Abstract
Highly coevolved pollination mutualism accompanied by reciprocal diversification has been known in only two plant genera, Ficus (Moraceae) and Yucca (Agavaceae), which are pollinated exclusively by obligate seed-parasitic wasps and moths, respectively. An additional, highly diversified, species-specific pollination mutualism between a monoecious tree genus, Glochidion (Euphorbiaceae), and a moth genus, Epicephala (Gracillariidae), is presented here. At night, the small female moth actively deposits pollen on the cryptic stigma of the female flower by using its proboscis, then oviposits into the style. The moth larva infests only a portion of the developing seeds within fruit. We confirmed that at least three Glochidion species are pollinated only by their respective seed-parasitic moth species, which could be distinguished by genitalic morphology and mitochondrial DNA sequences. These results and widespread evidence of limited seed infestation by the moths associated with Glochidion species suggest that speciation based on the highly specialized Glochidion stylar structure and moth oviposition behavior have promoted species diversification in Glochidion and its pollinators.
All species of Ficus (Moraceae; refs. 1–3) and Yucca (Agavaceae; refs. 4 and 5) are pollinated exclusively by obligate seed-parasitic wasps and moths, respectively. Among 220,000 angiosperm species, however, such obligate pollination mutualisms between plants and seed-parasitic insects are rare, probably because incipient mutualisms are dominated by abundant pollinators that visit flowers for nectar or pollen (6). Although several plant species have recently been found to be pollinated by seed-parasitic moths (6, 7) and flies (8), such mutualism is restricted to a few plant species in each genus. Accordingly, obligate plant–pollinator mutualism accompanied by reciprocal diversification is surprisingly rare and confirmed only in the fig/fig wasp (9, 10) and yucca/yucca moth (11, 12) systems.
Glochidion, a monoecious tree genus of Euphorbiaceae (recently treated as a separate family, Phyllanthaceae), has minute apetalous female flowers with highly specialized styles (13, 14). The genus consists of 318 species ranging from tropical Asia to Australia, Polynesia, and Madagascar (15). Although its pollination system was unknown, Glochidion trees usually bear many fruits, most of which are infested by small moth larvae. Our field observations revealed that flowers were actively pollinated by seed-parasitic gracillariid moths that oviposited into styles. Here we present evidence of a widespread obligate pollination mutualism between Glochidion trees and their seed-parasitic moths.
Materials and Methods
Field Observation.
We observed the pollination biology of three Glochidion species in Japanese evergreen forests: acuminatum at Nagakumo-toge on Amami-Ohshima Island (28°26′N, 129°35′E; May 6–8, 1997, May 8–10, 2001, and May 10–12, 2002); zeylanicum at Nabeguro on Amami-Ohshima Island (28°28′N, 129°42′E; May 9–11, 2002); and obovatum at Ena, Yura, Wakayama Prefecture (33°60′N, 135°07′E; May 19–20, 2002). Flower visitors and nectar secretion were observed during the day and at night. After field observations, we collected female flowers, counted the number of pollen grains attached to the stigmas, and dissected the flowers to detect eggs laid by the moths. We sampled fruits of the three Glochidion species to determine infestation by seed-parasitic moths on December 13, 1997, June 23, 2002, and July 7, 2002, respectively. Samples of the seed-parasitic moths for DNA analysis were taken at various localities in Japan and Taiwan: acuminatum from Amami and Okinawa Islands; zeylanicum from Yaku, Amami, Tokuno, Okinawa, Ishigaki, and Iriomote Islands; obovatum from Wakayama, Miyazaki and Yaku Islands; rubrum from Ishigaki Island and Taiwan; lanceolatum from Ishigaki, Iriomote, and Yonaguni Islands; and phillipicum from Nanjin and Manshu in Taiwan.
Analysis of Genetic Variation.
