Abstract
To better understand how innate and adaptive immune responses interact with each other, we combined 4-1BB T cell costimulation with specific adjuvants to determine whether these treatments would influence specific T cell expansion and function in vivo. In the presence of 4-1BB ligation and Toll-like receptor 3 (TLR)3 and/or TLR4 triggering, CD8 T cell clonal expansion and survival was augmented profoundly. Specific T cells primed in vivo with TLR ligands responded normally to in vitro recall stimulus, but, surprisingly, copriming with 4-1BB costimulation significantly impaired the recall response even though many more specific effector T cells were rescued in vivo. Here, we demonstrate that the rescued CD8 T cells suppressed CD4 T cell proliferation via a type β transforming growth factor-dependent mechanism. Thus, 4-1BB and TLR ligands induce survival of specific effector CD8 T cells with suppressive recall potential, which may explain the dual role that 4-1BB activation plays in mediating tumor clearance and prevention of autoimmune disease.
The activation-inducible T cell costimulatory receptor 4-1BB (also referred to as CD137) operates downstream of CD28 and is unique in its pattern of stimulation as compared with CD28. For example, 4-1BB is expressed earlier on activated CD8 than on CD4 T cells in vivo (1). Recently, it was shown that 4-1BB is expressed on dendritic cells but reported to be down-regulated after dendritic cell activation via CD40 ligation (2, 3). However, the function of 4-1BB on dendritic cells is not clear at this time (4). In contrast to CD28, 4-1BB is more effective at stimulating effector T cells than naïve T cells (5). Although it is clear that anti-4-1BB is very capable of stimulating CD4 T cells in vitro as well as CD8 T cells (6), it is evident that postactivation, 4-1BB-induced T cell survival preferentially occurs within the CD8 subpopulation (1, 7). Specifically, ligation of 4-1BB on activated CD8 T cells promotes in vivo long-term-specific T cell survival in the CD8 but not the CD4 compartment (1). This feature was not observed with other costimulatory molecules (8, 9). Thus, there appears to be specialized function unique to individual costimulatory molecules expressed on T cells.
The data presented in this article suggest that ligands specific for 4-1BB could be used therapeutically to generate potent vaccines, and based on data from several other groups, it is clear that the 4-1BB-stimulated T cells can effectively carry out effector functions (10–12). For example, 4-1BB has been shown to mediate eradication of established tumors and heighten cytokine secretion, proliferative capacity, and cytotoxic T lymphocyte activity. Thus, 4-1BB-costimulated T cells may serve as excellent effector cells endowed with extended longevity in vivo.
The view that 4-1BB costimulates T cells in a productive manner has been complicated in two recent observations. First, it was shown that 4-1BB-deficient T cells are hyperresponsive to mitogens compared with WT T cells (13). Second, it was demonstrated that an agonist mAb specific to 4-1BB, which enhances tumor rejection in mice, also was capable of suppressing T-dependent humoral immunity (14). A similar finding was reported when it was observed that agonistic anti-4-1BB mAbs were able to ameliorate experimental autoimmune encephalomyelitis (15). Because ligation of 4-1BB on T cells has been shown to enhance memory pools of influenza-specific CD8 T cells and broaden primary CD8 responses (7, 16), it is unclear how 4-1BB-mediated signals inhibit CD4+ T helper function that drives T-dependent antibody responses.
In this article, we show that Toll-like receptor (TLR) ligands and 4-1BB costimulation massively enhanced specific T cell clonal expansion in vivo but profoundly suppressed T cell recall responses. Neither addition of IL-2 nor extremely high or low levels of recall stimulus were able to inhibit suppression. It is shown that the 4-1BB-rescued CD8 T cells exerted suppressive effects on CD4 T cells and in vivo depletion of CD8 cells permitted rescue of specific CD4 T cells costimulated by 4-1BB. To elucidate the mechanism(s) that account for this form of suppression, we show that neutralization of transforming growth factor type β (TGF-β) could block suppression without having to deplete CD8+ T cells. These data show that triggering 4-1BB and TLR can generate effector CD8 T cells possessing suppressive recall function.
Materials and Methods
Mice, Reagents, mAbs, and Treatments.
B10.A, C57BL/6, and B10.BR mice were purchased from the National Cancer Institute (Frederick, MD) or The Jackson Laboratory. All mice were maintained under pathogen-free conditions in accordance with federal guidelines.
Staphylococcal enterotoxin A (SEA) and ovalbumin (Ova) were purchased from Sigma and were administered as i.p. injections in balanced salt solution (BSS). For part of the data in Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org, Ova was decontaminated away from lipopolysaccharide (LPS), but this was found not to influence our data at all. Anti-CD4, -CD8, and -CD11a flow cytometry staining mAbs were conjugated to either FITC, phycoerythrin, or allophycocyanin and purchased from PharMingen. The anti-T cell antigen receptor Vβ3 mAb [KJ25-607.7 (17) and anti-CD3 (145 2c11; ref. 18)] were purified from hybridoma supernatant over protein G agarose (Life Technologies, Grand Island, NY). The mAbs were conjugated to FITC as described (19). BrdUrd was purchased from Sigma, and murine IL-2 was purchased from Intergen (Purchase, NY). The anti-TGF-β mAb 1D11.16 and control Ig KG7 were kind gifts of Bruce Pratt and Steve Ledbetter (Genzyme; ref. 20). Tetramers for SIINFEKL specificity were a kind gift of Leo Lefrancois (University of Connecticut Health Center).
