiyama F, Yagami K, Murakami K. Biochem Biophys Res Commun. 1995;212:326–333. doi: 10.1006/bbrc.1995.1973. [DOI] [PubMed] [Google Scholar]
- 35.Nio Y, Matsubara H, Murasawa S, Kanasaki M, Inada M. J Clin Invest. 1995;95:46–54. doi: 10.1172/JCI117675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann S, Krause T, Pinto Y, Bulkema H, Bohlender J, Nishimura H, Inagami T, Ganten D, Urata H. Hypertension. 1998;28:535. (abstr.). [Google Scholar]
- 37.Hein L, Stevens M E, Barsh G S, Pratt R E, Kobilka B K, Dzau V J. Proc Natl Acad Sci USA. 1997;94:6391–6396. doi: 10.1073/pnas.94.12.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzolai L, Nussberger J, Aubert J F, Brunner D B, Gabbiani G, Brunner H R, Pedrazzini T. Hypertension. 1998;31:1324–1330. doi: 10.1161/01.hyp.31.6.1324. [DOI] [PubMed] [Google Scholar]
- 39.Sadoshima J, Izumo S. EMBO J. 1996;15:775–787. [PMC free article] [PubMed] [Google Scholar]
- 40.Adams J W, Sakata Y, Davis M G, Sah V P, Wang Y, Liggett S B, Chien K R, Brown J H, Dorn G W. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilal-Dandan R, Ramirez M T, Villegas S, Gonzalez A, Endo-Mochizuki Y, Brown J H, Brunton L L. Am J Physiol. 1997;272:H130–H137. doi: 10.1152/ajpheart.1997.272.1.H130. [DOI] [PubMed] [Google Scholar]
- 42.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Hiroi Y, Mizuno T, Maemura K, Kurihara H, Aikawa R, et al. J Biol Chem. 1996;271:3221–3228. doi: 10.1074/jbc.271.6.3221. [DOI] [PubMed] [Google Scholar]
- 43.Butt R P, Laurent G J, Bishop J E. Eur J Cell Biol. 1995;68:330–335. [PubMed] [Google Scholar]
- 44.Brilla C G, Zhou G, Matsubara L, Weber K T. J Mol Cell Cardiol. 1994;26:809–820. doi: 10.1006/jmcc.1994.1098. [DOI] [PubMed] [Google Scholar]
- 45.Eghbali M, Tomek R, Sukhatme V P, Woods C, Bhambi B. Circ Res. 1991;69:483–490. doi: 10.1161/01.res.69.2.483. [DOI] [PubMed] [Google Scholar]
- 46.Guarda E, Katwa L C, Myers P R, Tyagi S C, Weber K T. Cardiovasc Res. 1993;27:2130–2134. doi: 10.1093/cvr/27.12.2130. [DOI] [PubMed] [Google Scholar]
- 47.Rizvi M A, Katwa L, Spadone D P, Myers P R. J Mol Cell Cardiol. 1996;28:243–252. doi: 10.1006/jmcc.1996.0023. [DOI] [PubMed] [Google Scholar]
- 48.MacLellan W R, Brand T, Schneider M D. Circ Res. 1993;73:783–791. doi: 10.1161/01.res.73.5.783. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi T, Miyauchi T, Sakai S, Kobayashi M, Yamaguchi I, Goto K, Sugishita Y. Am J Physiol. 1999;276:H1197–H1206. doi: 10.1152/ajpheart.1999.276.4.H1197. [DOI] [PubMed] [Google Scholar]