Abstract
Cancer cachexia involves the loss of weight, mainly in skeletal muscle and adipose tissue, that is not caused simply by anorexia. The syndrome includes anemia and immunosuppression along with a number of biochemical changes indicating systemic effects of the cancer. It is a major factor in morbidity and mortality from cancer. For 30 years beginning in 1948, a large number of studies reported isolation from many tumors of a heterogeneous group of small peptides, generally labeled toxohormone, that caused various correlates of cachexia shortly after injection into mice. Interest in toxohormone-like peptides then fell off for diverse reasons that had little to do with their clinical significance and was shifted to cytokines, ILs, and ectopic hormones with catabolic consequences that were sporadically found in tumors. At the same time, evidence was accumulating for an important role of pericellular proteases in driving progressive stages of neoplastic development. A central part of that evidence was the inhibition of transformation-related changes by protease inhibitors, particularly the combination present in fetal bovine serum, which fully suppressed the expression of the transformed phenotype in discrete foci of chicken embryo fibroblasts (CEF) infected by Rous sarcoma virus against a confluent background of uninfected CEF. In contrast, CEF cultures heavily infected with Rous sarcoma virus in the same medium underwent pervasive transformation, which was correlated with the release of low molecular weight cytotoxic substances. Reevaluation of all of the evidence supports a central role for proteolytically generated peptides derived from tumors in producing cancer cachexia.
Cancer Cachexia, Toxohormones, and Small, Toxic Peptides
Cachexia occurs in about half of all cancer patients and is associated with a decreased survival time (1). It is more common in patients with lung and upper gastrointestinal tract cancer and less common in breast and lower gastrointestinal cancer (2). Cachexia is chiefly characterized by a loss of skeletal muscle mass and adipose tissue and cannot be fully explained by lowered intake of nutrient. It is often accompanied by anemia and immunodepression (3). There are several systemic biochemical effects of transplanted tumors in mice, including a decrease of liver catalase and plasma iron (4, 5), which taken together can be considered a cancer cachexia syndrome. Many attempts have been made to identify substances released from neoplastic tissue that could account for the systemic effects associated with cachexia. A milestone in this effort was established by Japanese workers when a chemical fraction concentrated from each of 14 human gastric and colorectal cancers depressed liver catalase activity within a day after injection into mice, whereas fractions from corresponding normal tissues of the same patients did not (6). Liver catalase depression was chosen as the assay because it was the most consistent biochemical correlate with experimental cancer (7). In subsequent studies by the same workers, all 10 different types of human cancer examined yielded material that was active in milligram quantities, whereas all normal tissue tested was negative (4). The active substance was designated “toxohormone,” initially defined as a substance released from tumors into the circulation that induced a clearly defined biochemical lesion. However, it also caused atrophy of the thymus, another common occurrence in experimental cancer (8). It was conjectured that toxohormone might be a polypeptide. An estimate based on amino acid components of active material isolated by alcohol precipitation and reprecipitation in high salt yielded a molecular mass of ≈4,000 Da. Because the activity in crude extracts was nondialyzable (6), but became dialyzable after acid treatment or proteolytic digestion, it was thought that toxohormone existed in cancer tissue as aggregates or bound to larger molecules (4). The basic results of the Japanese workers were confirmed by using more efficient methods of isolating the toxohormone in several laboratories, including the one that had established the relation between depression of liver catalase and cancer (9). Necrotic portions of cancer tissue yielded no greater amount of the active fraction than did the fresh non-necrotic portions, nor did 20 h of decomposition at 37°C in vitro of cancer tissue increase the activity of the fraction (6), indicating it was not produced by simple autolysis of the cancer. It was later reported, however, that appreciable quantities of toxohormone activity could be isolated from a variety of normal animal tissues that had undergone autolysis in vitro at 37°C for 4 days (10). Unlike the material from the living part of tumors, that isolated from the necrotic part of tumors killed the animals (9). It is noteworthy, however, that proteases and peptidases are found mainly in the peripheral parts (11) of tumors containing small, actively multiplying cells rich in cytoplasmic RNA and protein synthesis rather than the more central regions consisting of large, quiescent cells poor in cytoplasmic RNA and protein synthesis, or of necrosis (12). This finding implies that an abundant supply of proteolytic peptides occurs in the growing part of the tumor, which is in accord with the isolation of cytotoxic peptides from free fluids at the tumor periphery (13).