All injections were i.p., and day 0 was the time in which SEA or Ova was injected. T cells were stimulated in vivo with 3H3 mAb, which is an agonist rat mAb specific for murine 4-1BB (11, 21). As a control, rat IgG (Sigma) was used in place of anti-4-1BB. These antibodies were mixed separately with SEA or Ova and injected on day 0. The dose of SEA was 0.15 μg and Ova was 1 mg, and the dose of anti-4-1BB or rat IgG was 25 μg unless stated otherwise. The injections typically were given in 200 μl of sterile BSS. LPS and polyinosinic⋅polycytidylic acid (PIC) were purchased from Sigma and were injected at 20 and 150 μg, respectively.
Cell Processing and Flow Cytometry.
Spleens and peripheral lymph nodes (LNs) were teased through nylon-mesh sieves (Falcon; PharMingen). Red blood cells were lysed with ammonium chloride. Our procedure for liver cell analysis has been described (22).
For two-, three-, and four-color flow cytometry, cells were incubated on ice with 5% normal mouse serum, culture supernatant from 2.4.G.2 [anti-FcR (23)] hybridoma cells, and 10 μg/ml human γ globulin (Sigma) to block nonspecific binding of the added mAbs. The cells were incubated on ice for 30 min with staining buffer (BSS/3% FBS/0.1% sodium azide) containing primary mAbs or for 1.5 h at room temperature with tetramers. The cells were washed several times with staining buffer and then analyzed on a FACSCalibur (Becton Dickinson Immunocytometry Systems). Analysis was completed with cellquest software.
For BrdUrd staining, 10 μM BrdUrd was added at 48 h into the 72-h 37°C culture period. The triplicate cultures typically contained 5 × 105 responder cells and 1 × 106 effector cells in 200 μl of CTM (MEM/FBS/amino acids/salts/antibiotics). At 72 h, the cells were stained for CD4 and then stained for BrdUrd incorporation into DNA. The cells were stained with a modified version of a protocol in ref. 24. Cells were dehydrated and fixed in ice-cold 95% ethanol, permeabilized in BSS containing 1% paraformaldehyde and 0.01% Tween 20, followed by light DNA digestion with 50 Kunitz units of DNase I (Sigma). The cells were stained with an anti-BrdUrd-FITC mAb (PharMingen). A control sample (no BrdUrd, but stained) was used to standardize the technique.
Cell Proliferation.
For proliferation assays, cells were incubated in triplicate in CTM for 72 h in vitro at 37°C and 5% CO2. Various amounts of cells were assayed in 96-well plates, typically starting with 1 × 106 cells per well followed by 2- or 3-fold dilutions. The cultures were restimulated with 0.1 μg/ml SEA and, in several experiments, between 20 and 40 μg/ml anti-TGF-β or control Ig KG7 was included. During the last 8 h of culture, 1 μCi of [3H]thymidine was added to each well and the samples were harvested and counted by using a cell harvester (TomTec, Wallac, Gaithersburg, MD) and a 1450 Microbeta Trilux scintillation counter (Wallac).
Cell Purification and Depletion.
CD8 T cells were isolated by negative selection by using CD8 cell-enrichment columns from Cedarlane Laboratories.
Depletion of CD8 T cells was accomplished by positively selecting CD8 T cells away from LN and spleen cell populations. Whole LN and spleen cells were combined, washed, RBC-lysed, and incubated on ice for 30 min with rat anti-mouse CD8 mAb (PharMingen). At a ratio of 4:1, sheep anti-rat IgG beads were added to the cells for a secondary incubation (Dynal, Great Neck, NY). The cells and beads were placed onto a magnet apparatus (Dynal) for 2–5 min. The cells not attached to the magnet were purged of CD8 T cells and washed several times before being placed into culture. Typically, we find that <1% of T cells in the final population bear CD8 after this procedure.
In vivo depletion of CD8 T cells was accomplished by injecting either 1 mg of anti-CD8 mAb 53-6.72 or 100 μg of anti-CD8 depleting mAb from Cedarlane Laboratories. For a control, rat IgG was used in place of the CD8-depleting mAb at the same concentration. Two injections of the depleting mAbs were given on days −5 and −2, and SEA was injected on day 0 with anti-4-1BB. CD8 depletion was confirmed by flow cytometry, and typically <2% of cells were CD8+ at the time of assay on day 7.
Results
It is known that injection of peptides or superantigens into mice leads to significant clonal expansion followed by peripheral deletion of the responding T cells (25–28). We have shown that, unlike other costimulatory signals, 4-1BB stimulation blocks peripheral deletion of superantigen-specific CD8 T cells in vivo (1). It also has been demonstrated that activators of innate immunity such as the TLR ligands LPS and PIC can interfere with peripheral T cell deletion (29, 30). Therefore, we were interested in combining the functional capacity of both strategies to study the interplay between innate and adaptive immunity. It was hypothesized that the combination may lead to synergistic rescue of specific T cells, thereby being the basis for a very effective vaccination strategy for infectious pathogens and tumors.
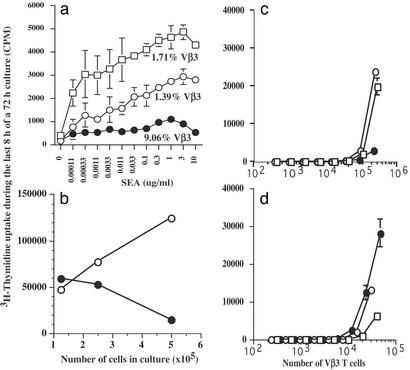
C57BL/6 mice were treated with Ova, the TLR-3-specific ligand PIC (31), and an agonist mAb specific to 4-1BB (21). Control mice were given Ova + rat IgG, Ova + anti-4-1BB, or Ova + PIC, and during a time course we stained peripheral blood lymphocytes for CD8, CD11a, and for SIINFEKL tetramer specificity to quantify activated Ova-specific CD8 T cells (Fig. 5, which is published as supporting information on the PNAS web site). Analyzing CD11ahi cells confirms that they were activated and were not unstimulated SIINFEKL-specific CD8 T cells. The effect of anti-4-1BB and PIC on Ova-specific CD8 T cells was very significant when compared with Ova + IgG primed mice, which generated few tetramer+ cells (Fig. 5a). This marked synergistic effect is shown on day 7, where TLR3 and 4-1BB triggering is at least severalfold higher than either agent alone with Ova. Nevertheless, there appeared to be a reduction in T cell numbers; however, we hypothesized that this may reflect migration of T cells from blood into other tissues. To address this issue, we examined spleen and liver 40 days after injection and found that the 4-1BB and PIC combination enhanced accumulation of tetramer CD11ahi CD8 T cells to a much higher level than any other combination (Fig. 5b).
