Abstract
The control of cell proliferation during organogenesis plays an important role in initiation, growth, and acquisition of the intrinsic size of organs in higher plants. To understand the developmental mechanism that controls intrinsic organ size by regulating the number and extent of cell division during organogenesis, we examined the function of the Arabidopsis regulatory gene AINTEGUMENATA (ANT). Previous observations revealed that ANT regulates cell division in integuments during ovule development and is necessary for floral organ growth. Here we show that ANT controls plant organ cell number and organ size throughout shoot development. Loss of ANT function reduces the size of all lateral shoot organs by decreasing cell number. Conversely, gain of ANT function, via ectopic expression of a 35S::ANT transgene, enlarges embryonic and all shoot organs without altering superficial morphology by increasing cell number in both Arabidopsis and tobacco plants. This hyperplasia results from an extended period of cell proliferation and organ growth. Furthermore, cells ectopically expressing ANT in fully differentiated organs exhibit neoplastic activity by producing calli and adventitious roots and shoots. Based on these results, we propose that ANT regulates cell proliferation and organ growth by maintaining the meristematic competence of cells during organogenesis.
Although plant growth is influenced greatly by external environmental factors, it appears that the intrinsic size of plant organs is determined by internal developmental factors. Evidence for intrinsic organ size control is seen by the remarkable uniformity of organ size among individuals within a species (e.g., Arabidopsis thaliana petal size) and by the conspicuous differences in organ size between related species (e.g., A. thaliana versus Brassica napus petal size). Previous genetic analysis has focused primarily on the role of polarized cell elongation in determining organ morphology (1, 2). However, disparity in size of a particular organ (e.g., petal) between species is primarily the result of differences in cell number, not cell size (3). Thus, the intrinsic organ size of a species is determined primarily by organ cell number. Population genetics has indicated that intrinsic plant organ size might be regulated by a polygenic system, and in some species quantitative trait loci that affect plant organ size have been studied (4). However, how intrinsic organ size is genetically controlled, or the nature of the developmental regulators involved in plant organ size control, is not well understood.
The total cell number of an organ is determined by the number of divisions of undifferentiated stem cells [i.e., meristematic cells (5)]. During shoot development, lateral organs are initiated as primordia from apical and lateral meristems (6, 7). Although most cells in organ primordia are meristematic and proliferate, cells lose meristematic competence and withdraw from the cell cycle as organs develop. Thus, the maintenance of meristematic competence of cells is a key mechanism that mediates organ growth and cell proliferation by defining total cell number and thereby the size of plant organs.
To understand how intrinsic organ size is controlled in Arabidopsis, we have studied the function of the AINTEGUMENTA (ANT) gene (8, 9). ANT encodes a transcription factor of the AP2-domain family that has been found only in plant systems (8–10). Previously, it was shown that loss-of-function ant mutants exhibit reduction in the number and size of floral organs, in addition to defects in the initiation and growth of the integuments during ovule development. These results suggested that ANT might control integument and floral organ initiation and growth (8, 9). However, in situ hybridization experiments revealed that ANT mRNA accumulates in primordia of all lateral shoot organs, not only floral organ and ovule primordia. Shortly after emergence of the primordium, ANT mRNA becomes localized at the growing zone of immature organs (9). This pattern of ANT expression suggested that ANT may play a general role in organ primordium initiation and/or organ growth throughout shoot development.
To understand ANT function in plant organogenesis, we have examined loss- and gain-of-function effects of ANT on organogenesis during shoot development. We demonstrate that ANT is an intrinsic organ size regulator that is necessary and sufficient to control cell number and growth of lateral organs throughout shoot development. Based on our results, we discuss possible mechanisms by which ANT regulates the cell number and size of mature organs. We propose that ANT may coordinate cell proliferation with cell growth by maintaining meristematic competence of cells during organogenesis.
Methods
Mutant Allele.
The null allele, ant-1 (8), back-crossed four times into the Col-0 background, was used. Plants were grown on soil under long-day conditions (16-h light) unless otherwise noted.
Plant Transformation and Propagation.
The cauliflower mosaic virus 35S promoter (11) was used for ectopic expression of ANT. Agrobacterium-mediated transformation of Arabidopsis plants was performed by vacuum-infiltration. Transgenic tobacco (Nicotiana tabacum, SR1 cultivar) plants were generated from leaf-disks by using tissue culture procedures as described (12). Because most Arabidopsis 35S::ANT transgenic plants expressing ANT ectopically were sterile, individual T1 plants were used for phenotypic analysis. In some experiments using floral organs, vegetatively propagated plants from T1 plants were used. Vegetative propagation of T1 plants was performed under long-day conditions on MS agar plates containing 1 × MS salts (GIBCO/BRL), 1% sucrose, and 0.8% Bacto-agar (Difco). Rooted plantlets were transferred into soil for full growth.
Microscopy.
Preparation of specimens for scanning electron microscopy and differential interference contrast microscopy was performed as described (8, 12).
Size and Number Measurement.
Most Arabidopsis organs consist of cells with polyploid nuclei attributable to endoreduplication that influences cell size (13). For comparison of cell size and numbers, the distal portion of the petal epidermis was analyzed because it has cells that are diploid and uniform in size (ref. 12; data not shown). For statistical analysis, images were digitized with a umax scanner (UMAX Technologies, Fremont, CA) and were analyzed by using the nih image program (http://rsb/info.nih.gov/nih-image). Statistical calculations were performed with Microsoft excel.