Later studies using different neoplastic tissues and different methodologies for fractionation also concluded that toxohormone consisted of small polypeptides. For example, fractions from human lung and liver tumors that depressed liver catalase activity in mice occurred in at least three chromatographic fractions with molecular masses between 4,200 and 6,400 Da (14). Toxohormone obtained from the Walker 256 rat carcinoma, which inhibited amino acid incorporation into diaphragm myofibrillar muscle protein, had molecular masses in the regions of 8,000 and 14,000 Da (15). Untreated cell-free fluid from Ehrlich ascites carcinomas in mice suppressed the immune response in healthy mice (16). It was fractionated by Diaflo ultrafiltration and gave five fractions with stepwise reductions in molecular size, of which the middle three size classes suppressed the immune response in Jerne's highly sensitive hemolytic plaque method. The molecular mass of the most active fraction was in the range of 1,000–10,000 Da, with moderate activity from the 500–1,000-Da fraction, and significant but low activity from the 10,000–100,000-Da fraction. There was no activity in the fraction <500 Da or >100,000 Da. Serum-free medium from a culture of mouse fibrosarcoma cells depressed plasma-bound iron levels in mice and inhibited cell-free protein synthesis in microgram doses (3). The active fractions eluted from a Sephadex G-15 column in a broad peak. The peak lost two-thirds of its activity on dialysis, indicating that the active fractions, which had at least three antigenically distinct components, were small peptides. Some activity capable of depressing liver catalase was found in fractions from normal spleen, but in much smaller amounts and different fractions than in tumors (17). No activity was detected in nonautolyzed normal liver and kidney (10). The inhibition of protein synthesis in striated muscle (15) and cell-free protein synthesis (3) by toxohormone are of particular significance as direct causes of cachexia because its most prominent manifestation is wasting of muscle (2).
It was claimed that the toxohormone activities of a transplanted rat carcinoma and a rat hepatoma line come not from the tumors but from contaminating bacteria (18, 19). Tumors initiated by cells that had been freed of bacteria by passage with antibiotics in culture exhibited no toxohormone activity. These reports were countered by the finding that a chemically induced primary rat sarcoma demonstrably free of bacterial contamination markedly depressed liver catalase activity in vivo, although it was confirmed that bacterial toxin prepared by the toxohormone method did indeed depress liver catalase activity (20). Toxohormone was also isolated from bacteria-free fibrosarcomas that had been chemically induced and serially transplanted in germ-free mice (21). Subsequently, a fibrosarcoma grown in culture with antibiotic-containing medium secreted a low molecular weight peptide that depressed plasma-bound iron levels in mice and inhibited cell-free protein synthesis, which were considered properties of toxohormone (3). Data will be presented later that show that cytotoxin becomes demonstrable in the unfractionated, antibiotic-containing medium of cells shortly after they undergo viral-induced transformation in culture (22). The cytotoxin is also lethal to the transformed cells that produce it in heavily infected cultures, but not to the producers in discrete transformed foci surrounded by normal cells in lightly infected cultures. This finding suggests that the cytotoxic material is produced at the cell surface or beyond and must exceed a threshold level in the medium to produce its lethal effect.
A small cytotoxic peptide was isolated by gel filtration and column chromatography of a dialyzate from the interstitial fluid of 10 different types of human tumors as well as a transplantable rat sarcoma and a mouse ascites mammary carcinoma (23). Its cytotoxic effect was determined on established cultures of mouse fibroblasts (13). This peptide with a molecular mass originally estimated between 3,000 and 10,000 Da (13), but later briefly noted as 3,500 Da (23), was thought to differ from Nakahara's toxohormone for several reasons, including its failure to depress liver catalase in mice (24). Unlike the toxohormone consisting of several components (14, 15), only a single peak of activity by column chromatography was described for the cytotoxic peptide from the interstitial fluid of tumors. It is possible that this disparity in number of cytotoxic factors may result from different methods of purification, which selected different peptides from a broad size range of those available (16). Other work has shown that toxohormone isolated in a single peak by column chromatography could be resolved into four spots by paper chromatography (14). Each of the four fractions exhibited toxohormone activity by depressing liver catalase activity in mice. In another case, a single broad peak from Sephadex filtration of a tumor culture toxohormone had at least three antigenically distinct components (3), so the presence of a single gel filtration peak from the interstitial fluid of tumors does not, in itself, distinguish its cytotoxic activity (23) from toxohormone.
The foregoing isolations of toxohormone were made directly from tumors or their cell cultures, but do not explain the systemic effects that would be necessary to produce cachexia and its correlates. However, the discovery of an immunoregulatory protein in normal human sera (25, 26) led to the detection of small amounts of immunodepressive peptides in serum of normal individuals (26) and to much larger amounts in serum of ≈50% of cancer patients (27, 28). The peptides were noncovalently bound exclusively to ∝-globulins in normal individuals, but much larger amounts were bound to all classes of serum proteins in the cancer patients. The immunodepressive components were initially nondialyzable, as would be expected from their binding to proteins, but could be dissociated by low pH or high ionic strength and had molecular masses in the range from 4,000 to 7,000 Da (26, 27). There are obvious parallels to the induced dialyzability of toxohormones (4, 9) and the range of their molecular masses (16).
Although the serum peptides occurred in a single peak upon ultracentrifugation, at least 10 peptides with varied mobility were found on paper electrophoresis (26). There was detectable immunodepressive activity in sera of 66% of cancer patients who failed to exhibit delayed hypersensitivity in skin tests (27). Immunodepressive activity was found in unfractionated ascites fluid of human tumors that had metastasized to the peritoneum, whereas unfractionated effusions from noncancer patients or unfractionated sera from cancer patients were negative (29). It was assumed that the immunodepressive activity of the ascites fluid was caused by the same peptides that were isolated from the serum of cancer patients. The peptides depressed cell-mediated, but not antibody-mediated, immune responses and would therefore interfere with immune rejection of the cancer. It is noteworthy that the peptides are in the same size range as the immunodepressive peptides found in the ascites fluid of Ehrlich carcinoma cells growing in mice (16). The depression of the immune response could be related to the thymic involution observed within 48 h after injection of toxohormone in mice (8) and the decrease in its size that accompanies the progressive growth of transplanted tumors in rats (30, 31). Indeed, the thymus completely disappears in 40% of rats with progressively growing sarcomas (30).