To test this hypothesis in a different model, B10.A or B10.BR mice were injected with the superantigen SEA, which specifically stimulates T cell receptor Vβ3-bearing T cells, and these mouse strains typically contain ≈3% CD8 Vβ3 and 6% CD4 Vβ3 T cells (data not shown). SEA was injected with various combinations of anti-4-1BB mAb, or control IgG, with TLR ligands, and 3 and 10 days after immunization the percentage of spleen CD4 or CD8 Vβ3 T cells was detected by flow cytometry (Table 1). In all cases, the presence of an adjuvant effect above that of SEA with control IgG enhanced day-3 clonal expansion and day-10 rescue from deletion in both CD4 and CD8 SEA-specific populations. The maximal level of expansion and rescue of CD4 Vβ3 T cells was achieved in the LPS/PIC/4-1BB-stimulated group. On day 10, there was an ≈7.5-fold increase in the percentage of CD4 Vβ3 T cells over SEA/IgG and a 3.5-fold increase over SEA/anti-4-1BB. Therefore, the adjuvants enhanced the effects of 4-1BB costimulation on the SEA-responsive CD4 population.
Table 1.
4-1BB costimulation combined with TLR ligands mediates enhanced clonal expansion and rescue of CD4 and CD8 SEA-specific T cells from deletion
| Treatment | % CD4 Vβ3+ spleen T cells
|
% CD8 Vβ3+ spleen T cells
|
||
|---|---|---|---|---|
| Day 3 | Day 10 | Day 3 | Day 10 | |
| SEA/IgG | 10.47 ± 0.4 | 2.21 ± 0.2 | 7.88 ± 1.1 | 1.78 ± 0.5 |
| SEA/α-41BB | 19.87 ± 2.4 | 4.68 ± 0.5 | 35.39 ± 3.4 | 17.63 ± 2.3 |
| SEA/LPS/IgG | 26.75 ± 1.1 | 8.25 ± 1.1 | 22.11 ± 1.1 | 8.61 ± 1.6 |
| SEA/LPS/α-41BB | 30.29 ± 1.1 | 8.38 ± 1.6 | 43.77 ± 0.8 | 30.49 ± 7.8 |
| SEA/PIC/IgG | 16.58 ± 1.3 | 3.77 ± 0.5 | 20.71 ± 2.2 | 4.4 ± 1.1 |
| SEA/PIC/α-41BB | 24.88 ± 2.5 | 6.1 ± 0.7 | 44.93 ± 2.1 | 32.75 ± 5.2 |
| SEA/PIC/LPS/IgG | 31.83 ± 1.4 | 12.98 ± 2.3 | 26.13 ± 1.6 | 15.09 ± 2.7 |
| SEA/PIC/LPS/α-41BB | 34.43 ± 0.4 | 16.73 ± 1.3 | 46.56 ± 1.4 | 38.48 ± 2.0 |
B10.A mice were injected i.p. with 0.15 μg of SEA and 25 μg of either control rat IgG or anti-4-1BB. Some groups received 20 μg of LPS and/or 150 μg of PIC. Three and 10 days after immunization, spleen cells were assayed for the presence of CD4, CD8 Vβ3 T cells by flow cytometry. Data are mean percent ± SEM from the combination of three independent experiments for a total of six mice at each time point.
In the CD8 population, there was profound rescue from deletion on day 10 (Table 1). The combination of LPS/PIC and 4-1BB costimulation yielded 38.48% CD8 Vβ3-bearing T cells on day 10, which is a 21-fold increase over SEA/IgG and a >2-fold increase when either the adjuvants or 4-1BB costimulation is negated. Perhaps the most significant result in analyzing the CD8 population was the low amount of deletion in the adjuvant/4-1BB groups between the clonal expansion phase, observed on day 3 to the deletion point observed on day 10.