Semiquantitative Reverse Transcription–PCR.
Total RNA isolation and reverse transcription was performed by using inflorescences with young floral buds (up to early-stage 9) or 7-week-old leaves to analyze expression levels of ANT and other genes. Four dilutions (100, 10−1, 10−2, 10−3) of 1/50 of the reverse transcription reactions were used for PCR. Oligonucleotide primers for reverse transcription–PCR were made according to the published cDNA sequence of ANT (8), CycD3 (14), CycB1b (15), and GaPDH (16).
Results
Loss of ANT Function Reduces Mature Organ Size by Decreasing Cell Numbers.
Because ANT mRNA accumulated in leaf (9), we examined the effect of a loss-of-function ant mutation on vegetative shoot development. Although there was no difference in the timing of leaf primordia initiation or the number of leaf primordia between ant-1 and control wild-type plants (not shown), the width and length of mature ant-1 leaves were both reduced in comparison with those of corresponding wild-type leaves (Fig. 1 A and B). Because ant mutant floral organs were found to be reduced in size (8, 9) (Fig. 1B), these observations demonstrate that loss of ANT function reduces organ size throughout shoot development.
Figure 1.
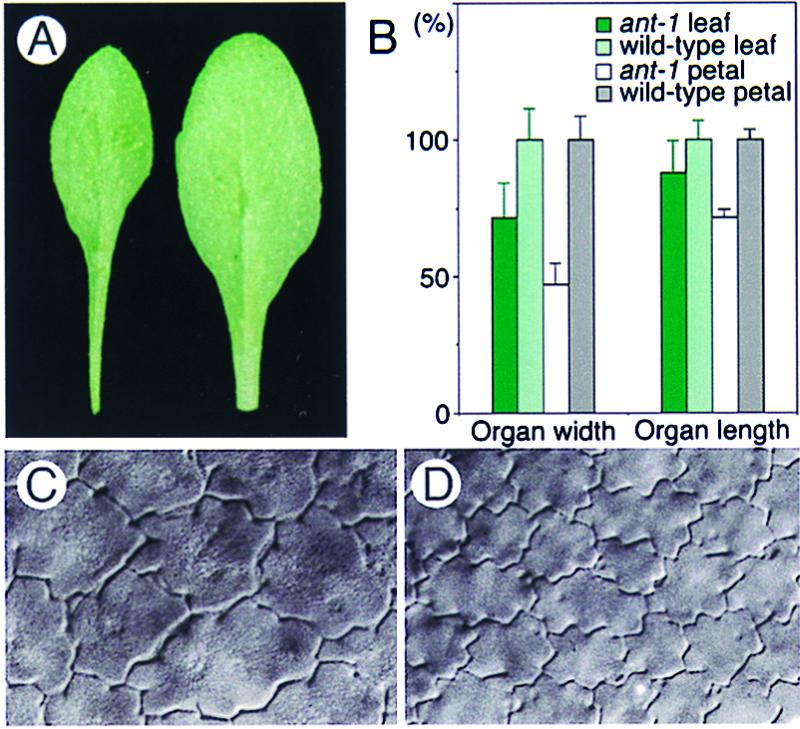
The loss-of-function ant-1 allele reduces mature organ size and cell number. (A) Fully grown seventh-rosette leaves from an ant-1 mutant (Left) and wild-type plant (Right). (B) Width and length of mature rosette leaves and petals. Wild-type (n = 12) and ant-1 (n = 12) mature third-rosette leaves, and wild-type (n = 58) and ant-1 (n = 41) mature petals (stage 15) were analyzed. Bars on columns show SD. (C and D) Epidermal cells (×150) from the abaxial, distal portion of mature (stage 15) ant-1 (C) and wild-type petals (D).
A change in organ size can reflect an alteration in the size or number of cells, or both. To understand why ant-1 organs are smaller, we examined the size and number of cells in mature ant-1 organs and compared them with those in wild-type controls. The distal portion of the petal epidermis was observed initially because it has cells that are diploid and uniform in size and shape. We found that ant-1 organs had fewer cells per unit area and per organ than the wild type; however, ant-1 cells were much larger than normal (Fig. 1 C and D). Essentially the same phenotype was observed in the epidermis and subdermal cell-layers of all ant-1 floral organs and leaves (Fig. 1; data not shown). Thus, the systemic reduction in size of ant-1 organs is associated with a decrease in cell number but not a decrease in cell size.
Because ant mutants reduce the number of floral organs, it has been suggested that ANT might be involved in organ primordium patterning as well as organ growth (8, 9). To evaluate this possibility, we observed the pattern of sepal primordia in developing wild-type and ant-1 floral buds under scanning electron microscopy. By the end of floral stage 4 (17), all four sepal primordia were initiated at the periphery of developing wild-type floral buds. In ant-1 floral buds at the comparable stage, the organ primordia initiated were arranged normally in ant-1 floral buds, although the number of floral organ was reduced (not shown). Thus, ANT appears to have little role in controlling the position of floral organ primordium in developing floral buds.
Gain-of-Function of ANT Increases Organ Size and Cell Numbers in Transgenic Arabidopsis Plants.