Despite the extensive literature describing toxohormone-like activity in tumors, ascites fluid and serum of cancer patients that produced effects correlated with cachexia, and general agreement that the activity was produced by small peptides, discussion of their possible role in cachexia has declined to the point of extinction in recent years. This decline can be seen in successive editions of Cancer Medicine, a standard work in oncology. In the 1973 edition, there is a full page on toxohormone suppression of liver catalase. The coverage of all toxohormone-related activities dropped to half a page in 1982 and to six half-column lines in 1993 and 1997, with no mention whatever in 2000. The reasons given for the dwindling interest in toxohormone given in the 1997 edition were that liver catalase depression, which was universally used as the assay for toxohormone only in the early literature, is nonspecific, the structure of toxohormone had not been determined, and transport to the liver had not yet been proven (32). However, none of these reasons are decisive, nor is any note taken of the many confirmatory reports using assays more directly concerned with the muscle loss of cachexia than liver catalase reduction (3, 5, 15, 16). Interest in the role of heterogeneous, small peptides may be restored in the light of the recent widespread acceptance of a central role for proteases in the initiation and progression of tumors (33, 34). As will be discussed, the size distribution of peptides from proteolytic digestion of fibrin and fibronectin by transformed cells qualifies them for consideration as toxohormones and justifies further investigation of their role in cachexia.
Cytokines, ILs, and Other Proteins
As interest declined in tumor-derived small peptides as agents of cancer cachexia, there was a rise of interest in several classes of proteins such as cytokines and ILs for the same role (1, 2). The term cytokine was initially used to separate a group of immunomodulatory proteins from other growth factors. This distinction can no longer be maintained; some cytokines are produced by a limited number of cell types and others are produced by almost the entire spectrum of known cell types (Cytokine Online Pathfinder Encyclopedia, www.copewithcytokines.de/cope.cgi?002222). ILs are a large group of cytokines produced mainly by leukocytes and are involved mainly in directing other immune cells to divide and differentiate (Cytokine Online Pathfinder Encyclopedia, www.albany.net/∼tjc/interleukin.html). The first cytokine to be associated with cachexia was secreted by a macrophage cell line in response to endotoxin (35). This cytokine is a lipoprotein designated cachectin because it suppressed lipoprotein lipase in preadipocyte cultures, which is one of several biochemical alterations associated with cachexia. Cachectin is identical to tumor necrosis factor, which caused hemorrhagic necrosis of tumors in endotoxin-treated animals (36). Tumor necrosis factor is one of a number of proinflammatory cytokines, including IL-1, IL-6, IFN-γ, ciliary neurotropic factor, and proteolysis-inducing factor that cause cachexia-related effects upon inoculation into mice (2, 37). Some tumor lines produce cytokines in culture, but it is rare to detect circulating concentrations of cytokines in cancer patients, even those with cachectic loss of weight (38). Elevated circulating concentrations of IL-6 are associated with weight loss in some patients with lymphoma, lung cancer, and colorectal cancer. Production of proinflammatory cytokines also induces the production of corresponding antiinflammatory cytokines. It appears that a complex network of cytokines in combination with other factors would be required if they are to produce cachexia (2).
In addition to cytokines, the infusion of hormones such as cortisol, glucagon, and adenalin in humans will produce some features of cachexia (2). Ectopic production of hormones by tumors is not an uncommon finding in cancer (39, 40). The ectopic hormones cause symptoms related to the function of the hormones, but differ in that there is no feedback regulation of the ectopic production. As many as seven different hormones have been detected from a single tumor, and lesser numbers of ectopic hormones are occasionally found in some human tumors (39). Unlike the toxohormone and cytotoxic peptides, however, which are reportedly found in all advanced tumors (4), the catabolic cytokines and ectopic hormones occur sporadically, and many tumors have none.
Proteases Secreted by Tumors and Transformed Cells
One of the earliest and clearest demonstrations of protease release from tumors came from the lysis of plasma clots by Rous chicken sarcomas that were embedded in the clots (41). Experiments indicated that the tumor cells activated a proenzyme in the plasma to degrade the fibrin that provides the solid framework of the clot (42). A suggestion that proteases were involved in the transformation of chicken embryo fibroblasts (CEF) by Rous sarcoma virus (RSV) came from the observation that moderate concentrations of FBS, or two to three times higher concentrations of calf serum (CS) in an agar overlay medium, suppressed the development of discrete, multilayered transformed foci in low-dose infection by the Bryan strain of RSV (B-RSV) (43). The high serum concentrations did permit full replication of the virus and exponential proliferation of the infected cells, but they retained their normal appearance and were subject to contact inhibition at confluence. In contrast, even the highest concentrations of FBS tested failed to prevent transformation of heavily infected cultures. It is well known that bovine sera have a wide range of protease inhibitors (44). The implication was that protease inhibitors in the bovine sera effectively neutralized proteases released from the infected cells only when those cells constituted a small minority of the population; when they were a dominant element in the population, the serum inhibitors were ineffective. These suggestions that proteases played a prominent role in transformation prompted the addition of low doses of trypsin and certain other serine proteases to normal, confluent CEF cultures where they stimulated proliferation of the contact-inhibited cells without detaching them (45). Dialyzed conditioned medium obtained from cultures heavily transformed by RSV infection did the same (46). Thrombin was the only protease among them, however, that stimulated proliferation of contact-inhibited mammalian cells as well as the CEF (47). The same proteases also stimulated glucose transport in contact-inhibited CEF (48), thereby reproducing another typical characteristic of transformed cells.