We analyzed nucleotide sequence variation among the six Epicephala from fruits of six Glochidion species. We extracted total DNA from live or ethanol-preserved adults and larvae. A fragment of the mitochondrial cytochrome oxidase subunit I gene (COI) was PCR amplified by using the primers 5′-ATAATTTTTTTTATAGTTATAC-3′ (19) and 5′-ATAAATGGGGCTTAAATCC-3′ and directly sequenced using the internal sequencing primers 5′-GAAGTTTATATTTTAATTTTACC-3′ and 5′-GATGAGCTCAAACAATAAAACCTA-3′. All sequences were deposited in the GenBank database (accession nos. AY221964–AY221981).
Results and Discussion
Glochidion flowers are dimorphic, consisting of a pedunculate male flower with unfolded sepals and connate ellipsoid stamens, with a sessile or shortly pedunculate female flower composed of folded sepals and a united columnar style. The style has a narrow pit at its lobed tip. The inner surface of the pit is the stigma. This cryptic stigma is unlikely to be pollinated by wind or ordinary insect visitors. We therefore made field observations of the pollination of three Glochidion species in Japan.
Trees of G. acuminatum have male and female flowers in separate axillary clusters on each branch (Fig. 1a). Male flowers are aggregated at the base, and the female flowers are at the apical part of each branch. Female flowers are generally six-ovuled. Neither male nor female flowers secrete nectar. In the daytime, the flowers were rarely visited by insects, whereas various insects passed by the inflorescence.
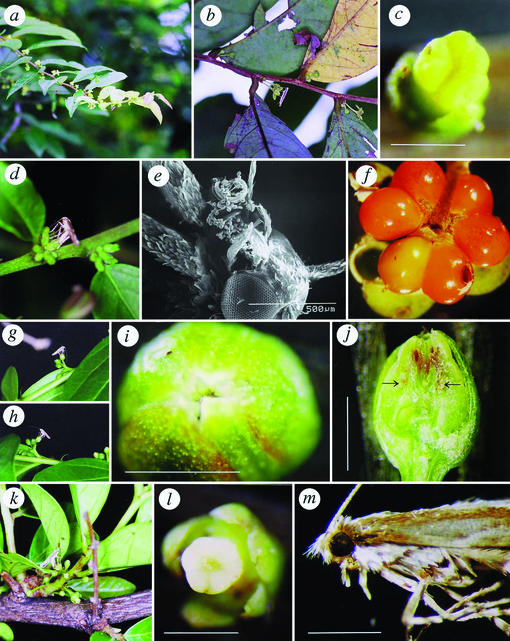
Figure 1.
Flowers and pollinators of G. acuminatum (a–f), G. zeylanicum (g–j), and G. obovatum (k–m). (a) A branch bearing many male and female flowers, one of which is visited by a moth, Epicephala sp. 1. (b) A female moth visiting a male flower. (c) Pollinated stigma. (d) An ovipositing Epicephala moth. (e) Pollen-loaded proboscis of a female moth. (f) A fruit with two seeds infested by a moth larva, which had emerged from the hole. (g) An Epicephala sp. 2 moth actively pollinating a female flower. (h) An ovipositing Epicephala moth. (i) Dorsal view of a female flower, showing the pollinated cryptic stigma. (j) Cross section of a female flower with two eggs (shown by arrows). (k) An ovipositing Epicephala sp. 3 moth. (l) Pollinated stigma. (m) Lateral view of a female moth, whose proboscis is loaded with pollen. [Scale bars, 1 mm (except in e).]
However, beginning in the evening and continuing until midnight, the flowers were visited frequently by a gracillariid moth, Epicephala sp. 1. Female moths visited male flowers to collect pollen by inserting their proboscis into the anthers (Fig. 1b). After flight migration among trees or within a tree, female moths visited female flowers. All female Epicephala moths netted around the Glochidion trees had G. acuminatum pollen grains attached to their ciliated proboscides (mean pollen number 88 ± 66, n = 25; Fig. 1e), whereas proboscides of males had no pollen grains (0 ± 0, n = 19). The behavior of the female moths on female flowers was remarkable. Visiting a cluster of female flowers, a female uncoiled its proboscis, deposited the pollen grains onto the cryptic stigma, and bent its abdomen to insert its long ovipositor into the stylar pit (Fig. 1d). The time spent in pollination and oviposition was 46 ± 10 s (n = 14). The female walked along the branch, visiting each female flower sequentially and repeating the stereotypic behavior.