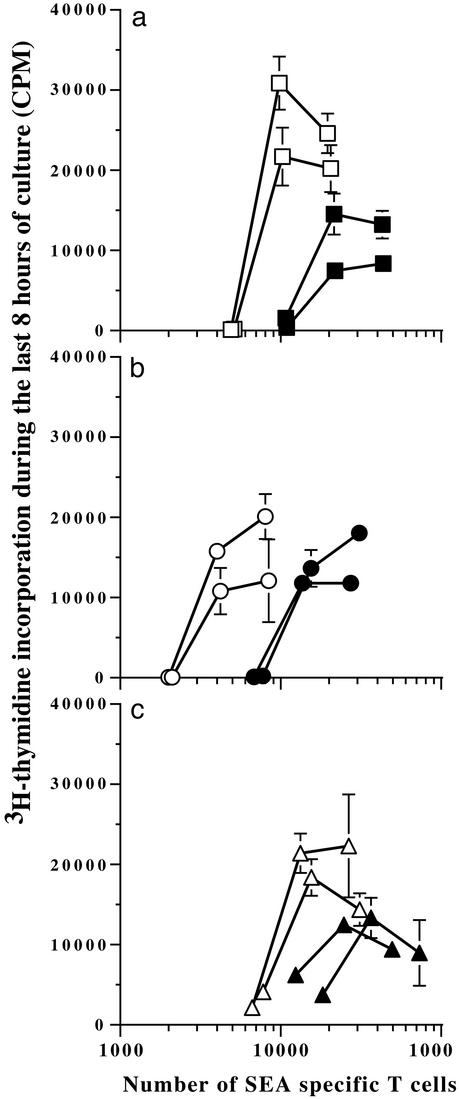
The functional capacity of the rescued T cells was examined by using the day-10 spleen cells from Table 1. Cells from all groups were seeded equally into wells and serially diluted 2-fold. The percentage of T cell antigen receptor Vβ3+ T cells was measured by flow cytometry so that an accurate count of SEA-specific T cells could be enumerated in each well (x axis). A saturating amount of SEA was added in the wells, and on day 3, [3H]thymidine was added for the last 8 h of incubation (Fig. 1). The SEA/LPS-primed cells incorporated [3H]thymidine in response to SEA as we observed in the past (29). Perhaps unexpectedly, however, we found that copriming SEA/LPS mice in vivo with anti-4-1BB significantly impaired the recall response (Fig. 1a). This was true when the magnitude of the response was evaluated as well as when counts were analyzed on a Vβ3 T cell basis. 4-1BB costimulation inhibited the PIC-primed cells and the LPS/PIC-primed cells as well (Fig. 1 b and c).
Figure 1.
TLR3- and TLR4-specific adjuvants do not prevent the 4-1BB-suppressed recall proliferative response. Mice were immunized as stated in Table 1, and on day 10 spleen cells from SEA/LPS (□) and SEA/LPS/anti-4-1BB (■) (a), SEA/PIC (○) and SEA/PIC/anti-4-1BB (●) (b), and SEA/PIC/LPS (▵) and SEA/PIC/LPS/anti-4–1BB (▴) (c) mice were compared in a 3-day [3H]thymidine incorporation assay as described in Materials and Methods. The x axis shows the number of Vβ3 T cells (SEA-specific) in each well. Each line represents cells from one mouse, and the data points are mean ± SEM from triplicate cultures. These experimental results are comparable to the results from another identical experiment.
The data in Fig. 2a show that this effect was not limited to adjuvant primed cells because spleen cells from 7 days earlier with SEA proliferated vigorously to recall SEA, but proliferation was almost eliminated when the mice were coprimed with anti-4-1BB. Even cells taken from normal mice, which are likely to be naïve, incorporated much more [3H]thymidine in response to SEA. This was the case even though, proportionally, there was a severalfold increase in the numbers of Vβ3-bearing T cells in the cell population taken from 4-1BB-costimulated mice. Furthermore, suppression of proliferation was observed over a several log titration of SEA, strongly suggesting that SEA was neither in limiting nor in overabundant concentration. Collectively, these data are in direct contrast to the proliferative capacity of cells taken from the same mouse strain but primed in vivo with SEA and anti-CD40 (32).
Figure 2.
Impaired proliferation by 4-1BB-primed T cells. (a) B10.BR mice were separated into three groups and treated with nothing (□), SEA/rat IgG (○), or SEA/anti-4-1BB (●) as described in Materials and Methods. After 7 days, spleen cells were removed from the mice, the percentage of Vβ3+ T cells was measured by flow cytometry (shown under the corresponding line), and 250,000 cells were set up in culture for 72 h with a titration of SEA. During the last 8 h of culture, [3H]thymidine was added, and shown are the mean ± SEM from triplicate wells. (b) Two groups of mice were treated either with SEA/rat IgG (○) or SEA/anti-4-1BB (●), and 5 days later the cells were cultured for proliferation capacity. Initially, each culture received 0.1 μg/ml murine IL-2 and proliferation was measured at 72 h. (c and d) For depletion studies, mice were treated with nothing (□), SEA/IgG (○), or SEA/anti-4-1BB (●), and 5 days later spleen and LN cells were combined from two mice in each group. The populations were depleted of nothing (c) or CD8 T cells (d) and set up in culture for proliferation capacity. The x axis shows the number of Vβ3 T cells (SEA-specific) in each well. The data shown are mean ± SEM from triplicate cultures and are representative of three separate experiments.
An explanation for the lack of proliferation in this system may stem from the possibility that the CD8 T cells either consume the majority of IL-2 produced and fail to proliferate, or IL-2 was not produced in appreciable amounts. We tested this notion by adding a saturating amount of IL-2 (0.1 μg/ml) at the beginning of culture, thereby circumventing these effects. To ensure that there were saturating amounts of IL-2, we demonstrated by ELISA that IL-2 was readily detectable at the end of culture (data not shown). In cultures that contained cells taken from day-5 SEA/rat IgG-treated mice, IL-2 alone led to very robust proliferation (Fig. 2b), and adding SEA made little difference, further demonstrating that the amount of IL-2 was saturating (data not shown). This is in contrast to cells taken from day-5 SEA/anti-4-1BB-treated mice. Although these cultures contained saturating amounts of IL-2, there was no increase in proliferation even as more cells were added to the culture (Fig. 2b). Therefore, we conclude that IL-2 consumption is not a feasible explanation for the lack of proliferation and neither is competition for SEA because SEA was not included in these cultures. Additionally, anergy or Ag-induced nonresponsiveness is not a viable explanation because the 4-1BB-primed cells proliferated poorly in response to IL-2 as cell density was increased, which would not be expected if these mechanisms were operating (33, 34).