Transformation of CEF by RSV (Schmidt–Ruppin strain) was accompanied by a very large increase in the secretion of plasminogen activator (PA), a serine protease that converted serum plasminogen into plasmin, which degraded fibrin and thereby lysed plasma clots (49, 50). An increase of PA was also associated with transformation of mammalian fibroblasts (51), and the resultant fibrinolytic activity was correlated with morphological transformation (52). Transformed cultures degraded a fibrin film to peptides of heterogeneous size (49). A large fraction of the resulting fragments was acid-insoluble, indicating that the enzyme(s) responsible had a restricted specificity for peptide sequence, size, conformation, or a combination of these.
There was fibrinolytic activity in cell-free medium conditioned on RSV-transformed cells if it contained chicken serum, but not if it contained FBS, which inhibited the PA in the medium (49). However, direct contact between the transformed cells in the presence of FBS and the fibrin film on which they rested did result in degradation of the film, indicating that the fibrinolytic PA was largely bound to the cell surface. RSV-transformed CEF also degrade fibronectin, the major glycoprotein on the surface of fibroblasts (53) and the major structural protein of extracellular matrix (54). The degradation of fibronectin in the presence of 10% FBS, like the digestion of fibrin, only occurs at points of contact with the cell surface (55). The released peptides from fibronectin, unlike those from fibrin, are not acid-precipitable, consistent with a molecular mass of <30,000 Da (55). Antibody to PA inhibited by 60–65% the degradation of extracellular matrix by plasminogen-free conditioned medium from the transformed cells, which indicated a direct catalytic role of PA in the process (56).
General interest in the role of proteases in transformation declined in the mid-1970s when a number of cases were found in which there was no correlation between PA and transformation. For example, there was no correlation between PA production by rat embryo fibroblasts transformed by polyoma virus and their capacity to produce colonies when suspended in agar (57). Although PA activity is high in most sarcomas, it is absent in most carcinomas (58, 59), the major form of cancer in humans. It is also present in some normal tissues and nontumorigenic cell lines (58). These and other observations were inconsistent with a dominant causal role for PA and fibrinolysis in neoplastic growth. However, there was a resurgence of interest in proteases in the 1980s, especially with regard to the role of matrix metalloproteinases in the neoplastic process (60), which prompted a search for their presence in RSV-transformed cells. RSV-transformed CEF were found to secrete high levels of a 70,000-Da metallogelatinase, which degrades denatured collagens but not native collagen (61). Two other matrix metalloproteinases were later isolated from RSV-transformed CEF, suggesting that proteases play diverse roles in neoplastic behavior (62). The activation of multiple matrix metalloproteinases associated with RSV transformation of CEF would, of course, add to the sum of small peptides released by the PA of these cells and highlights the possibility of their involvement in the toxicity of medium conditioned by these cells.
The Production of Toxic Substances by B-RSV-Transformed CEF
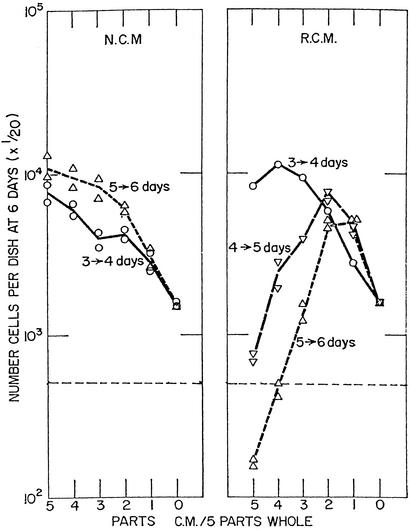
Very low population densities of primary and secondary cultures of CEF multiply poorly unless supported by normal conditioned medium (NCM) from higher densities of cells that support their own exponential proliferation (Fig. 1 Left). The growth-enhancing material in NCM consists of one or more large, heat-labile proteins, which also help to normalize the appearance and growth-regulatory behavior of RSV-transformed foci when accumulated for several days in an otherwise permissive medium (43). In sharp contrast to conditioned medium from normal CEF, medium conditioned by cultures fully transformed after B-RSV infection (RCM) was not only inhibitory, but toxic to the proliferation of CEF (Fig. 1 Right). Indeed, once the heavily infected cultures underwent widespread transformation, they themselves stopped multiplying, indicating they were also damaged by the toxic material they produced. With increasing dilution, however, the RCM exhibits growth-enhancing activity that was hidden by the toxic activities of higher concentrations of RCM. It is of prime interest that the first signs of transformation were usually seen in scattered cells at 3 days, in many more cells at 4 days, and most of the cells at 5 days when the toxic activity first became prominent (Fig. 1 Right). Therefore, the toxic activity of the medium was closely correlated with pervasive transformation of the culture.
