Abstract
Polycystic kidney disease (PKD) is the most common genetic cause of renal failure in humans. Several proteins that are encoded by genes associated with PKD have recently been identified in primary cilia in renal tubular epithelia. These findings have suggested that abnormalities in cilia formation and function may play a role in the pathogenesis of PKD. To directly determine whether cilia are essential to maintain tubular integrity, we conditionally inactivated KIF3A, a subunit of kinesin-II that is essential for cilia formation, in renal epithelia. Constitutive inactivation of KIF3A produces abnormalities of left–right axis determination and embryonic lethality. Here we show that tissue-specific inactivation of KIF3A in renal tubular epithelial cells results in viable offspring with normal-appearing kidneys at birth. Cysts begin to develop in the kidney at postnatal day 5 and cause renal failure by postnatal day 21. The cyst epithelial cells lack primary cilia and exhibit increased proliferation and apoptosis, apical mislocalization of the epidermal growth factor receptor, increased expression of β-catenin and c-Myc, and inhibition of p21CIP1. These results demonstrate that the absence of renal cilia produces both the clinical and cell biological findings associated with PKD. Most generally, the phenotype of Kif3a mutant mice suggests a role for primary cilia in the maintenance of lumen-forming epithelial differentiation.
Autosomal dominant polycystic kidney disease (PKD) is a common genetic disorder that affects 1 in 500 individuals and is characterized by the accumulation of fluid-filled cysts in the kidney, liver, and other organs (1). The renal cysts originate from the epithelia of the nephrons and collecting ducts and are distinguished by increased proliferation, poor differentiation, and abnormalities in cell polarity, fluid secretion, and extracellular matrix (2). Similar clinical phenotypes occur due to mutations of either PKD1 on chromosome 16 or PKD2 on chromosome 4. The PKD1 gene product, polycystin-1, is an integral membrane protein containing a large extracellular domain that is postulated to have receptor function (3). Polycystin-2, which is encoded by PKD2, is a calcium channel that interacts with polycystin-1 and functions in a common signaling pathway (1, 3).
Although the genetics of autosomal dominant PKD has been defined, the pathogenesis of the disease remains unclear. Recent observations suggest that abnormalities of primary cilia play a role in cyst formation. Primary cilia are hair-like organelles that consist of an axoneme containing nine peripheral pairs of microtubules (9 + 0 pattern) surrounded by a ciliary membrane (4). Most cells, including all renal epithelial cells except intercalated cells, contain a single, immotile primary cilium on the cell surface. Renal cilia project into the tubule lumen and respond to flow or mechanical bending by stimulating an increase in cytosolic calcium, which suggests that they have a mechanosensory function (5). Polycystin-2 is located in primary cilia in cultured renal cells and in native kidney tubules (6, 7). Polycystin-1 is also expressed in renal cilia, and cells lacking polycystin-1 do not increase calcium influx in response to flow (7, 8). Several mouse models of recessive PKD involve proteins that are also localized in renal cilia (9–11).
The synthesis of primary cilia involves a process known as intraflagellar transport in which large particles or “rafts” containing protein cargo are transported bidirectionally along the ciliary axoneme (12). The anterograde movement of proteins along the axoneme is mediated by kinesin-II, a heterotrimeric protein composed of two motor subunits (KIF3A and KIF3B) and one nonmotor subunit (KAP3). Conventional knockout mice that completely lack KIF3A fail to synthesize cilia in the embryonic node and exhibit randomization of left–right asymmetry and structural abnormalities of the neural tube, pericardium, branchial arches, and somites (13, 14). KIF3A is expressed in the kidney but its role there has not been established, because conventional Kif3a knockout mice are embryonic lethal before the onset of renal organogenesis. To determine the function of KIF3A in the kidney and the role of cilia in the pathogenesis of PKD, we created a kidney-specific knockout of Kif3a in transgenic mice.