To examine the effects of gain of ANT function on organ growth, we generated Arabidopsis transgenic plants in which the ANT cDNA is expressed under the control of the constitutive cauliflower mosaic virus 35S promoter (11). Approximately 50% (10 of 22) of kanamycin-resistant (KmR) T1 plants highly expressed the ANT transgene and displayed multiple organ hyperplasia. That is, leaves, stems, pedicels, sepals, petals, stamens, gynoecia, ovules, and fruits were dramatically enlarged without altering their superficial morphology (Fig. 2 A–G). Mass of leaves and flowers was increased as much as three times over those in control plants (Fig. 2 B and D). This demonstrates that ectopic ANT expression is sufficient to increase organ size and mass by enhancing organ growth that is usually coordinated with organ morphogenesis in Arabidopsis plants.
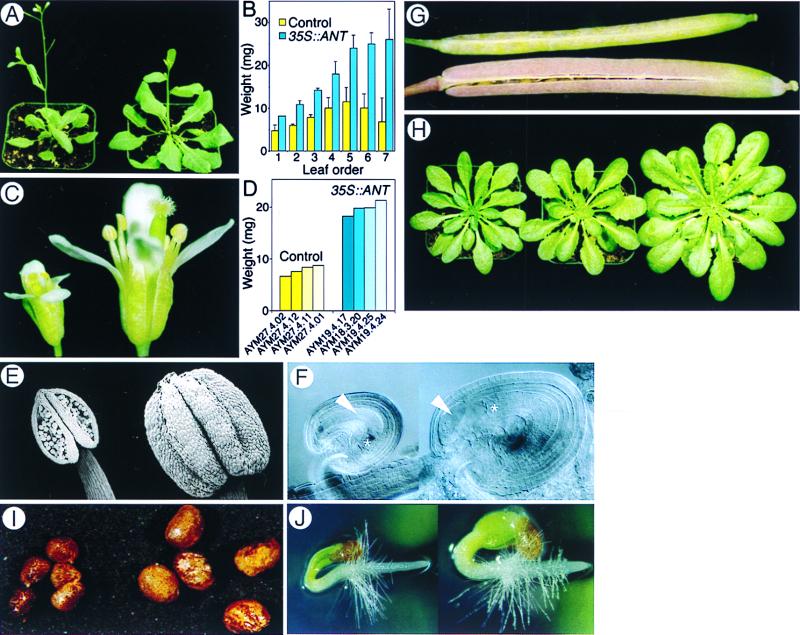
Figure 2.
Gain-of-function 35S::ANT transgene expression causes multiple organ hyperplasia. (A–H) Arabidopsis. (I and J) tobacco. A, C, and E–G show plants or organs of 35S-vector-only control (Left or Upper) and 35SANT (Right or Lower) transformants grown under the long-day conditions. Specimens in each panel were photographed together (A, C, G, and H), or are shown at the same magnification (E and F). (A) Whole plants after bolting. (B) Leaf mass. The average from four independent 35S::ANT and control transgenic plants is shown. Bars indicate SD. (C) Mature flowers. (D) Mass of 10 flowers each from four independent 35S::ANT (AYM 18, 19) and control (AYM 27) T1 transgenic plants. (E) Mature anthers. The 35S::ANT anther fails to dehisce. (F) An enlarged ovule from the weak 35S::ANT transformant AYM19.4.17 with abnormal growth of nucellus cells. Embryo sac (asterisk) and nucellus cells (arrowhead) are indicated. (G) A fruit obtained after hand-pollination of an AYM19.4.17 pistil with wild-type pollen and an autonomously self-pollinated control fruit. (H) ant-1 (left), control (middle), and 35S::ANT (right) plants grown under short-day (8-h light) conditions. (I) KmR R1 tobacco seeds obtained from hand-pollination. (J) Two-day-old tobacco seedlings.
Ectopic ANT expression resulted in male sterility in Arabidopsis plants, primarily because anthers failed to dehisce (Fig. 2E). Most 35SS::ANT transgenic plants were female sterile as well, because of abnormally extended proliferation of the chalazal nucellular cells (Fig. 2F). However, T1 plants expressing ANT at relatively low levels could generate seeds when pollinated by hand with wild-type pollen. The enlarged 35S::ANT fruit (Fig. 2G) included T2 seeds that were larger than normal (not shown), because of enlarged embryos. These seeds gave rise to mature KmR T2 plants exhibiting multiple organ hyperplasia (not shown). This hyperplasia in the T2 generation reveals that ectopic ANT expression increases embryonic growth as well, and ectopic ANT effects on organ size are heritable.
We observed similar loss- and gain-of-function effects on organ size when plants were grown under short-day (Fig. 2H), continuous-light conditions, and in poor or rich media (not shown). Thus, ANT function seems to be independent of the perception of external growth signals.
Differentiated cells in fully mature 35S::ANT petals were the same size as those in wild-type petals (Fig. 3 B and C). Similarly, no obvious difference in cell size was detected in the epidermis between control and 35S::ANT organs other than petals (not shown). Thus, an increase of cell numbers, and not cell size, is associated with the enlarged 35S::ANT organs
Figure 3.