Figure 1.
Progressive changes in the growth-enhancing or growth-inhibiting effects of media conditioned on normal CEF (NCM) and RSV-infected CEF (RCM). About 2 × 106 CEF freshly dispersed from 10-day-old embryos attached to the surface of 100-mm Petri dishes and initiated proliferation, dividing on average about once a day. Half of the cultures were infected with 106 focus-forming units of B-RSV in synthetic medium 199 with antibiotics plus 10% tryptose phosphate broth (TPB) and 2% CS, and the other half were kept as controls in the same medium. The medium was replaced daily from the third day forward with 199 plus 10% TPB, 4% CS, and 1% chicken serum. It was centrifuged to remove any floating cells and released virus. It was then mixed in stepwise decreasing ratios with fresh medium in a total of 5 ml as shown in the abscissa and used to support the growth of 104 CEF seeded in 60-mm dishes. The values on the ordinate are 1/20th the total number of cells per culture. The horizontal broken line indicates the total number of cells seeded, i.e., 104 cells. (Left) NCM. (Right) RCM. The daily intervals at which the medium was collected accompany the curves of cell counts at 6 days. The point furthest to the right represents the number of cells in fresh medium, and the points above and to its left represent enhancement by increasing parts of NCM or RCM in 5 ml total. Points below the broken horizontal line indicate toxicity of the RCM. [Modified with permission from ref. 22 (Copyright 1966, Elsevier).]
A very different picture was seen when the infected cells were only a small minority in an otherwise normal population of CEF in a transformation-permissive medium. Each of the infected cells multiplied to form a discrete focus of transformed cells surrounded by normal cells (43). The cells in the transformed foci continued to proliferate to form multilayered, tumor-like groups against a monolayered background of confluent, contact-inhibited CEF. As previously noted, a moderate concentration of FBS, or an especially high concentration of CS, causes the cells in transformed foci to take on the morphology and regulated growth of normal, noninfected CEF. It appears either that toxic factors released by a few scattered, infected cells amidst a multitude of uninfected cells do not raise toxicity of the medium to a threshold required to damage cells even locally or that conditioning factors produced by surrounding normal cells decrease extracellular proteolysis sufficiently to maintain toxic peptides below effective levels. Moderate concentrations of FBS or high concentrations of CS in the medium appear to inhibit the proteases even more strongly and thereby prevent the transformation. This prevention does not occur in heavily transformed cultures, presumably because the antiprotease conditioning factors and serum inhibitors are inadequate to effectively inhibit the high concentrations of proteases produced by overwhelming numbers of infected cells. It is noteworthy that Rous sarcomas growing in chickens, which form a tumorous mass of transformed cells, yield effective concentrations of toxohormone (63).
A >2-fold dilution of RCM with fresh medium removes the cytotoxic effects of the former and reveals its cell-derived, growth-enhancing activity (Fig. 1 Right). Because there is no reduction in growth-enhancing activity in dilutions of RCM when compared with the activity present in equal dilutions of NCM, protease digestion of serum proteins is likely to be the major source of toxic peptides in cell culture. It is of interest in this regard that albumin is the most common protein of plasma, and a marked reduction of its concentration regularly occurs in cancer patients (7), which suggests the reduction may result from digestion by tumor proteases.
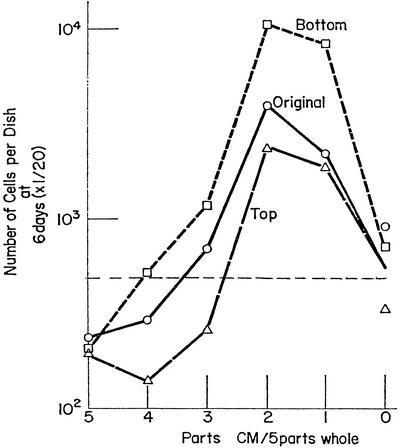
Centrifugation for 16 h at 50,000 × g increased the growth-enhancing activity in the bottom half of the centrifuge tube and decreased it in the top half (Fig. 2). At the same time the inhibitory activity of the RCM is increased in the top half, presumably by subtraction of the large, growth-enhancing molecules, reflecting the small size of the toxic material. Another indication of the small size of the toxic material is that dialysis of RCM removes its inhibitory activity (46). The growth inhibitory activity of RCM therefore exhibits the same low molecular weight characteristics of the peptides associated with toxohormone (3, 4, 14–16) and other cytotoxic material isolated from tumors (13, 23).
Figure 2.
The effect of high-speed centrifugation on the distribution of growth-enhancing and toxic effects of RCM. A highly inhibitory RCM was centrifuged at 50,000 × g for 30 min to remove most of the virus, and the supernatant was similarly centrifuged for 16 h. The bottom half was collected through a pinhole and the top half was removed by pipette. The supernatant of the brief centrifugation (original), and the bottom and top halves of the prolonged centrifugation were tested for their growth-supporting and toxic activities with 104 CEF as described in Fig. 1. The horizontal line indicates the number of cells seeded. The values on the ordinate are 1/20th the number of cells per culture. [Modified with permission from ref. 22 (Copyright 1966, Elsevier).]