Materials and Methods
Transgenic Mice.
creKsp transgenic mice containing 1.3 kb of the Ksp-cadherin promoter linked to the coding region of Cre recombinase were produced as described (15). Kif3afl/+ mice containing loxP sites flanking exon 2 of the Kif3a gene were produced by homologous recombination in embryonic stem cells, and Kif3a+/− mice were produced by deletion of exon 2 as described (14). The genetic background of the mice was C57BL/6. Some animals were treated with [1-deamino-8-d-arginine]vasopressin (ddAVP; 0.4 μg/kg, i.p., twice 30 min apart) 30 min before analysis or BrdUrd (50 μg/g, i.p.) 2 h before analysis. All experiments were performed under the auspices of the Institutional Animal Care and Research Advisory Committee.
Ab Staining.
Formalin-fixed, paraffin-embedded tissue sections and paraformaldehyde-fixed cryosections were stained with Abs to acetylated tubulin (Sigma), aquaporin-2 (AQP2; gift from M. Knepper, National Heart, Lung, and Blood Institute) (16), Na-K-Cl cotransporter (M. Knepper), Na-Cl cotransporter (M. Knepper), Tamm–Horsfall protein (gift from J. Hoyer, University of Pennsylvania), AQP3 (Chemicon), epidermal growth factor receptor (EGFR; Sigma), β-catenin (BD Biosciences), p21CIP1 (Upstate, Charlottesville, VA), and p16INK4a (Santa Cruz Biotechnology) as described (17). Secondary Abs were conjugated to Cy3 (Jackson ImmunoResearch), Alexa Fluor 594, or Alexa Fluor 488 (Molecular Probes). BrdUrd incorporation was measured by using an FITC-coupled primary Ab (BD Biosciences). Photomicrographs were obtained with a Zeiss Axioplan 2 microscope, and image deconvolution was performed with openlab software (Improvision, Lexington, MA).
Scanning Electron Microscopy.
Scanning electron microscopy was performed by the University of Texas Southwestern Molecular and Cellular Imaging Facility. Kidneys were perfused with Karnovsky's fixative, sectioned at 2–4 mm, postfixed with Karnovsky's fixative, and washed with 0.1 M cacodylate buffer containing 7.5% sucrose. Samples were incubated in 1% osmium tetroxide, rinsed, dehydrated through graded ethanol, and critical-point dried. Dried samples were mounted on stubs, sputter-coated with palladium/gold, and visualized with a JEOL JSM-840A microscope.
RT-PCR and Northern Blot Analysis.
RNA extraction and RT-PCR were performed by using methods similar to those described (18). The primers used to amplify Kif3a exon 2 were CGGAAAGCTGCGATAATGTGAAG and GCCTTCCAGAACAGAATCAATAATTGG. Gapdh primers were used as a positive control. Northern blot analysis was performed as described (18). Filters were hybridized with a 32P-labeled Kif3a cDNA (exon 2) or a Gapdh cDNA as a loading control.
Immunoblot Analysis.
Kidneys were homogenized with a Potter–Elvehjem homogenizer in medium containing 10 mM Hepes (pH 7.6), 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and protease and phosphatase inhibitors. Subcellular fractions were purified as described by DeBose-Boyd et al. (19). Whole-cell extracts and subcellular fractions were analyzed by immunoblotting with Abs to KIF3A (BD Biosciences), β-tubulin (National Institute of Child Health and Human Development, Hybridoma Bank), β-catenin, E-cadherin (BD Biosciences), c-Myc (Santa Cruz Biotechnology), or polycystin-2 (20) as described (17).
Terminal Transferase-Mediated Fluorescein-dUTP Nick End Labeling (TUNEL) Analysis.
TUNEL was performed as described (21).