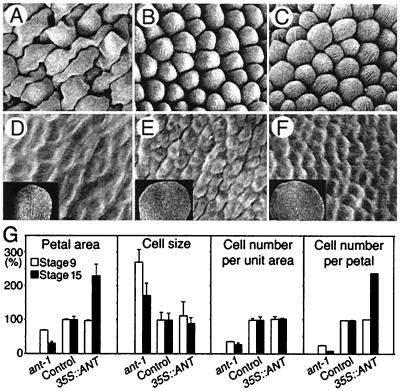
Loss- or gain-of-ANT-function influences the extent of cell proliferation during organogenesis. (A–C) Fully differentiated cells (×135) with centripetal ridges characteristic of the adaxial epidermis of mature petals at stage 15. (D–F) Undifferentiated epidermal cells (×450) from the adaxial, distal portion of mid-stage 9 petals (Insets, ×45). (A and D) ant-1 petal. (B and E) Control (wild type). (C and F) 35S::ANT. (G) Comparison in petal area, cell size, cell number per unit area, and numbers per petal. Percentages of results from ant-1 and 35S::ANT petals to those from control petals are shown. Bars indicate SD. The analysis was performed with the abaxial, distal portion of petals by using more than 20 flowers.
In contrast to the striking effects on final organ size, ectopic ANT expression did not alter the size or structure of apical and lateral meristems and the size or number of organ primordia (not shown). Therefore, ectopic ANT expression is not sufficient to increase organ primordium size or number.
Ectopic ANT Expression Enlarges Tobacco Organs.
To investigate whether ANT function in organ size regulation is conserved in other species, we examined gain-of-ANT-function effects in tobacco. Ten of 63 KmR R0 transgenic tobacco plants highly expressed the 35S::ANT transgene and exhibited multiple organ hyperplasia. KmR R1 seed (Fig. 2I) and seedlings (Fig. 2J) from hand pollinated R0 plants were larger than control. Hence, the effects of ANT on organ size seem to be conserved and heritable in a heterologous plant system.
ANT Affects the Duration of Cell Proliferation in Developing Petals.
How does ANT control cell number during organogenesis? In general, plant organ growth involves neither cell migration nor cell death; thus, organ cell number essentially depends on proliferation of the meristematic cells in the developing organ (6, 7). Because ANT is expressed in cells at the growing domain of the developing organs (9), it might modulate cell proliferation during organogenesis and thereby determine the total cell number in mature organs. To test this idea, we compared the extent of cell proliferation in control and ant-1 organs by measuring cell numbers and cell size of both developing and fully mature petals. During mid-floral stage 9 (17), the adaxial epidermal cells of wild-type petals were not differentiated (Fig. 3E) and divided frequently whereas ant-1 petals had fewer undifferentiated cells than normal (Fig. 3 D and E) per unit area and per organ (Fig. 3G). This reduction in cell number became more pronounced in fully differentiated ant-1 petals at stage 15 (17) (Fig. 3G). Thus, there are fewer cell divisions than normal in ant-1 petals throughout organogenesis, particularly during later developmental stages before maturation. Cell growth occurred without cell division in ant-1 petals, resulting in extremely large cells (Fig. 3 A and G). These results suggest that ANT is required for the normal extent of cell proliferation, but not primarily for cell growth.
There are at least two possible ways by which ANT regulates the extent of cell proliferation during organogenesis. First, ANT may determine the period of cell proliferation without altering the intrinsic cell cycle time. In this case, ANT may function to maintain meristematic competence of cells, thereby determining the developmental period in which cells are competent to proliferate. Alternatively, ANT might regulate the intrinsic cell cycle time. In this scenario, ANT determines the frequency of cell divisions before losing meristematic competence at the proper developmental time. To test these two models, we analyzed how ectopic ANT expression affects organ size, cell size, and cell numbers during petal development. In contrast to the early effect on cell numbers in ant-1 petals, cell number and cell size (Fig. 3G) in 35S::ANT petals at stage 9 were normal (Fig. 3 E, F, and G). This demonstrates that ectopic ANT expression does not increase cell growth or the frequency of cell proliferation during early stages of petal development and suggests that increased ANT activity does not alter the intrinsic cell cycle time. By stage 15, however, the total cell number of fully mature 35S::ANT petals reached ≈2.5 times that of controls (Fig. 3G), indicating that additional cell divisions occurred in 35S::ANT petals before organ maturation, yet only after stage 9. Extra cell divisions must be coordinated with cell growth because cell size in mature 35S::ANT petals is normal (Fig. 3 B, C, and G). Therefore, it is likely that ectopic ANT expression allows petal cells to proliferate for a longer period than normal without altering the intrinsic cell cycle time.
Leaf Growth Is Extended by Ectopic ANT Expression.
To confirm our hypothesis in another plant organ, we compared growth of rosette leaves in 35S::ANT and control seedlings (Fig. 4A). At 16 days after germination, both 35S::ANT and control seedlings had the same number of rosette leaves, and all leaves of 35S::ANT seedlings were the same size as corresponding control leaves (Fig. 4A). However, 35S::ANT leaves continued to grow beyond the period in which corresponding control leaves ceased to grow (Fig. 4A, first and second control leaves at 16 and 19 days, respectively, after germination), eventually giving rise to larger leaves than normal (Fig. 4A, first and second 35S::ANTA leaves). This observation supports our hypothesis that prolonged cell proliferation coordinated with cell growth causes hyperplasia in 35S::ANT plants.