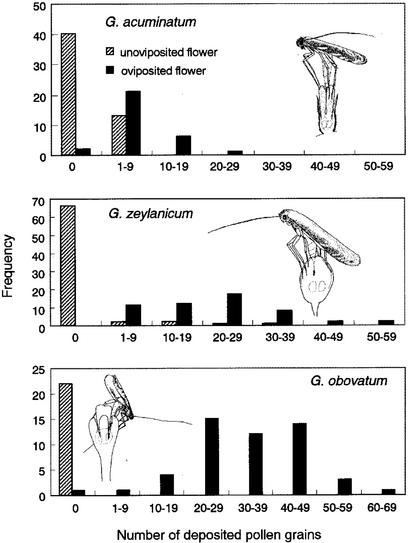
We examined pollen attachment and moth eggs in female flowers by dissecting the styles under a microscope. Normally, a female laid an egg in a flower just above the ovules at the base of each oviposited style, and an average female flower received 1.71 eggs (Table 1). Oviposited flowers were consistently pollinated (Fig. 1c), whereas unoviposited flowers were very rarely pollinated (Fig. 2). The mean number of pollen grains deposited on an oviposited style was 7.8 ± 4.8, significantly greater than that of an unoviposited style (1.2 ± 2.4, Mann–Whitney U test; U = 154, P < 0.0001). Although few, the pollen grains attached to each style were enough to fertilize all six ovules in an ovary.
Table 1.
Comparison of moth pollination, oviposition, and seed infestation among three Glochidion species
| Glochidion species | Pollen grains | Laid eggs | Ovules | Intact seeds | Infested seeds | Sterile/aborted seeds |
|---|---|---|---|---|---|---|
| acuminatum | 7.8 ± 4.8 (30) | 1.7 ± 0.8 (262) | 6.1 ± 0.4 (364) | 3.3 ± 1.7 (364) | 1.8 ± 1.7 (364) | 0.9 ± 1.2 (364) |
| zeylantcum | 32.4 ± 12.3 (51) | 2.3 ± 1.1 (51) | 10.3 ± 0.9 (104) | 2.1 ± 2.7 (104) | 7.6 ± 2.9 (104) | 0.6 ± 1.0 (104) |
| obovatum | 26.7 ± 11.2 (40) | 1.6 ± 0.6 (40) | 11.9 ± 0.4 (56) | 3.1 ± 2.6 (56) | 4.8 ± 2.5 (56) | 1.3 ± 0.6 (56) |
Although the typical ovule number is different among the three species, 20–54% of seeds were intact even after multiple oviposition by Epicephala moths and infestation by nonpollinating seed-parasitic moths. Means ± SD are shown. Numbers in parentheses represent the number of examined fruits.
Figure 2.
Frequency distributions of the number of pollen grains attached to oviposited (solid) and unoviposited (shaded) stigmas of three Glochidion species (acuminatum, zeylanicum, and obovatum). The typical ovipositing postures and oviposited eggs of each Epicephala moth species are shown in each Inset.
Active pollination and the oviposition into styles by gracillariid moths was also observed on G. zeylanicum and G. obovatum, which have different style structures (Figs. 1 and 2). G. zeylanicum has ovoid bud-like female flowers whose styles are almost completely enclosed by sepals (Fig. 1i). The flowers have small openings at the apical tip that lead to the narrow stigma pit of the fused styles. Female flowers were visited at night by Epicephala sp. 2, which actively pollinated the female flower (Fig. 1g), inserted its long ovipositor into the narrow stigma pit (Fig. 1h), and laid an egg (Fig. 1j). Female flowers of G. obovatum are columnar like those of G. acuminatum, but differ in having distinctly swollen ovaries. At night the flowers were actively pollinated by Epicephala sp. 3, which inserted its abdomen between the style and calyx and laid an egg into the locules directly through the ovary wall, rather than through the stigma (Fig. 1 k–m).