Results
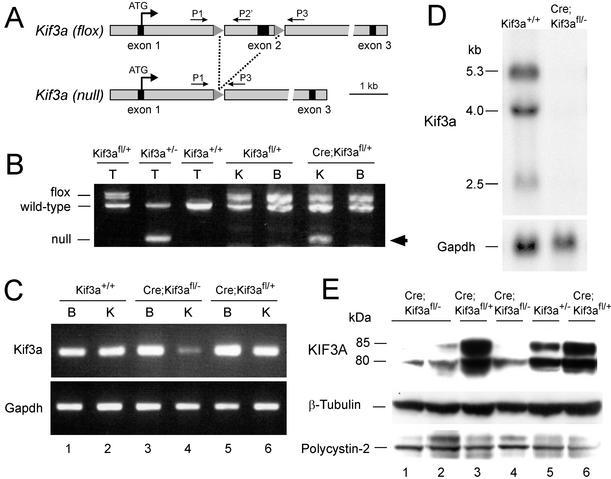
To test whether inhibition of renal ciliogenesis produced PKD, we generated a kidney-specific knockout of Kif3a by using Cre/loxP recombination. creKsp transgenic mice (15) that express Cre recombinase exclusively in tubular epithelial cells in the kidney and developing genitourinary tract were crossed with Kif3afl/+ mice (22) containing two loxP sites flanking exon 2 of the Kif3a gene (Fig. 1A). Cre-mediated recombination between the loxP sites results in deletion of exon 2, which introduces a frameshift in the coding region that results in premature termination of translation. PCR analysis of cre;Kif3afl/+ progeny showed that the recombined (null) allele was present in genomic DNA from the kidney but was absent in the brain, consistent with the known tissue specificity of the creKsp transgene (15) (Fig. 1B). Competitive quantitative PCR (22) showed that recombination could be detected at postnatal day (P)1 and increased to 40% at P28. Incomplete recombination was anticipated because the creKsp transgene is expressed only in tubular epithelial cells in the kidney. cre;Kif3afl/+ mice exhibited no kidney abnormalities.
Figure 1.
Kidney-specific inactivation of the Kif3a gene. (A) Map of the Kif3afl allele before and after recombination. Primers P1 and P3 used for genotyping have been described (22). The sequence of primer P2′ was GGCAGATGGATCTCTGTGAGTTTG. (B) PCR products obtained after amplification of DNA from tails (T) of mice with the genotypes indicated and the kidneys (K) and brains (B) from Kif3afl/+ mice and cre;Kif3afl/+ littermates. Arrow indicates the band corresponding to the recombined (null) allele. (C) RT-PCR analysis of Kif3a and Gapdh mRNA expression in kidney (K) and brain (B) from Kif3a mutants (lanes 3 and 4) and controls (lanes 1 and 2 and 5 and 6) at P28. (D) Northern blot analysis of RNA from mutant kidneys (right lane) and control kidneys (left lane) at P28. (E) Immunoblot analysis of kidney extracts from Kif3a mutants (lanes 1, 2, and 4) and controls (lanes 3, 5, and 6) at P28.
To produce a kidney-specific Kif3a knockout, cre;Kif3a+/− mice were crossed with cre;Kif3afl/+ mice, yielding cre;Kif3afl/− progeny (designated Kif3a mutants). The expression of Kif3a mRNA was decreased in the kidneys of Kif3a mutants compared with their control littermates (Fig. 1 C and D). No differences in expression were observed in the brain. Real-time PCR showed that the mRNA levels in mutant kidneys were 51% of controls at P1 and 7% of controls at P21. The expression of KIF3A protein was decreased in the kidneys of Kif3a mutants at P28 (Fig. 1E), whereas no differences in expression were detected at P1. Kif3a mutants were born in the expected Mendelian ratios and did not show situs inversus or other gross organ abnormalities. By P35 the mutant mice exhibited lethargy and growth retardation and were killed.