The strong cytotoxic activity of RCM is readily detectable in a 2-fold dilution even though the ratio of cell volume to medium volume in a confluent CEF culture is considerably <1:1,000. Therefore, it is plausible that a 10-g tumor could produce systemic damage and cachexia in a 50-kg human during the long period of tumor development. The combination of protease activity in the interstitial fluid of a tumor and a local gradient of toxic peptides would also account for the severe local tissue damage along the path of invasion of malignant cells (11). One marked difference between heavily transformed cultures of B-RSV-transformed primary CEF and serially transplanted mammalian tumors is that the former are apparently damaged by the proteases and their cytotoxic products, whereas the latter are unaffected by the cytotoxic peptides that kill normal cells (13). The resistance of the transplanted tumors probably arises by cell selection during serial transplantations of the tumors, but a similar resistance could arise over the decades required for evolution of most human carcinomas.
Discussion
Despite the overriding importance of cachexia in cancer morbidity and mortality (1, 2), little coherent work has been done since the 1970s on a general causal agency of the condition once the idea of toxohormones and other small, toxic peptides produced by the tumors, fell into disfavor. Some of the slack has been taken up, mainly by tumor-derived cytokines and their subclass of ILs, that have catabolic effects, but these have so far not accounted for the wide prevalence of cachexia in cancer because they occur only sporadically in tumors. There had also been a decline of interest in the role of proteases in the genesis of cancer in the late 1970s, but there has been an upsurge of interest with the recognition of increasing numbers of matrix metalloproteinases with probable causal roles in tumor development (34, 60, 64, 65), and the recognition that 2–3% of the human genome codes for proteases (66) that might also be involved in carcinogenesis. Awareness of a crucial role for proteases in tumor development should focus attention on the peptides that result from their action and their possible significance in cachexia. One of the problems in the early work on toxohormone-like peptides is that they were sampled from mature tumors, and the studies were scattered among many different kinds of tumors. There was no opportunity to study the genesis of the peptides during all stages of neoplastic development from normal to malignant cell, so a strict correlation could not be drawn between the onset of the neoplastic phenotype and cytotoxic peptide production. An additional problem was that the diverse array of assays for toxicity used in different studies were not precisely quantifiable nor were they comparable with one another.
The transformation of CEF by B-RSV offers many of the features lacking in the analysis of the cytotoxicity of factors released by individual tumors. There is a ready and reproducible source of target cells from chicken embryos. Stocks of different RSV strains and characteristics are available, including temperature-sensitive mutants that can turn transformation on or off with a simple manipulation, and have been profitably applied to the study of protease effects in transformation (49). There are also a number of partial transformation mutants that have been used to characterize the relation of various characteristics to the morphology and growth behavior of the neoplastic phenotype (67). The entire population of CEF can be infected in a single step, and they transform within 3–5 days, in parallel with the appearance of cytotoxic material in the medium (22). The number of infected cells can be determined at any time by the infective center assay (68), and the transformed phenotype can be turned on or off in low multiplicity infection by changing the concentration of bovine serum (43). The assay of toxin production by growth inhibition of small numbers of CEF is extremely sensitive and gives quantitative results over a wide range (22). Neither fractionation nor concentration of the cytotoxic factors is required to produce their effect, so the full range of their sizes can be determined directly by Diaflo ultrafiltration, as it was in the fluid of ascites carcinoma cells (16).
Given the advantages of the RSV transformation system, it should be possible to elucidate some fundamental features of cytotoxin production and apply that knowledge to an understanding of cachexia. The use of inhibitors specific for each class of proteases, as was done for digestion of extracellular matrix (54), should help to identify the protease types and their protein substrates involved in toxin production. This would in turn help to distinguish between cytotoxic peptides from surface proteases involved in the process of transformation and those involved in autolysis. Application of modern techniques of proteomics (69) should help to identify both the proteolytic enzymes and the protein substrates that give rise to the cytotoxic peptides. The RSV transformation system could be used efficiently to find ways to inhibit toxin production, not only by protease inhibition but by antibodies to peptides and by other methods that suggest themselves as detailed information is generated. The system offers promise of new approaches to controlling cachexia, which could alleviate some of the most serious clinical effects of cancer.
Acknowledgments
Helpful comments on the manuscript were made by Prof. Morgan Harris. Special thanks go to Dorothy Rubin for editing the manuscript throughout its various drafts. Support for the present work came from National Institutes of Health Grant G13 LM07483-01.