Figure 4.
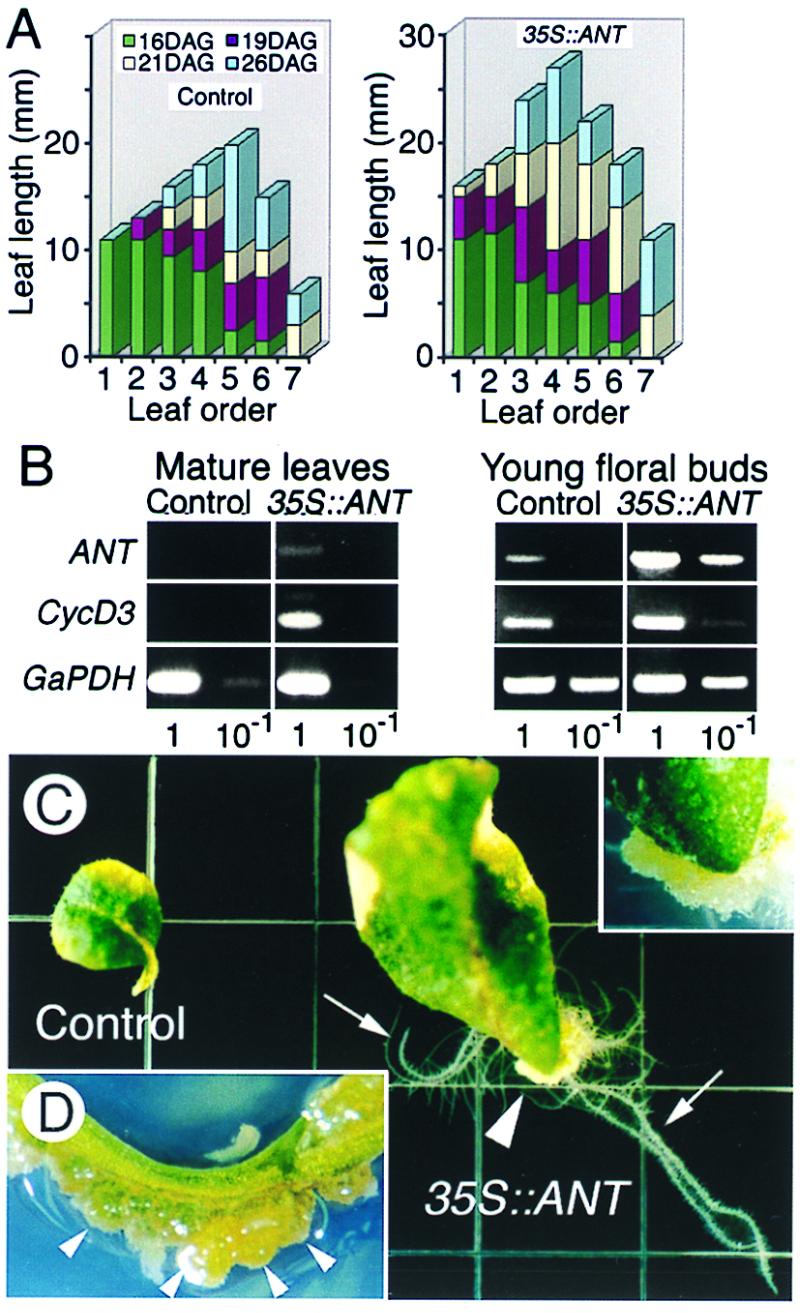
Gain-of-ANT-function prolongs meristematic potential in developing and mature organs. (A) Growth of leaves in control and 35S::ANT seedlings. Length of leaves from six vector-only controls and six 35S::ANT plants was measured at 16, 19, 21, and 26 days after germination (DAG). (B) Effect of ANT activity on CycD3 RNA level in line AYM19.4.7. (C) Ectopic ANT expression causes abnormal outgrowth of cells, or neoplasia. Nine-week-old control and 35S::ANT leaves were excised and placed on an MS-agar plate without phytohormones and were photographed two weeks after excision. Calli (arrowhead) and adventitious roots (arrows) were formed around the cut surface of the 35S::ANT leaf. Inset shows an enlargement of the mass of calli in the 35S::ANT leaf. (D) A stem of a 19-week-old, 35S::ANT plant shows clusters of green growing calli (arrowheads).
Ectopic CycD3 Expression Was Observed in Fully Differentiated, Mature 35S∷ANT Leaves.
To begin to understand how ANT maintains the meristematic competence of cells at the molecular level, we analyzed the transcription of CycD3, a G1 cyclin gene induced in response to growth stimuli and involved in the initiation and progression of the cell cycle (14). In fully differentiated 9-week-old leaves, where endogenous ANT expression was no longer detected, CycD3 transcripts were still observed in 35S::ANT leaves, but not in control or ant-1 leaves (Fig. 4B; data not shown). This demonstrates that prolonged ANT expression from the 35S::ANT transgene extends the period of cell cycle gene expression, suggesting that fully differentiated 35S∷ANT leaves still had cells with the meristematic competence. However, comparable levels of CycD3 mRNA were detected in control and 35S∷ANT inflorescences with very young floral buds composed primarily of proliferating cells (Fig. 4B), suggesting that ectopic ANT expression does not further increase CycD3 expression in tissue in which most cells are meristematic. Similar results were obtained in comparing mRNA levels of CycB1b (Cyc1bAt) (15), a mitotic cyclin gene (not shown). These results are consistent with the hypothesis that ANT maintains the meristematic competence of cells and consequently sustains expression of cell cycle regulators.