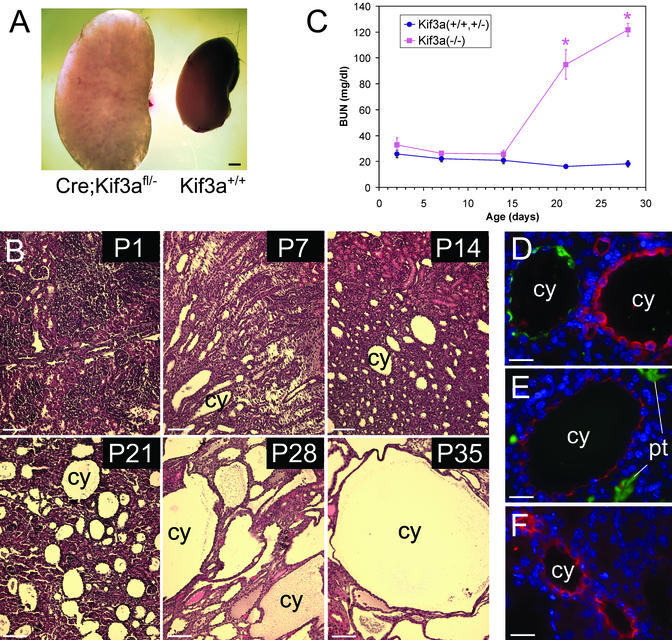
The kidneys of adult Kif3a mutants were grossly enlarged (Fig. 2A). Microscopic examination revealed that the kidneys contained multiple, fluid-filled cysts in both the cortex and medulla. Cysts first appeared at P5 as fusiform dilatations of the collecting ducts in the renal medulla. By P35 the renal parenchyma was completely replaced with large cysts (Fig. 2B). No renal cysts were observed in control (cre;Kif3afl/+, Kif3afl/−, or Kif3a+/+) littermates. During early stages of cyst formation the intervening parenchyma was relatively well preserved. More advanced cysts were surrounded by atrophic tubules and areas of interstitial fibrosis. Measurements of blood urea nitrogen showed that the renal function of Kif3a mutants was not different from that of their control littermates up to P14. Thereafter, the mutant mice rapidly developed azotemia (Fig. 2C). Male and female mice were affected equally, and there were no differences in the phenotype among different age-matched mutants.
Figure 2.
PKD in Kif3a mutant mice. (A) Gross appearance of kidneys from a Kif3a mutant (left) and a wild-type littermate (right) at P28. (B) Hematoxylin/eosin-stained sections of kidneys from Kif3a mutants at different ages. (C) Blood urea nitrogen of mutant mice (red) and control littermates (blue). Error bars = SEM. *, statistically different from control (P < 0.001, Student's t test). (D–F) Staining of cystic kidneys at P21 for AQP2 (green) and Tamm–Horsfall protein (red) (D), Lotus tetragonolobus agglutinin (green) and Na-K-Cl cotransporter (red) (E), and Na-Cl cotransporter (red) (F). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). cy, cyst; pt, proximal tubule. [Bars = 1 mm (A), 80 μm (B), and 30 μm (D–F).]
To determine the tubular origins of the cysts, the mutant kidneys were stained with Abs or lectins that label specific nephron segments (Fig. 2 D–F). Of the cysts, 73% expressed AQP2 and were labeled with Dolichos biflorus agglutinin, indicating an origin from renal collecting ducts. Five percent of the cysts expressed the Na-K-Cl cotransporter and Tamm–Horsfall protein, indicating an origin from thick ascending limbs of loops of Henle. Eight percent of the cysts expressed the Na-Cl cotransporter, indicating an origin from distal convoluted tubules. Fourteen percent of the cysts did not express any of the markers tested, and no glomerular cysts or cysts labeled with the proximal tubular marker Lotus tetragonolobus agglutinin were found. These results are consistent with the known high expression of the creKsp transgene in the distal nephron and collecting ducts (15).
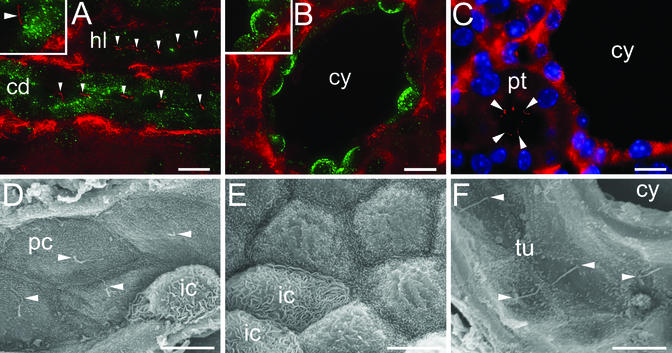
To determine the effects of Kif3a inactivation on the formation of renal cilia, kidney sections were stained with an Ab to acetylated tubulin that preferentially labeled the ciliary axoneme. In the renal medulla of control mice, primary cilia were present on the luminal surface of cells in the loops of Henle and collecting ducts (Fig. 3A). In contrast, Kif3a mutants exhibited no tubulin-positive cilia on the luminal surfaces of most cyst epithelial cells derived from the same nephron segments (Fig. 3B). In the cortex, cilia were present in normal proximal tubules adjacent to the cysts (Fig. 3C). Scanning electron microscopy showed that principal cells in the collecting ducts of control mice contained a single cilium projecting into the tubule lumen (Fig. 3D). In Kif3a mutants, cilia were absent from the surface of epithelial cells lining the cysts but were present in adjacent noncystic tubules (Fig. 3 E and F). Cilia were present in newborn collecting ducts before the onset of cyst formation, indicating that they were formed initially then were lost during postnatal development.