Fertilized ovules began to develop, whereas unpollinated female flowers abscised shortly thereafter. The hatched moth larva bored into the ovary and consumed a few developing seeds within a fruit. In G. acuminatum, a larva usually consumed two seeds to complete larval growth and escaped from the fruit to pupate on the litter (Fig. 1f). The life cycles of both the plant and its pollinator moth are inseparably linked (Fig. 3). Seed destruction was caused mostly by Epicephala moths, but nonpollinating seed-parasitic moths of Pyralidae and Tortricidae also infested seeds. On average, one fruit had 6.09 ovules, of which 1.80 were infested by moth larvae, 3.33 were intact, and 0.91 were sterile or aborted. The overall outcomes were similar among the three Glochidion species (Table 1).
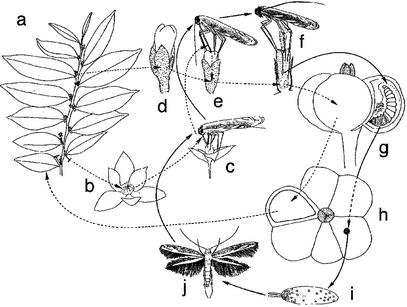
Figure 3.
Life cycles of G. acuminatum (broken arrows) and its pollinator moth, Epicephala sp. 1 (solid arrows). a, Branch bearing male and female flowers; b, male flower; c, female moth collecting pollen on a male flower; d, female flower; e, female moth depositing pollen on stigma; f, moth laying an egg into a style; g, moth larva infesting seeds; h, fruit with intact and infested seeds; i, moth puparium with an exuvia; j, emerged moth.
Surveying other Glochidion species, we found that all six species harbored an individual, undescribed, seed-parasitic Epicephala species that could be distinguished by its genitalic morphology. Host-specificity of the moths was not surprising because several Glochidion species often co-occur at our study sites without apparent hybridization. We confirmed host-specificity of the moths by investigating nucleotide sequence variation within 1,325 bp of the mitochondrial cytochrome oxidase subunit I gene (COI) among Epicephala. Pairwise sequence divergence between Epicephala moths reared from different Glochidion species was 3.1–9.1% (mean 7.3%), whereas sequence divergence between moths reared from the same Glochidion species in different locations was <0.5%.
These findings suggest that at least three Glochidion species are pollinated by a species-specific seed-parasitic Epicephala species, at the cost of infested seeds. It is notable that the female moth has an exceptionally long ovipositor to insert an egg into a style and a ciliated proboscis to collect pollen. The moth actively pollinated flowers, similar to fig wasps and yucca moths. The seed-parasitic habit of Epicephala is unique in Gracillariidae, most species of which are leaf miners. Other Epicephala larvae infest Phyllanthus seeds and Caesalpinia flower buds (17).
Twelve additional Glochidion species in New Caledonia, Fiji, Australia, Malaysia, and Myanmar, all had traces of limited seed infestation by the moths. Thus, Glochidion–Epicephala mutualism may be widespread among the 318 known Glochidion species, whereas Epicephala has not yet been found in Madagascar. This mutualism shares many characteristics with fig/fig wasp and yucca/yucca moth mutualisms, because the reward for the pollinator consists of developing ovules or seeds. Furthermore, the sister groups of these pollinator taxa are endophytic herbivores and the pollinators are females with elongate ovipositors (1–5). Outstanding diversification has occurred only in Ficus (700 spp.) and Glochidion, both of which are tropical monoecious or gynodioecious woody plants that have highly specialized styles into which small pollinating insects oviposit (3).