Cells in 35S∷ANT Organs Exhibit Neoplastic Activity.
Another striking finding that connects ANT function with the maintenance of meristematic competence is neoplasia found in the Arabidopsis 35S::ANT organs. That is, clusters of undifferentiated cells (i.e., calli) were generated from wounds or senesced-surfaces of 35S::ANT plants, or detached-ends of fully differentiated 35S::ANT organs without external phytohormone treatment (Fig. 4 C and D). These calli often differentiated into organs, such as roots (Fig. 4C), leaves, or shoots (not shown). This neoplasia was observed consistently in 35S::ANT organs but never was seen in control organs treated in the same way (Fig. 4C). It is well established that differentiated plant tissue can induce calli after phytohormone treatment (18). Perhaps, ectopic ANT expression in differentiated cells that are normally quiescent preserves meristematic competence and decreases their dependence on phytohormones for reentry into the cell cycle.
Discussion
Even in animal systems, where growth takes place relatively autonomously, how organ and body size is controlled remains largely unknown (19). This issue is more complicated in plants, where growth can be significantly influenced by signals from the external environment. That is, height, shoot structure, and organ size or shape of plants can be modified depending on their growth conditions. Nevertheless, as in animal systems, there is genetic information that controls the intrinsic size of plants or organs during development. This developmental information interacts with those from the external environment and determines the eventual size of plants or plant organs. The complex nature of organ size control in plants, and classical genetic analysis of organ and plant size, has suggested that a polygenic system is responsible for plant organ size control. Here, we demonstrate that the Arabidopsis transcription factor ANT is an intrinsic organ size regulator that is necessary and sufficient to control plant organ size. Our results provide evidence that plant organ size, as well as organ cell number, can be controlled to a great extent by modulation of a single gene activity.
Effect of ANT Function on Organ Cell Number and Cell Size.
Through loss- and gain-of-function analysis, we showed that ANT positively regulates organ size through the alteration of cell number. That is, ant-1 organs are smaller and have fewer cells (Figs. 1 and 3) whereas 35S::ANT organs are larger and have more cells (Fig. 2). Thus, ANT activity is both necessary and sufficient to control organ size and cell number. In contrast, ANT function, cell size, and organ size do not appear to be simply correlated. We have found that ant-1 cells are larger than normal, even in immature organs in which undifferentiated cells are actively dividing in control organs (Fig. 3). When ANT was ectopically expressed, however, we observed an increase in the number of cells that were normally sized in both immature and mature organs (Fig. 3). Hence, ANT does not appear to control organ size control through cell size regulation.
Effect of ANT Function on Cell Proliferation and Cell Growth.
In plants, cell number is determined primarily by cell proliferation. The positive correlation found between ANT function and organ cell number suggests that ANT positively regulates cell proliferation. Furthermore, comparison of organ growth pattern and cell number increase during organogenesis in wild-type versus 35S::ANT transgenic plants revealed that the difference in cell number was less significant in immature growing organs but was obvious in mature organs (Fig. 3 and 4). This suggests that ANT regulates the extent of cell proliferation, rather than the rate of cell proliferation, to control organ cell numbers.
Cell size is regulated by the coordination of cell growth with cell proliferation in dividing cells, as well as by cell expansion in differentiated nondividing cells (20). The larger ant-1 cells partially compensated for the decrease in cell numbers and minimized the reduction in overall ant-1 organ growth (Fig. 3). This increase in cell size in ant-1 mutant plants suggests that ANT function may not be necessary for cell growth. However, ectopic ANT expression appeared to extend the period of cell division in petals (Fig. 3) and leaves (Fig. 4), and the cell divisions were coupled normally with cell growth throughout organogenesis. This demonstrates that increased ANT function is sufficient to positively regulate cell growth. One way to explain the contradiction between loss- and gain-of-function phenotypes is that ANT regulates growth as well as cell proliferation, but redundant gene activities allow for cell growth when ANT is absent. Alternatively, ANT may coordinate growth with cell proliferation such that they are no longer coupled when ANT function has been eliminated (see below).
Coordination of Cell Proliferation and Growth.
In most tissues, cell proliferation is coordinated with growth such that cells double their size before dividing in two. In general, mutations that block the cell cycle generally do not interfere with cell growth. Conversely, mutations affecting metabolism coordinately arrested both cell growth and division (21). Hence, cell growth and cell cycle progression are separable processes, and growth is dominant and rate-limiting (21, 22).
We observed that the loss-of-function ant-1 mutation uncouples cell proliferation and growth, resulting in organs with fewer cells whose size is larger than normal (Figs. 1 and 3). Similar compensation of organ and cell growth was observed in other plant and animal systems in which cell division is restricted by defects in cell cycle progression (21, 23). Thus, the ant loss-of-function phenotype appears to be consistent with the hypothesis that ANT is a regulator of cell cycle progression. However, as described below, other lines of evidence show that ANT function is not merely involved in cell proliferation.