Figure 3.
Absence of renal cilia in Kif3a mutant mice. (A–C) Immunostaining of acetylated tubulin (red) and AQP2 (green) in kidneys from control mice (A) and Kif3a mutants (B and C) at P21. Arrowheads, primary cilia; cd, collecting duct; hl, loop of Henle; pt, proximal tubule; cy, cyst. Insets show higher-magnification images. (Bar = 10 μm.) (D–F) Scanning electron micrographs of kidneys from control mice (D) and Kif3a mutants (E and F) at P21. Arrowheads, primary cilia; pc, principal cell; ic, intercalated cell; tu, tubule. [Bars = 10 (A–C) and 5 μm (D–F).]
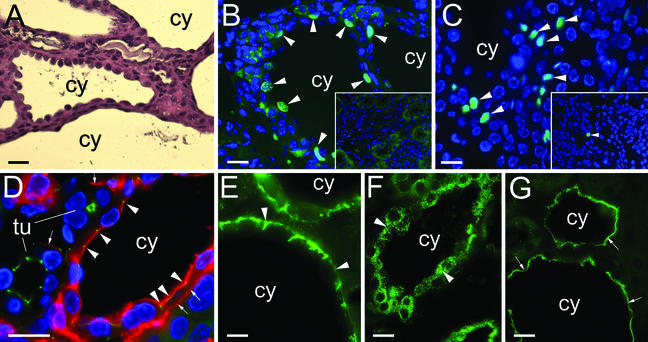
The renal cysts in Kif3a mutant mice were lined by a single layer of columnar, cuboidal, or squamous epithelial cells (Fig. 4A). Cyst epithelial cells in humans with PKD exhibit abnormalities in cell proliferation, apoptosis, and epithelial polarity (2). Cyst epithelial cells in Kif3a mutant mice incorporated BrdUrd, indicating active DNA synthesis (Fig. 4B). After a single injection of BrdUrd, 9.5 ± 1.2% of cyst epithelial cells showed incorporation, whereas no incorporation was detected in renal tubules in control littermates. Terminal transferase-mediated fluorescein-dUTP nick end labeling (TUNEL) analysis showed that apoptosis was also increased in the cystic kidneys (Fig. 4C). Of the cells in cystic kidneys, 4.0 ± 0.6% were TUNEL-positive compared with 0.2 ± 0.1% in normal controls. Polycystin-2 was expressed at equal levels in kidneys from mutant mice and control littermates (Fig. 1E), and the expression in cyst epithelial cells was in a reticular pattern similar to wild-type tubules, suggesting that cyst formation was not due to loss of cytoplasmic polycystin-2.
Figure 4.
Abnormalities of proliferation, apoptosis, and cell polarity in Kif3a mutant mice. (A) Hematoxylin/eosin-stained kidney from a Kif3a mutant at P35. cy, cyst. (B) BrdUrd incorporation (arrowheads) in kidneys from a Kif3a mutant and control littermate (Inset) at P28. (C) Apoptotic cells (arrowheads) in kidneys from a Kif3a mutant and control littermate (Inset) at P21. (D) Localization of EGFR (red) and ZO-1 (green) in kidneys from a Kif3a mutant at P21. Arrowheads, colocalization in apical membrane; arrows, basolateral membrane; tu, tubule. (E) Expression of AQP3 in a cystic kidney at P28. Arrowheads, basolateral membrane. (F and G) Expression of AQP2 in cystic kidneys before (F) and 30 min after (G) treatment with vasopressin. Arrowheads, cytoplasmic vesicles; arrows, apical membrane. [Bars = 20 (A) and 10 μm (B–G).]