Abbreviations
- RSV
Rous sarcoma virus
- B-RSV
Bryan strain of RSV
- CEF
chicken embryo fibroblasts
- CS
calf serum
- NCM
normal conditioned medium
- PA
plasminogen activator
- RCM
medium conditioned by CEF cultures fully transformed by RSV infection
References
- 1.Tisdale M J. J Natl Cancer Inst. 1997;89:1763–1773. doi: 10.1093/jnci/89.23.1763. [DOI] [PubMed] [Google Scholar]
- 2.Fearon K C H, Moses A G W. Int J Cardiol. 2002;85:73–81. doi: 10.1016/s0167-5273(02)00235-8. [DOI] [PubMed] [Google Scholar]
- 3.Blakeslee D, Raymond M J, Ward T, Bandy P. Cancer Lett. 1978;5:49–54. doi: 10.1016/s0304-3835(78)80010-x. [DOI] [PubMed] [Google Scholar]
- 4.Nakahara W, Fukuoka F. Adv Cancer Res. 1958;5:157–177. doi: 10.1016/s0065-230x(08)60411-x. [DOI] [PubMed] [Google Scholar]
- 5.Kampschmidt R F, Adams M E, McCoy T A. Cancer Res. 1959;19:236–239. [PubMed] [Google Scholar]
- 6.Nakahara W, Fukuoka F. Jpn Med J. 1948;1:271–277. [Google Scholar]
- 7.Greenstein J R. Biochemistry of Cancer. New York: Academic; 1954. [Google Scholar]
- 8.Fukuoka K, Nakahara W. Gann. 1952;43:55–62. [PubMed] [Google Scholar]
- 9.Greenfield R E, Meister A. J Natl Cancer Inst. 1951;11:997–1005. [PubMed] [Google Scholar]
- 10.Olivares J, Callao V, Montova E. Science. 1967;157:327–328. doi: 10.1126/science.157.3786.327. [DOI] [PubMed] [Google Scholar]
- 11.Sylvén B, Malmgren H. Acta Radiol, Suppl. 1957;154:1–124. [PubMed] [Google Scholar]
- 12.Caspersson T, Santesson L. Acta Radiol, Suppl. 1942;46:1–105. [Google Scholar]
- 13.Holmberg B. Nature. 1962;195:45–47. doi: 10.1038/195045a0. [DOI] [PubMed] [Google Scholar]
- 14.Yunoki K, Griffin A C. Cancer Res. 1961;21:537–544. [PubMed] [Google Scholar]
- 15.Goodlad G A J, Raymond M J. Eur J Cancer. 1973;9:139–146. doi: 10.1016/0014-2964(73)90083-2. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki H, Nitta K, Umezawa H. Gann. 1973;64:83–92. [PubMed] [Google Scholar]
- 17.Yunoki K, Griffin A C. Cancer Res. 1960;20:533–540. [PubMed] [Google Scholar]
- 18.Kampschmidt R F, Schultz G A. Cancer Res. 1963;23:751–755. [PubMed] [Google Scholar]
- 19.Kampschmidt R F, Upchurch H F. Cancer Res. 1963;23:756–761. [PubMed] [Google Scholar]
- 20.Matsuoka K, Hozumi M, Koyama K, Kawachi T, Nagao M, Sugimura T. Gann. 1964;55:411–421. [PubMed] [Google Scholar]
- 21.Nakahara W, Hozumi M, Pollard M. Proc Soc Exp Biol Med. 1966;123:124–125. doi: 10.3181/00379727-123-31419. [DOI] [PubMed] [Google Scholar]
- 22.Rubin H. Exp Cell Res. 1966;41:149–161. doi: 10.1016/0014-4827(66)90555-6. [DOI] [PubMed] [Google Scholar]
- 23.Sylvén B, Holmberg B. Eur J Cancer. 1965;1:199–202. doi: 10.1016/0014-2964(65)90049-6. [DOI] [PubMed] [Google Scholar]
- 24.Holmberg B. Z Krebsforsch. 1964;66:65–72. doi: 10.1007/BF00525562. [DOI] [PubMed] [Google Scholar]
- 25.Cooperband S R, Badger A M, Davis R C, Schmid K, Mannick J A. J Immunol. 1972;109:154–163. [PubMed] [Google Scholar]
- 26.Occhino J C, Glasgow A H, Cooperband S R, Mannick J A, Schmid K. J Immunol. 1973;110:685–694. [PubMed] [Google Scholar]
- 27.Glasgow A H, Nimberg R B, Menzoian J O, Saporoschetz I, Cooperband S R, Schmid K, Mannick J A. N Engl J Med. 1974;291:1263–1267. doi: 10.1056/NEJM197412122912401. [DOI] [PubMed] [Google Scholar]
- 28.Nimberg R B, Glasgow A H, Menzoian J O, Constantian M B, Cooperband S R, Mannick J A, Schmid K. Cancer Res. 1975;35:1489–1494. [PubMed] [Google Scholar]
- 29.Badger A M, Cooperband S R, Merluzzi V J, Glasgow A H. Cancer Res. 1977;37:1220–1226. [PubMed] [Google Scholar]
- 30.Murphy J B, Sturm E. Cancer Res. 1948;8:139–140. [PubMed] [Google Scholar]
- 31.Begg R W. Cancer Res. 1951;11:341–344. [PubMed] [Google Scholar]
- 32.Ohnuma T. In: Cancer Medicine. 4th Ed. Holland J F, Frei E, editors. New York: Williams and Wilkins; 1997. pp. 3091–3109. [Google Scholar]
- 33.McCawly L J, Matrisian L M. Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 34.Egeblad M, Werb Z. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 35.Beutler B, Mahoney J, LeTrang N, Pekala P, Cerami A. J Exp Med. 1985;161:984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beutler B, Cerami A. Nature. 1986;320:584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- 37.Tisdale M J. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 38.Socher S H, Martinez D, Craig J B, Kuhn J G, Oliff A. J Natl Cancer Inst. 1988;80:595–598. doi: 10.1093/jnci/80.8.595. [DOI] [PubMed] [Google Scholar]