In general, strategies that simply increase expression of cell cycle regulators have not lead to increased growth and organ size. In these experiments, either cell cycle duration was adjusted by an unknown mechanism, or an increased cell number was offset by a reduction in cell size. For example, in Drosophila, modulation of the cell cycle rate by ectopic expression of dE2F, when cell death was suppressed, increased cell numbers but failed to stimulate growth (21). Similarly, in Arabidopsis, ectopically expressed CycD3, a G1 cyclin, under the control of the 35S promoter failed to increase organ size: that is, the increased cell division disturbed morphogenesis, resulting in producing twisted organs, not enlarged organs (24). In contrast, we showed that ectopic expression of ANT increased the number of normal sized cells that resulted in net organ growth (Figs. 3 and 4). This result suggests that ANT does more than simply control cell cycle progression, influencing cell growth as well.
There are instances in which modification of specific cell cycle regulators appears to affect cell proliferation as well as organ growth, however. In Arabidopsis, ectopic CYCB1a (Cyc1At) expression driven by the cdc2aAt promoter stimulated root growth through the stimulation of cell proliferation (25). An important difference in phenotype between 35S::ANT∷ and cdc2aAt∷Cyc1At transgenic plants, however, is that 35S::ANT did not increase the initial growth rate of organs, including roots (not shown), as cdc2aAt∷Cyc1At did with roots (25). Furthermore, improved growth of shoot organs by cdc2aAt∷Cyc1At has not been reported. Another example is found in mice, where elimination of the G1 cyclin-CDK inhibitor p27kip1 or p18INK4c causes both hyperplasia and neoplasia by preventing cells from exiting the cell cycle and extending the period of cell proliferation without altering cell size (26, 27). By analogy, ANT may act by inactivating a cell cycle inhibitor(s) like p27kip1. This could also explain how ectopic ANT expression during late organogenesis allows cells to divide for a longer period. Further investigation will reveal whether the inactivation of negative cell cycle regulators is a mechanism for modulating plant organ size.
ANT May Maintain Meristematic Competence of Cells During Organogenesis.
In plant and animal systems, growth signaling pathways and the cell cycle machinery appear to share many common factors (28, 29). Nevertheless, given the immobile attributes of plant life and plant cells, which are surrounded by rigid cell walls (5), some aspects of plant growth and cell proliferation are likely to be regulated and coordinated in a different way from those of animals (19). Thus, it may not be surprising that ANT is a plant-specific regulator (8–10) that uniquely coordinates cell proliferation with growth to control organ size.
A clue to address how ANT controls the extent of cell proliferation and growth during organogenesis may be found in the regulation of activity of the plant-specific growth domain, meristems. The meristem is composed of stem cells (i.e., meristematic cells) and forms new cells by division (5, 6). Most cells in an organ primordium are meristematic, and thus competent to divide, when initiated from the periphery of the apical shoot meristem. As the organ develops, cells gradually lose meristematic competence and cease to divide. Thus, it is likely that there is a developmental switch that regulates cells to maintain or relieve the meristematic competence, thereby determining the extent of organ growth as well as the total cell number of organs (7). The ANT expression pattern (9), as well as loss- and gain-of-function ANT phenotypes (this study), suggests that ANT may function as such a factor.
We propose a model of ANT action in maintenance of meristematic competence of cells during organogenesis (Fig. 5). In this model, developmental growth signals activate growth regulators, which positively regulate ANT during organogenesis. ANT functions to maintain meristematic competence of cells, thereby modulating the expression of cell cycle and cell growth regulators. As a result, ANT sustains cell proliferation that is coupled to cell growth in developing organs. Ectopically expressed ANT, therefore, results in the abnormal retention of meristematic competence of cells and causes hyperplasia and neoplasia whereas the absence of ANT causes precocious termination of cell proliferation and organ growth.
Figure 5.
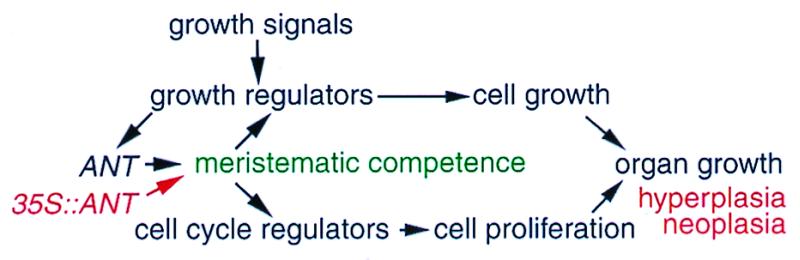
A model of ANT function in plant organ size control.
Organ Size Control in Plant Evolution.
One of the most intriguing questions in evolution is how plants within the same genus or family attained significant size differences, even though they maintain essentially the same architecture. Regulation of meristematic competence by ANT, at least in part, may be a mechanism that is responsible for the size diversity observed in higher plant species. It has been suggested that the genetic basis for plant interspecies diversity of phenotype might be minor changes in the structure or expression of orthologous regulatory genes (30, 31). Recently, we have isolated a gene encoding a potential B. napus ANT ortholog, with 84% amino acid identity to Arabidopsis ANT, and have shown that it also causes multiple hyperplasia when ectopically expressed in Arabidopsis plants (data not shown). Future studies on differences in structure and expression pattern of ANT and its orthologs from the Brassicaceae species (e.g., B. napus) may contribute to understanding the interspecies diversity of organ size in higher plants. Finally, increasing organ mass by ectopic ANT expression might be a new powerful method for improving the yield of agriculturally important plants.