The polarity of the cyst epithelial cells was assessed by labeling with markers specific for the apical or basolateral membrane. The EGFR was located in the basolateral membrane of normal renal tubules but was located in both the apical and basolateral membranes of cyst epithelial cells (Fig. 4D). Similar apical “mislocalization” of the EGFR has been observed in human PKD and in cpk, orpk, bpk, and Pkd1 mutant mice (23, 24). The aberrant localization of EGFR did not reflect a generalized defect in cell polarity because the basolateral water channel, AQP3, was located in the basolateral membrane of cyst epithelial cells (Fig. 4E). Likewise, the apical proteins Na-K-Cl cotransporter and Na-Cl cotransporter were found in the apical membrane of cyst epithelial cells (Fig. 2 E and F). In neurons, KIF3A mediates axonal transport of membrane vesicles (25). However, inactivation of Kif3a in the kidney did not prevent AQP2-containing vesicles from translocating to the apical membrane in response to vasopressin (Fig. 4 F and G).
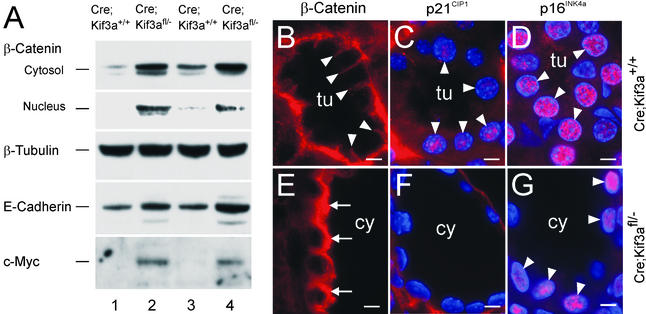
To explore the mechanism of the renal abnormalities in Kif3a mutants, we examined the expression of β-catenin, an important regulator of epithelial cell adhesion and proliferation. In renal tubules from control mice, β-catenin was located primarily in the basolateral cell membrane, consistent with its association with E-cadherin in adherens junctions (Fig. 5B). In cystic kidneys, the expression of β-catenin in the cytosol and nucleus was increased (Fig. 5 A and E). Cystic kidneys showed increased nuclear expression of c-Myc (Fig. 5A) and decreased expression of p21CIP1 (Fig. 5 C and F). p16INK4a was present in the nuclei of both control renal tubules and cysts, indicating that the effects on p21CIP1 were specific (Fig. 5 D and G).
Figure 5.
Abnormal expression of β-catenin, c-Myc, and p21CIP1 in Kif3a mutants. (A) Immunoblot analysis of kidney extracts from Kif3a mutants (lanes 2 and 4) and control littermates (lanes 1 and 3) at P21. (B and E) Expression of β-catenin in control (B) and cystic (E) kidneys at P21. Arrowheads, basolateral membrane; arrows, cytosol. (C and F) Expression of p21CIP1 (red) in control (C) and cystic (F) kidneys at P21. Arrowheads, expression in nuclei that were counterstained with 4′,6-diamidino-2-phenylindole (blue). (D and G) Expression of p16INK4a (red) in control (D) and cystic (G) kidneys at P21. tu, tubule; cy, cyst. (Bars = 5 μm.)
Discussion
We conclude that Kif3a is required for the maintenance of primary cilia and that the loss of cilia in the kidney produces renal cysts with characteristics similar to those found in human PKD. The absence of cilia was observed in both large and small cysts and was first detected at P5 when cysts began to appear. Cilia were absent in the cysts but were present in adjacent noncystic tubules, further supporting the relationship between cilia expression and cystic disease. Cilia are present in renal cysts of cpk, inv, and Pkd2 mutant mice, indicating that the absence of cilia in Kif3a mutant mice is not secondary to cyst formation itself (ref. 26 and S.S., unpublished data). Although Cre recombinase is expressed in the developing metanephros as early as embryonic day 15.5, Kif3a mutants did not exhibit loss of renal cilia and cyst formation until P5. The delayed appearance of the phenotype may be due to the half-life of KIF3A and/or inefficiency of Cre/loxP recombination.