In the Malay Archipelago, Glochidion is the largest genus (150 spp.) of Euphorbiaceae (15), and the principal species diagnostic characteristic is the structure of the style (13, 14). Because pollinating moths oviposit into styles by using diverse and specific methods, the length of their ovipositor and their oviposition behavior are crucial for such specialization. Thus, the specialized structure of the Glochidion style and the specialized oviposition behavior of the moths may well serve as barriers against both Glochidion hybridization and host-shift by the moths. In turn, plant speciation based on these traits provides a selective basis for speciation and high diversity. The coevolution of species is one of the major processes contributing to the earth's biodiversity (18, 19), and the Glochidion–Epicephala relationship will provide a model system for comparative analyses of coevolutionary processes and mutualistic interactions.
Acknowledgments
We thank D. W. Roubik and S. Sakai for critical reading of the manuscript; T. Kumata for taxonomical suggestion on the moth; T. Terachi, T. Sota, and T. Sugiyama for technical support; and A. Naiki and H. Samejima for plant materials. This work was supported by Grant-in-Aid for Scientific Research 02440217 from the Japan Ministry of Education, Science, and Culture.
Footnotes
References
- 1.Wiebes J T. Annu Rev Ecol Syst. 1979;10:1–12. [Google Scholar]
- 2.Janzen D H. Annu Rev Ecol Syst. 1979;10:13–51. [Google Scholar]
- 3.Weiblen G D. Annu Rev Entomol. 2002;47:299–330. doi: 10.1146/annurev.ento.47.091201.145213. [DOI] [PubMed] [Google Scholar]
- 4.Riley C V. Missouri Bot Gard Annu Rep. 1892;3:99–158. [Google Scholar]
- 5.Powell J A. Trends Ecol Evol. 1992;7:10–15. doi: 10.1016/0169-5347(92)90191-D. [DOI] [PubMed] [Google Scholar]
- 6.Thompson N J, Pellmyr O. Ecology. 1992;73:1780–1791. [Google Scholar]
- 7.Fleming T H, Holland J N. Oecologia. 1998;114:368–375. doi: 10.1007/s004420050459. [DOI] [PubMed] [Google Scholar]
- 8.Pellmyr O. Oecologia. 1989;78:53–59. doi: 10.1007/BF00377197. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama J. In: Biodiversity and Evolution. Arai M, Kato M, Doi Y, editors. Tokyo: National Science Museum Foundation; 1995. pp. 115–130. [Google Scholar]
- 10.Herre E A, Machado C A, Bermingham E, Nason J D, Windsor D M, McCafferty S S, Van Houten W, Bachmann K. J Biogeogr. 1996;23:521–530. [Google Scholar]
- 11.Pellmyr O, Thompson J N, Brown J M, Harrison R G. Am Nat. 1996;148:827–847. [Google Scholar]
- 12.Pellmyr O. Syst Entomol. 1999;24:243–271. [Google Scholar]
- 13.Airy Shaw H K. Kew Bull. 1978;32:370–379. [Google Scholar]
- 14.Chakrabarty T, Gangopadhyay M. J Econ Taxon Bot. 1995;19:173–234. [Google Scholar]
- 15.Govaerts R, Frodin R G, Radcliffe-Smith A. World Checklist and Bibliography of Euphorbiaceae. Kew, London: Royal Botanic Gardens; 2000. [Google Scholar]
- 16.Tanaka H, Roubik D W, Kato M, Liew F, Gunsalam G. Insectes Soc. 2001;48:44–51. [Google Scholar]
- 17.Fletcher T B. Mem Dep Agric India Bot Ser. 1920;6:137–167. [Google Scholar]
- 18.Thompson J N. The Coevolutionary Process. Chicago: Univ. of Chicago Press; 1994. [Google Scholar]
- 19.Thompson J N. Science. 1999;284:2116–2118. doi: 10.1126/science.284.5423.2116. [DOI] [PubMed] [Google Scholar]