Another possibility suggested that one T cell subpopulation inhibited the other from responding. To test this idea, we generated lymphocyte cultures taken from mice that were immunized with SEA ± anti-4-1BB 5 days earlier. The lymphocytes were purged of CD8 T cells and then restimulated in vitro with SEA. As expected, it is shown that undepleted lymphocytes from the SEA/anti-4-1BB mice proliferated poorly in vitro in response to SEA (Fig. 2c). In contrast, nondepleted cells from untreated animals proliferated robustly as did nondepleted cells from mice injected with SEA alone. These data are consistent with the fact that lymphocytes from SEA/anti-4-1BB mice make very little IL-2 compared with cells taken from SEA alone or untreated mice (data not shown).
In contrast, results from Fig. 2d demonstrate that CD8-depleted day-5 SEA/anti-4-1BB cells responded vigorously to recall SEA. In fact, responsiveness was very similar to the nondepleted cell populations from untreated and mice treated with SEA alone (Fig. 2c). The effect of CD8 depletion in cells taken from SEA/anti-4-1BB mice is very evident when one compares cpm levels between Fig. 2 c and d. This is in direct contrast to untreated or SEA-treated cell populations. Furthermore, IL-2 production was enhanced when CD8 T cells were depleted in the SEA/anti-4-1BB population compared with no depletion, which was not observed in the SEA-alone group (data not shown). These data demonstrate that CD4 T cells derived from SEA/anti-4-1BB mice were not anergic when restimulated with SEA in vitro in the absence of CD8 T cells as shown by their ability to incorporate [3H]thymidine.
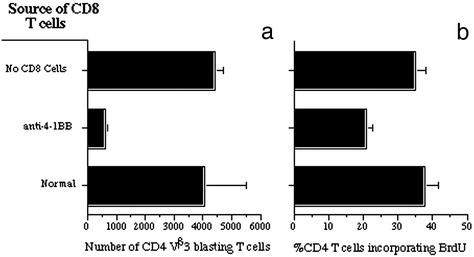
To examine how the CD8 T cells suppress the CD4 T cells, we developed a CD8 “swapping” assay, where various populations of purified CD8 T cells were combined with CD8-depleted day-5 SEA/4-1BB-primed cells. Thus, the CD8-depleted population contained their normal constituency of antigen-presenting cells and CD4 T cells, and the numbers of these cells were the same in each culture. The data show adding SEA alone with no CD8 T cells resulted in a substantial accumulation of blasting CD4 Vβ3 T cells in the cultures (Fig. 3a). Cells were determined to be blasting based on forward scatter by comparison with the same cell population in normal mice. Consistent with the data in Fig. 2 c and d, addition of purified CD8 T cells taken from the SEA/anti-4-1BB day-5 mice resulted in profound inhibition of blasting CD4 Vβ3 T cell numbers (>6-fold decrease). This was also the case for the percentage of blasting CD4 Vβ3 T cells (data not shown). CD8 T cells from untreated normal mice were used as a comparison and demonstrated to possess no suppressive effect unlike the same cells that had been primed with SEA and anti-4-1BB.
Figure 3.
The number and proliferative capacity of SEA-specific blasting CD4 T cells was reduced in the presence of 4-1BB-primed CD8 T cells. Cells taken from day-5 SEA/anti-4-1BB-treated mice were depleted of CD8 T cells. The CD8-depleted cells were mixed in culture (with SEA) for 72 h with purified CD8 T cells taken from normal untreated mice or day-5 SEA/anti-4-1BB mice, or they were not offered any CD8 T cells. (a) The number of blasting CD4 T cells that express Vβ3 was determined after 72 h in culture. (b) At 48 h, each culture was spiked with 10 μM BrdUrd, and at 72 h, cells were stained for CD4 and BrdUrd incorporation. The data shown represent blasting CD4 BrdUrd+ T cells. The data are combined from three separate experiments and are shown as mean ± SEM.
In a second assay, we tested whether the CD8 populations exerted a suppressive effect on SEA-driven CD4 T cell proliferation. Because [3H]thymidine incorporation does not distinguish between CD4 and CD8 T cells, blasting or resting, we chose BrdUrd incorporation as a more accurate and specific assay to examine whether the blasting CD4 T cells were blocked from proliferation. The data show that SEA plus purified CD8 T cells from normal mice or no CD8 T cells led to a significant percentage of blasting CD4 T cells incorporating BrdUrd (Fig. 3b). However, addition of SEA and purified CD8 T cells taken from day-5 SEA/anti-4-1BB mice inhibited BrdUrd incorporation by almost 50%.
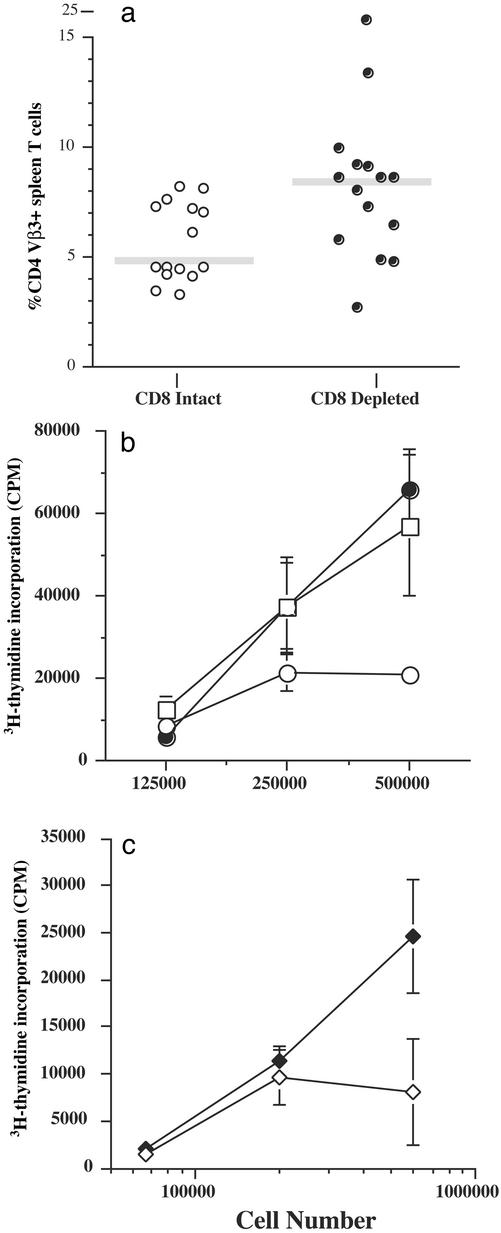
Based on these in vitro experiments, we designed an in vivo experiment to test the hypothesis that 4-1BB-primed CD8 T cells may exert a suppressive effect on CD4 T cells in vivo. Mice were, or were not, depleted of CD8 T cells and then immunized with SEA/anti-4-1BB. On day 7 after immunization, spleen cells were analyzed for the presence of CD4 Vβ3 T cells. There was an ≈2-fold increase in the percentage of CD4 Vβ3+ spleen T cells in CD8-depleted mice vs. intact animals (Fig. 4a). Also, statistical analysis for this data showed that in a two-tailed, nonpaired Student's t test, the probability value was 0.030, demonstrating that the difference between the groups is statistically significant.