A role of primary cilia in the pathogenesis of autosomal dominant PKD was originally suggested by studies in Caenorhabditis elegans in which the homologues of polycystin-1 and -2 (LOV-1 and PKD-2, respectively) were localized in the cilia of male-specific sensory neurons (27). Subsequently, polycystin-1 and -2 were localized in primary cilia in mammalian cells (6–8). orpk mutant mice that develop renal cysts during embryonic development carry mutations of polaris (Tg737), which is expressed in ciliary axonemes and basal bodies and is involved in intraflagellar transport (10, 28). cpk mutant mice also present with congenital PKD and have mutations of cystin, a protein of unknown function expressed in ciliary axonemes (9). inv mutant mice develop PKD and situs inversus attributable to partial deletion of the ciliary protein inversin (11). In orpk mutant mice the renal cilia are malformed and shortened, and they overexpress polycystin-2 (6, 28). In contrast, cpk, inv, and Pkd2 mutant mice contain renal cilia that are morphologically normal (11, 26). The present study demonstrates that the complete absence of renal cilia produces cysts after tubules are initially formed and provides direct evidence for the role of primary cilia in the pathogenesis of PKD. Additional unknown effects of Kif3a inactivation may also contribute to cyst formation.
The mechanism by which loss of primary cilia produces renal cysts is not known. However, the phenotype of Kif3a mutant mice suggests a link between primary cilia and β-catenin signaling. β-Catenin mediates the interaction between E-cadherin and the cytoskeleton and accumulates in the cytosol in response to activation of the Wnt signaling pathway. Cytosolic β-catenin translocates to the nucleus, where it interacts with T cell factor/lymphoid enhancer factor transcription factors and regulates gene expression. The involvement of β-catenin in PKD was suggested by studies showing that the cytoplasmic domain of polycystin-1 stabilizes β-catenin and activates a TCF-responsive promoter (29) and that transgenic mice expressing an activating mutant of β-catenin develop PKD (30). β-Catenin plays a pivotal role in regulating decisions between cell proliferation and differentiation in colonic epithelia (31). Stabilization and nuclear translocation of β-catenin activates TCF-responsive target genes, including c-Myc, which represses the transcription of specific cyclin-dependent kinase inhibitors, thereby promoting cell proliferation. Consistent with this model, we observed that inactivation of Kif3a leads directly or indirectly to increased levels of cytosolic and nuclear β-catenin, increased c-Myc, inhibition of p21CIP1, and increased cell proliferation. These results suggest that primary cilia play a role in maintaining the differentiation of lumen-forming epithelia via a pathway that may involve β-catenin.
Acknowledgments
We thank A. Kaup, B. McKnight, B. Sthapit, and Y. Bai for expert technical assistance; B. Thomson, Y. Cai, and X. Tian for advice and protocols; M. Knepper and J. Hoyer for Abs; and R. Quigley for helpful suggestions. This work was supported by grants from the National Institutes of Health (to P.I. and S.S.).