- 39.O'Neal L W, Kipnis D M, Luse S A, Lacy P E, Jarett L. Cancer. 1968;21:1219–1231. doi: 10.1002/1097-0142(196806)21:6<1219::aid-cncr2820210625>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 40.Liddle G W, Ball J H. In: Cancer Medicine. Holland J F, Frei E, editors. Philadelphia: Lea & Febiger; 1973. pp. 1046–1057. [Google Scholar]
- 41.Fischer A. Arch Entwicklungsmech Org (Wilhelm Roux) 1925;104:210–261. [Google Scholar]
- 42.Fischer A. Biology of Tissue Cells. Cambridge, U.K.: Cambridge Univ. Press; 1946. [Google Scholar]
- 43.Rubin H. Virology. 1960;12:14–31. doi: 10.1016/0042-6822(60)90146-x. [DOI] [PubMed] [Google Scholar]
- 44.Astrup T, Alkjaersig N. Nature. 1952;169:314–316. doi: 10.1038/169314a0. [DOI] [PubMed] [Google Scholar]
- 45.Sefton B M, Rubin H. Nature. 1970;227:843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- 46.Rubin H. Science. 1970;167:1271–1272. doi: 10.1126/science.167.3922.1271. [DOI] [PubMed] [Google Scholar]
- 47.Carney D H, Glenn K C, Cunningham D D. J Cell Physiol. 1978;95:13–22. doi: 10.1002/jcp.1040950103. [DOI] [PubMed] [Google Scholar]
- 48.Sefton B M, Rubin H. Proc Natl Acad Sci USA. 1971;68:3154–3157. doi: 10.1073/pnas.68.12.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unkeless J C, Tobia A, Ossowski L, Quigley J P, Rifkin D B, Reich E. J Exp Med. 1973;137:85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quigley J P, Ossowski L, Reich E. J Biol Chem. 1974;249:4306–4311. [PubMed] [Google Scholar]
- 51.Ossowski L, Quigley J P, Kellerman G M, Reich E. J Exp Med. 1973;138:1056–1064. doi: 10.1084/jem.138.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ossowski L, Quigley J P, Reich E. J Biol Chem. 1974;249:4312–4320. [PubMed] [Google Scholar]
- 53.Yamada K M, Olden K. Nature. 1978;275:179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- 54.Fairbairn S, Gilbert R, Ojakian G, Schwimmer R, Quigley J P. J Cell Biol. 1985;101:1790–1798. doi: 10.1083/jcb.101.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W-T, Olden K, Bernard B A, Chu F-F. J Cell Biol. 1984;98:1546–1555. doi: 10.1083/jcb.98.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sullivan L M, Quigley J P. Cell. 1986;45:909–915. doi: 10.1016/0092-8674(86)90565-9. [DOI] [PubMed] [Google Scholar]
- 57.Perbal B. J Virol. 1980;35:420–427. doi: 10.1128/jvi.35.2.420-427.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearlstein E, Hynes R O, Franks L M, Hemmings V J. Cancer Res. 1976;36:1475–1479. [PubMed] [Google Scholar]
- 59.Varani J, Orr W, Ward P A. J Cell Sci. 1978;34:133–144. doi: 10.1242/jcs.34.1.133. [DOI] [PubMed] [Google Scholar]
- 60.Stetler-Stevenson W G, Aznavoorian S, Liotta L. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 61.Chen J-M, Aimes R T, Ward G R, Youngleib G L, Quigley J P. J Biol Chem. 1991;266:5113–5121. [PubMed] [Google Scholar]
- 62.Hamaguchi M, Yamagata S, Thant A A, Xiao H, Iwata H, Mazaki T, Hanafusa H. Oncogene. 1995;10:1037–1043. [PubMed] [Google Scholar]
- 63.Osawa K. Folia Pharmacol Japonica. 1954;50:38–42. [Google Scholar]
- 64.Coussens L M, Werb Z. Chem Biol. 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- 65.McCawly L J, Matrisian L M. Mol Med Today. 2000;6:149–156. doi: 10.1016/s1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- 66.Barrett A J, Rawlings N D, Woessner J F. Handbook of Proteolytic Enzymes. San Diego: Academic; 1998. [Google Scholar]
- 67.Kahn P, Nakamura K, Shin S, Smith R E, Weber M J. J Virol. 1982;42:602–611. doi: 10.1128/jvi.42.2.602-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubin H. Virology. 1960;10:29–49. doi: 10.1016/0042-6822(60)90004-0. [DOI] [PubMed] [Google Scholar]
- 69.Miklos G L G, Maleszka R. Proteomics. 2001;1:30–41. doi: 10.1002/1615-9861(200101)1:1<30::AID-PROT30>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]