Figure 4.
How 4-1BB-primed CD8 T cells suppress SEA-specific CD4 T cells. (a) B10.BR mice were given either CD8-depleting or rat IgG control mAb and then immunized with SEA and anti-4-1BB. Seven days after immunization, spleen cells were stained for CD4, CD8, CD3, and Vβ3 and then analyzed by flow cytometry. Any mouse in the CD8-depletion group with >5% CD8+ cells was not used, although typically <2% CD8+ cells were detected in the spleen. The data are from three separate experiments, and each point is data from a single mouse. The gray bar represents the average. (b) Spleen cells from the CD8-depletion group (●) and the CD8 intact control group (○) were pooled separately for a 3-day proliferation assay. Thymidine incorporation was measured in response to 0.1 μg/ml SEA and either 20 μg/ml anti-TGF-β (□) or control Ig (circles), starting with 500,000 cells followed by 2-fold dilutions. The data show the mean cpm ± SD from triplicate cultures. Data are representative of two experiments. (c) Mice were immunized with SEA/anti-4-1BB/LPS, and 7 days later spleen cells were tested for their ability to incorporate [3H]thymidine. Three sets of triplicate cultures were stimulated with nothing (background), SEA + control IgG (⋄), or SEA + anti-TGF-β (♦). The data show the mean cpm minus background ± SD from the triplicate cultures for each titration of cells. These data are similar to five other experiments.
We tested whether CD8 depletion in vivo would rescue the in vitro recall responsiveness. The data in Fig. 4b show that no depletion in vivo led to suppressed in vitro recall responses compared with cells taken from mice in which CD8 T cells were depleted. Collectively, these data show that suppressed recall responsiveness because of 4-1BB can be rescued if CD8 T cells are removed either ex vivo or in vivo before in vitro restimulation with SEA (Figs. 2d and 4b).
Because we established that CD8 T cells derived from 4-1BB/SEA-stimulated mice possess the ability to suppress CD4 T cell responses, we were interested in studying the mechanism of how the CD8 T cells mediated CD4 T cell unresponsiveness. We hypothesized that antiinflammatory cytokines such as IL-4 or TGF-β may suppress SEA-driven proliferation. We did not detect IL-4 in supernatants taken from cell cultures derived from populations of SEA/anti-4-1BB-injected mice (data not shown). On the other hand, TGF-β was detected, but in all cultures, regardless of the cellular constituents. This may be complicated by the fact that TGF-β is present in FCS, can be membrane-associated and released after apoptosis, and requires acidification for activity (35). Therefore, we tested a direct role for active TGF-β by first neutralizing this cytokine with a mAb that blocks chronic erosive polyarthritis and inflammatory cell accumulation (36). Neutralization of TGF-β in restimulation cultures containing SEA/anti-4-1BB-primed cells (without CD8 depletion in vivo) significantly enhanced proliferation by severalfold (Fig. 4b). Second, we found that SEA-mediated recall proliferation of SEA/anti-4-1BB/LPS-primed cells also was rescued in the presence of neutralizing anti-TGF-β (Fig. 4c).
Discussion
Our data show that signaling through TLRs had immune-boosting effects in vivo, and in vitro recall responses were potent, consistent with earlier reports (29). Combining TLR triggering with 4-1BB costimulation led to massive, specific CD8 T cell clonal expansion and survival (Fig. 5 and Table 1), which was as robust as with other T cell costimulatory receptors such as OX40 and CD40 (9, 32, 37). Surprisingly, in vivo inclusion of costimulation through 4-1BB dampened the magnitude of the in vitro recall proliferative response and, based on a specific cell-to-cell basis, profoundly inhibited proliferation by severalfold (Fig. 1). Thus, it is shown that 4-1BB costimulation with, or without, TLR triggering generates effector CD8 T cells, which possess potent suppressive function.
T cell costimulation through 4-1BB results in T cell effector responses marked by proliferation, cytokine production, and cytolytic activity (10–12). Recently, however, several studies have documented surprising episodes of inhibitory activities after 4-1BB costimulation or the lack thereof. In particular, 4-1BB-deficient T cells are hyperresponsive to mitogens such as Con A and anti-CD3 (13), and agonist 4-1BB mAb has been shown to ameliorate experimental autoimmune encephalomyelitis even though the same mAb enhances tumor rejection (15). Our goal was to uncover a mechanism of suppression and distinguish between an active vs. cell-autonomous process.