Acknowledgments
We thank L. Reiser, J. Colasanti, M. Tsiantis, and T. Durfee for discussions and comments on the manuscript, M. Pastuglia for the GaPDH primers, and P. J. Lee for help in maintaining tobacco plants. R.L.F. is supported by a grant from the National Science Foundation (Grant IBN-9817992).
Abbreviation
- KmR
kanamycin-resistant
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kim G-T, Tsukaya H, Saito Y, Uchimiya H. Proc Natl Acad Sci USA. 1999;96:9433–9437. doi: 10.1073/pnas.96.16.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuge T, Tsukaya H, Uchimiya H. Development (Cambridge, UK) 1996;122:1589–1600. doi: 10.1242/dev.122.5.1589. [DOI] [PubMed] [Google Scholar]
- 3.Sachs J. Flora. 1893;77:46–81. [Google Scholar]
- 4.Mian M A R, Wells R, Carter T E, Ashley D A, Boerma H R. Theor Appl Genet. 1998;96:354–360. doi: 10.1007/s001220050748. [DOI] [PubMed] [Google Scholar]
- 5.Esau K. Anatomy of Seed Plants. New York: Wiley; 1977. [Google Scholar]
- 6.Steeves T A, Sussex I M. Patterns in Plant Development. New York: Cambridge Univ. Press; 1989. [Google Scholar]
- 7.Meyerowitz E M. Cell. 1997;88:299–308. doi: 10.1016/s0092-8674(00)81868-1. [DOI] [PubMed] [Google Scholar]
- 8.Klucher K M, Chow H, Reiser L, Fischer R L. Plant Cell. 1996;8:137–153. doi: 10.1105/tpc.8.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott R C, Betzner A S, Huttner E, Oakes M P, Tucker W Q J, Gerentes D, Perez P, Smyth D R. Plant Cell. 1996;8:155–168. doi: 10.1105/tpc.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigel D. Plant Cell. 1995;8:388–389. doi: 10.1105/tpc.7.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers S G, Klee H J, Horsch R B, Fraley R T. Methods Enzymol. 1987;153:253–277. [Google Scholar]
- 12.Mizukami Y, Ma H. Cell. 1992;71:119–131. doi: 10.1016/0092-8674(92)90271-d. [DOI] [PubMed] [Google Scholar]
- 13.Koornneef M. In: Arabidopsis. Meyerowitz E M, Somerville C R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. pp. 89–120. [Google Scholar]
- 14.Soni R, Carmicael J P, Shah Z H, Murray J A H. Plant Cell. 1995;7:85–103. doi: 10.1105/tpc.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day I S, Reddy A S, Golovkin M. Plant Mol Biol. 1996;30:565–575. doi: 10.1007/BF00049332. [DOI] [PubMed] [Google Scholar]
- 16.Shih M C, Heinrich P, Goodman H M. Gene. 1991;104:133–138. doi: 10.1016/0378-1119(91)90242-4. [DOI] [PubMed] [Google Scholar]
- 17.Smyth D R, Bowman J L, Meyerowitz E M. Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salisbury F B, Ross C W. Plant Physiology. Belmont, CA: Wadsworth; 1985. [Google Scholar]
- 19.Conlon I, Raff M. Cell. 1999;96:235–244. doi: 10.1016/s0092-8674(00)80563-2. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs T. Plant Cell. 1997;9:1021–1029. doi: 10.1105/tpc.9.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neufeld T P, de la Cruz A F A, Johnston L A, Edgar B A. Cell. 1998;93:1183–1193. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- 22.Johnstone G C, Pringle J R, Hartwell L H. Exp Cell Res. 1977;105:79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- 23.Hemerly A S, Ferreira P, de Almeida Engler J, Van Montagu M V, Engler G, Inze D. Plant Cell. 1993;5:1711–1723. doi: 10.1105/tpc.5.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riou-Khamlichi C, Huntley R, Jacqmard J, Murray A H. Science. 1999;283:1541–1544. doi: 10.1126/science.283.5407.1541. [DOI] [PubMed] [Google Scholar]
- 25.Doerner P, Jorgensen J-E, You R, Steppuhn J, Lamb C. Nature (London) 1996;380:520–523. doi: 10.1038/380520a0. [DOI] [PubMed] [Google Scholar]
- 26.Kiyokawa H, Kineman R, Mantova-Torova K O, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 27.Franklin D S, Godfrey V L, Lee H, Kovalev G I, Schoonhoven R, Chen-Kiang S, Su L, Xiong Y. Genes Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishihama R, Banno H, Shibata W, Hirano K, Nakashima M, Usami S, Machida Y. Plant Cell Physiol. 1995;36:749–757. doi: 10.1093/oxfordjournals.pcp.a078818. [DOI] [PubMed] [Google Scholar]
- 29.Burssens S, Van Montague M, Inze Plant Physiol Biochem. 1998;36:9–19. [Google Scholar]
- 30.Doebley J, Lukens L. Plant Cell. 1998;10:1075–1082. doi: 10.1105/tpc.10.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somerville S, Somerville C. Science. 1999;285:380–383. doi: 10.1126/science.285.5426.380. [DOI] [PubMed] [Google Scholar]