Abbreviations
- PKD
polycystic kidney disease
- EGFR
epidermal growth factor receptor
- Pn
postnatal day n
- AQP2
aquaporin-2
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Igarashi P, Somlo S. J Am Soc Nephrol. 2002;13:2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 2.Murcia N S, Sweeney W E, Jr, Avner E D. Kidney Int. 1999;55:1187–1197. doi: 10.1046/j.1523-1755.1999.00370.x. [DOI] [PubMed] [Google Scholar]
- 3.Harris P C. Curr Opin Nephrol Hypertens. 2002;11:309–314. doi: 10.1097/00041552-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Wheatley D N. Pathobiology. 1995;63:222–238. doi: 10.1159/000163955. [DOI] [PubMed] [Google Scholar]
- 5.Praetorius H A, Spring K R. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 6.Pazour G J, San Agustin J T, Follit J A, Rosenbaum J L, Witman G B. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 7.Yoder B K, Hou X, Guay-Woodford L. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 8.Nauli S M, Alenghat F J, Luo Y, Williams E, Vassilev P, Li X, Elia A E H, Lu W, Brown E M, Quinn S J, et al. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 9.Hou X, Mrug M, Yoder B K, Lefkowitz E J, Kremmidiotis G, D'Eustachio P, Beier D R, Guay-Woodford L M. J Clin Invest. 2002;109:533–540. doi: 10.1172/JCI14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taulman P D, Haycraft C J, Balkovetz D F, Yoder B K. Mol Biol Cell. 2001;12:589–599. doi: 10.1091/mbc.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan D, Eley L, Sayer J, Strachan T, Yates L M, Craighead A S, Goodship J A. Hum Mol Genet. 2002;11:3345–3350. doi: 10.1093/hmg/11.26.3345. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum J L, Witman G B. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 13.Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. J Cell Biol. 1999;145:825–836. doi: 10.1083/jcb.145.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marszalek J R, Ruiz-Lozano P, Roberts E, Chien K R, Goldstein L S B. Proc Natl Acad Sci USA. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao X, Somlo S, Igarashi P. J Am Soc Nephrol. 2002;13:1837–1846. doi: 10.1097/01.asn.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
- 16.DiGiovanni S R, Nielsen S, Christensen E I, Knepper M A. Proc Natl Acad Sci USA. 1994;91:8984–8988. doi: 10.1073/pnas.91.19.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao X, Johnson J E, Richardson J A, Hiesberger T, Igarashi P. J Am Soc Nephrol. 2002;13:1824–1836. doi: 10.1097/01.asn.0000016443.50138.cd. [DOI] [PubMed] [Google Scholar]
- 18.Quaggin S E, Vanden Heuvel G B, Igarashi P. Mech Dev. 1998;71:37–48. doi: 10.1016/s0925-4773(97)00201-3. [DOI] [PubMed] [Google Scholar]
- 19.DeBose-Boyd R A, Brown M S, Li W P, Nohturfft A, Goldstein J L, Espenshade P J. Cell. 1999;99:703–712. doi: 10.1016/s0092-8674(00)81668-2. [DOI] [PubMed] [Google Scholar]
- 20.Cai Y, Maeda Y, Cedzich A, Torres V E, Wu G, Hayashi T, Mochizuki T, Park J H, Witzgall R, Somlo S. J Biol Chem. 1999;274:28557–28565. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- 21.Quaggin S E, Yeger H, Igarashi P. J Clin Invest. 1997;99:718–724. doi: 10.1172/JCI119216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marszalek J R, Liu X, Roberts E A, Chui D, Marth J D, Williams D S, Goldstein L S B. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney W E, Jr, Avner E D. Am J Physiol. 1998;275:F387–F394. doi: 10.1152/ajprenal.1998.275.3.F387. [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Fan X, Basora N, Babakhanlou H, Law T, Rifai N, Harris P C, Perez-Atayde A R, Rennke H G, Zhou J. Nat Genet. 1999;21:160–161. doi: 10.1038/5944. [DOI] [PubMed] [Google Scholar]
- 25.Marszalek J R, Goldstein L S B. Biochim Biophys Acta. 2000;1496:142–150. doi: 10.1016/s0167-4889(00)00015-x. [DOI] [PubMed] [Google Scholar]
- 26.Ricker J L, Gattone V H, II, Calvet J P, Rankin C A. J Am Soc Nephrol. 2000;11:1837–1847. doi: 10.1681/ASN.V11101837. [DOI] [PubMed] [Google Scholar]
- 27.Barr M M, DeModena J, Braun D, Nguyen C Q, Hall D H, Sternberg P W. Curr Biol. 2001;11:1341–1346. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 28.Pazour G J, Dickert B L, Vucica Y, Seeley E S, Rosenbaum J L, Witman G B, Cole D G. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim E, Arnould T, Sellin L K, Benzing T, Fan M J, Grüning W, Sokol S Y, Drummond I, Walz G. J Biol Chem. 1999;274:4947–4953. doi: 10.1074/jbc.274.8.4947. [DOI] [PubMed] [Google Scholar]
- 30.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Oncogene. 2001;20:5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 31.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis A P, et al. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]