In the last few years, there have been reports documenting the existence of Tr1 cells that produce antiinflammatory cytokines (38). These cells are CD4+ and typically express CD25. We tested a role for Tr1 cells in our system by staining for CD25 on SEA-specific cells and did not find any evidence for their presence during culture (data not shown). We also found that depletion of CD4 T cells from cultures did not enhance proliferation (data not shown). However, in contrast to CD4 T cell depletion, we found that depletion of CD8 T cells rescued the proliferative response (Fig. 2 c compared with d). This was surprising because others have shown, using the same type of [3H]thymidine incorporation assay, that previously activated CD8+ T cell clones respond vigorously to SEB (39). Our in vivo CD8-depletion experiments confirmed this idea because rescue of CD4 Vβ3 T cells was enhanced in the presence of 4-1BB costimulation and SEA immunization (Fig. 4a). This is consistent with another study demonstrating that some of the superantigen deletion observed in vivo is CD8-driven, CD95-based death (40), but, importantly, our study shows that 4-1BB costimulation does not directly lead to CD4 T cell deletion. These data show that 4-1BB-induced CD4 T cell deletion in vivo depends on CD8 T cells.
To uncover further the mechanism of how CD8 T cells were mediating suppression, we demonstrated that the effects were directed against CD4 T cells. SEA-specific CD4 T cells responded vigorously when mixed with CD8 T cells taken from normal mice or not offered CD8 T cells at all, but purified CD8 T cells from SEA/4-1BB-primed mice suppressed CD4 T cell blastogenesis and entry into the cell cycle (Fig. 3). Each culture can be compared with each other because all cultures contained the same number, and constituency, of responding CD4 T cells. Competition for SEA is an unlikely explanation for the suppressive effect because normal CD8 T cell populations contain ≈3% Vβ3-bearing cells, and, yet, normal CD8 T cells did not suppress CD4 T cell responses at all. Additionally, the data in Fig. 2a show that saturating amounts of SEA could not rescue the response.
A possible explanation for these results is the release of a suppressive factor, which may inhibit activation and, thus, cellular division. For example, it was shown recently in an elegant series of studies that TGF-β is released from cells undergoing apoptosis, contributing to an immunosuppressive environment (35). Specifically, apoptotic cells release latent as well as bioactive TGF-β but, nonetheless, do not necessarily up-regulate TGF-β mRNA during the apoptotic process. This may explain why we were able to detect varying levels of TGF-β in all cultures by ELISA (data not shown). Nonetheless, a neutralizing anti-TGF-β mAb was able to block suppression (Fig. 4 b and c). Therefore, it is possible that the reduction of blastogenesis and proliferation of our primed cells may be a function of TGF-β release (or membrane-bound) and death induction. How this mechanism operates in vivo is unclear, but it is known that tumor cells can guard themselves against attack from the immune system by assembling an immunosuppressive environment through the secretion of TGF-β (41), which may be augmented by tumor cell apoptosis (35).
In our previous studies, we showed that OX40 costimulation in combination with LPS enhanced CD4 T cell clonal expansion and survival over that of CD8 T cells (9). The same is true for CD40, which is in direct contrast to the data shown in this study concerning 4-1BB (32). The data in Table 1 show that 4-1BB costimulation had a potent rescuing effect on CD8 T cells as opposed to CD4 T cells (line 2). This difference was enhanced further in the presence of LPS or PIC (lines 4 and 6). In the case of SEA/LPS-immunized mice, rescue of the CD4 SEA-specific T cells was enhanced over that of the SEA/4-1BB-treated mice, which suggests that LPS likely is operating through more than just 4-1BB costimulation (21). Thus, it is possible that costimulatory molecules have evolved for optimized function in specific T cell subpopulations. In the case of 4-1BB, this is particularly important to resolve because based on the data in this article, CD8 T cells primed with 4-1BB can have suppressive effects on CD4 T cells. Therefore, 4-1BB may activate CD4 T cells, but activation is masked in the presence of CD8 T cells. Our in vivo CD8-depletion data show that CD4 T cells respond better in the absence of CD8 T cells (Fig. 4 a and b), and the in vitro data extend these observations by demonstrating that normal CD8 T cells do not suppress the CD4 responses (Fig. 3).
Although these data may harken back to earlier ideas of CD8 suppressor T cells (42, 43), it is more likely to reflect that 4-1BB costimulation induces TGF-β release possibly by inducing death. One can envision this as a mechanism of modulating viral immunity, such as that during lymphocytic choriomeningitis virus or HIV infection, where the virus can infect CD4 T cells, which, in turn, can present MHC class I viral peptides (44). Thus, although the readout of the proliferative response is suppression, the actual mechanism may be dominance of one effector activity (cytotoxicity and TGF-β release) over another. This may explain why a therapeutic anti-4-1BB mAb can have immune-boosting and immune-suppressing effects at the same time. Uncovering how to control one activity over the other likely will have important therapeutic value.
Supplementary Material
Acknowledgments
We thank Drs. A. Adler, J. R. Maxwell, S. McSorley, and L. Lefrancois (University of Connecticut Health Center) for careful review and discussion of the manuscript. This work was supported in part by National Institutes of Health Grants A142858A and R21AI52108-01 (to A.T.V.). L.M. is supported by National Institutes of Health Immunology Training Grant T32-AI07080.
Abbreviations
- TLR
Toll-like receptor
- SEA
staphylococcal enterotoxin A
- LPS
lipopolysaccharide
- PIC
polyinosinic⋅polycytidylic acid
- TGF-β
transforming growth factor type β
- Ova
ovalbumin
- BSS
balanced salt solution
- LN
lymph node
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Takahashi C, Mittler R S, Vella A T. J Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 2.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K. Int Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox R A, Chapoval A I, Gorski K S, Otsuji M, Shin T, Flies D B, Tamada K, Mittler R S, Tsuchiya H, Pardoll D M, et al. J Immunol. 2002;168:4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 4.Diehl L, van Mierlo G J, den Boer A T, van der Voort E, Fransen M, van Bostelen L, Krimpenfort P, Melief C J, Mittler R, Toes R E, et al. J Immunol. 2002;168:3755–3762. doi: 10.4049/jimmunol.168.8.3755. [DOI] [PubMed] [Google Scholar]
- 5.Cannons J L, Lau P, Ghumman B, DeBenedette M A, Yagita H, Okumura K, Watts T H. J Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 6.Gramaglia I, Cooper D, Miner K T, Kwon B S, Croft M. Eur J Immunol. 2000;30:392–402. doi: 10.1002/1521-4141(200002)30:2<392::AID-IMMU392>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Bertram E M, Lau P, Watts T H. J Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell J R, Campbell J D, Kim C H, Vella A T. J Immunol. 1999;162:2024–2034. [PubMed] [Google Scholar]
- 9.Maxwell J R, Weinberg A, Prell R A, Vella A T. J Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 10.Pollok K E, Kim Y J, Zhou Z, Hurtado J, Kim K K, Pickard R T, Kwon B S. J Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- 11.Melero I, Shuford W W, Newby S A, Aruffo A, Ledbetter J A, Hellstrom K E, Mittler R S, Chen L. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 12.Shuford W W, Klussman K, Tritchler D D, Loo D T, Chalupny J, Siadak A W, Brown T J, Emswiler J, Raecho H, Larsen C P, et al. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon B S, Hurtado J C, Lee Z H, Kwack K B, Seo S K, Choi B K, Koller B H, Wolisi G, Broxmeyer H E, Vinay D S. J Immunol. 2002;168:5483–5490. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 14.Mittler R S, Bailey T S, Klussman K, Trailsmith M D, Hoffmann M K. J Exp Med. 1999;190:1535–1540. doi: 10.1084/jem.190.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Lin X, Chen H M, Wu Q, Subudhi S K, Chen L, Fu Y X. J Immunol. 2002;168:1457–1465. doi: 10.4049/jimmunol.168.3.1457. [DOI] [PubMed] [Google Scholar]
- 16.Halstead E S, Mueller Y M, Altman J D, Katsikis P D. Nat Immunol. 2002;3:536–541. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 17.Pullen A M, Marrack P, Kappler J W. Nature. 1988;335:796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- 18.Leo O, Foo M, Sachs D H, Samelson L E, Bluestone J A. Proc Natl Acad Sci USA. 1987;84:1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinney R, Thacker L, Hebert G A. J Dent Res. 1976;55:A38–A44. doi: 10.1177/002203457605500117011. [DOI] [PubMed] [Google Scholar]
- 20.Dasch J R, Pace D R, Waegell W, Inenaga D, Ellingsworth L. J Immunol. 1989;142:1536–1541. [PubMed] [Google Scholar]
- 21.Takahashi C, Mittler R S, Vella A T. Immunol Lett. 2001;76:183–191. doi: 10.1016/s0165-2478(01)00188-2. [DOI] [PubMed] [Google Scholar]
- 22.Masopust D, Vezys V, Marzo A L, Lefrancois L. Science. 2001;291:2413–2417. [PubMed] [Google Scholar]
- 23.Unkeless J C. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tough D F, Sprent J. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liblau R S, Tisch R, Shokat K, Yang X, Dumont N, Goodnow C C, McDevitt H O. Proc Natl Acad Sci USA. 1996;93:3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormack J E, Callahan J E, Kappler J, Marrack P. J Immunol. 1993;150:3785–3792. [PubMed] [Google Scholar]
- 27.MacDonald H S, Baschieri S, Lees R K. Eur J Immunol. 1991;21:1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- 28.Webb S, Morris C, Sprent J. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 29.Vella A T, McCormack J E, Linsley P S, Kappler J W, Marrack P. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 30.Tough D F, Borrow P, Sprent J. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 31.Alexopoulou L, Holt A C, Medzhitov R, Flavell R A. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell J R, Ruby C, Kerkvliet N I, Vella A T. J Immunol. 2002;168:4372–4381. doi: 10.4049/jimmunol.168.9.4372. [DOI] [PubMed] [Google Scholar]
- 33.DeSilva D R, Urdahl K B, Jenkins M K. J Immunol. 1991;147:3261–3267. [PubMed] [Google Scholar]
- 34.Tham E L, Mescher M F. J Immunol. 2001;167:2040–2048. doi: 10.4049/jimmunol.167.4.2040. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Frank M E, Jin W, Wahl S M. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 36.Wahl S M, Allen J B, Costa G L, Wong H L, Dasch J R. J Exp Med. 1993;177:225–230. doi: 10.1084/jem.177.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weatherill A R, Maxwell J R, Takahashi C, Weinberg A D, Vella A T. Cell Immunol. 2001;209:63–75. doi: 10.1006/cimm.2001.1783. [DOI] [PubMed] [Google Scholar]
- 38.Shevach E M. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 39.Fuller C L, Braciale V L. J Immunol. 1998;161:5179–5186. [PubMed] [Google Scholar]
- 40.Noble A, Pestano G A, Cantor H. J Immunol. 1998;160:559–565. [PubMed] [Google Scholar]
- 41.de Visser K E, Kast W M. Leukemia. 1999;13:1188–1199. doi: 10.1038/sj.leu.2401477. [DOI] [PubMed] [Google Scholar]
- 42.Dorf M E, Benacerraf B. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- 43.Dorf M E, Benacerraf B. Immunol Rev. 1985;83:23–40. doi: 10.1111/j.1600-065x.1985.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 44.Zinkernagel R M, Planz O, Ehl S, Battegay M, Odermatt B, Klenerman P, Hengartner H. Immunol Rev. 1999;168:305–315. doi: 10.1111/j.1600-065x.1999.tb01300.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.