Abstract
Modulation of the cytotoxicity and mutagenicity of 4-hydroxyestradiol (4-OHE2), an oxidative metabolite of estrogen, by antioxidants was assessed in human MCF7 cells and TK-6 lymphoblast cells. The cytotoxicity of the catecholic estrogens was potentiated by depletion of intracellular glutathione and was independent of oxygen concentration. Agents such as the nitroxide Tempol can facilitate the oxidation of the semiquinone to the Q and enhanced 4-OHE2 cytotoxicity. Conversely, reducing agents such as ascorbate, cysteine, and 1,4-dihydroxytetramethylpiperidine (THP) protected against cytotoxicity and decreased mutation induction, presumably by reducing the semiquinone to the hydroquinone. Our results support the proposition that oxidation of the semiquinone to the corresponding Q is crucial in eliciting the deleterious effects of catecholic estrogens. Furthermore, because the deleterious effects of 4-OHE2 were abrogated by dietary and synthetic antioxidants, our results would support the chemopreventive use of diets rich in reducing substances (vitamins and added synthetic antioxidants) as a means of decreasing the risks associated with estrogen exposure and developing of breast cancer.
Estrogen exerts hormonal effects by binding to the estrogen receptor and subsequently regulates the synthesis of proteins with important cellular functions (1). As a result of the oxidative metabolism of estrogens, undesired products can be formed and have altered hormonal activity and can chemically react with and damage DNA (2–5). Sequential accumulation of genetic alterations resulting from damage to DNA can result in the activation of oncogenes and/or inactivation of tumor suppressor genes (6). Recent reports suggest that endogenous estrogenic hormones or their metabolites can promote breast cancer carcinogenesis (2, 4, 7, 8). Initially, such hypotheses were based on the fact that high serum levels and long exposure (early menarche and late menopause) to estrogens were associated with higher incidence of breast cancer (9, 10). Additionally, prolonged exposure to mitogenic stimuli by natural or synthetic estrogens has long been considered an important factor in estrogen-induced carcinogenesis in experimental animals (11, 12). Such an association prompted evaluation of mechanisms underlying the estrogen-mediated carcinogenic activity.
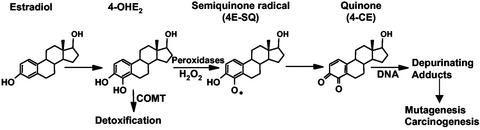
Estrogens are metabolized by conjugative and oxidative reactions. The predominant oxidative metabolites result from hydroxylation at the 2, 4, 6, 7, 15α, and 16α positions by the cytochrome P450 class of enzymes (13). Catecholic estrogens (CEs) are produced by hydroxylation at the 2 and 4 positions. Hydroxylation at the 2 position occurs predominantly in hepatic tissue (13). However, it was suggested that 4-hydroxylation was a dominant pathway for CE formation in several extra-hepatic target tissues by CYP 1B1 enzyme (14). Both CEs are detoxified by several enzymatic processes such as O-methylation, sulfation, glucoronidation, and methylation. Catechol-O-methyl transferase has been identified as a major inhibiting factor in estrogen-induced carcinogenesis (ref. 13; see Fig. 1).
Figure 1.
Partial scheme of oxidative conversion of estrogens to mutagenic agents.
CEs can be oxidized by sequential 1-electron oxidations to produce the estrogen semiquinone (SQ) radical and estrogen quinone (Q). The SQ radical has been suggested to undergo redox cycling to produce reactive oxygen species, which are capable of causing DNA damage (15, 16). The Q, on the other hand, is significantly electrophilic to react with DNA to form covalent adducts or even cause depurination. For example, the Q of 2-hydroxyestradiol (2-OHE2) forms stable DNA adducts, whereas the Q of 4-hydroxyestradiol (4-OHE2) reacts at the N-3 and N-7 positions of guanine and causes ultimate depurination (ref. 2; see Fig. 1). Such modifications in critical genes can induce mutations that lead to carcinogenesis. Tumor induction in the kidneys of Syrian hamsters treated with CEs was assessed to estimate the carcinogenic potential of the oxidative metabolites of estrogen. Although 2-OHE2 was ineffective in tumorigenesis, treatment with 4-OHE2 resulted in kidney tumors (17). These studies suggest that the Q of 4-OHE2 is a potent carcinogen. Most recently, it was suggested that an elevated ratio of 4-OHE2/2-OHE2 is a useful marker/predictor of mammary tumor development (9).
Several facts associated with the relative expression of the “activating” enzymes, such as the CYP 1B1 or the deactivating enzymes such as the catechol O-methyltransferase (COMT), may determine tumorigenesis from estrogen. For example, CYP 1B1 localizes with aromatases in target tissue such as breast, making the effective concentration of estrogen much higher than the systemic concentration (18). On the other hand, higher risk for breast cancer was found in women with low activities of COMT (19). Taken together, these data support the hypothesis that oxidative metabolism of estrogen in specific tissues leads to the formation of CEs that can generate reactive oxygen species in route to formation of DNA damaging electrophilic species such as the Qs.
While enzymes such as COMT and GST can detoxify CEs and estrogen Qs, respectively, the estrogen SQ may be a molecular target for intervention with chemopreventive agents. In the present study, the effect of chemopreventive agents on the cytotoxicity and mutagenicity of CEs was assessed by clonogenic assays as well as mutational frequency estimates. Supporting experiments with electron paramagnetic resonance (EPR) spectroscopy to directly detect the SQ radicals were performed. The results support the hypothesis that oxidation of the CE to the SQ is a critical step in formation of the Q, and the SQ radical can be targeted by agents that can be readily added to nutritional supplementation.
Materials and Methods
Chemicals.
Horseradish peroxidase (HRP), hypoxanthine, 4-OHE2, 4-OH-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol), zinc acetate, trifluorothymidine, cysteine, sodium ascorbate, sulfosalicyclic acid, diethylenetriaminopentaacetic acid (DTPA), and 5,5-dimethyl-1-pyrroline N-oxide (DMPO) were purchased from Sigma. Xanthine oxidase and CuZn superoxide dismutase (CuZn-SOD) were obtained from Roche Molecular Biochemicals. l-buthionine sulfoximine (BSO) was purchased from Schweizerhall (South Plainfield, NJ). 1,4-Dihydroxytetramethylpiperidine (TPH) was purchased from Molecular Probes. DMPO was purified as described (20). Subsequently, DMPO concentration was determined spectroscopically. Hydrogen peroxide (H2O2) was obtained from Fisher Scientific and standardized by using an ɛ240 of 43.6 M−1⋅cm−1. Deionized water from a Millipore (MILLI-Q water system) apparatus was used for all experiments.
EPR.
For EPR measurements, samples were drawn into a gas-permeable Teflon capillary tube (Zeus Industries, Orangeburg, SC) with a 0.81-mm inner diameter, a 0.38-mm wall thickness, and a 15-cm length. Each capillary was folded twice, inserted into a narrow quartz tube open on both sides (2.9-mm inner diameter), and placed in the cavity of a Varian E-109 X-band spectrometer. EPR parameters were 3368 G Field set, 9.39 GHz microwave frequency, 1.25 G modulation amplitude, 100 kHz modulation frequency, and 10 mW microwave power. The formation of Tempol from TPH (5 mM) during HRP-catalyzed oxidation of 4-OHE2 was initiated by 6 mM H2O2 in the presence of DTPA (0.1 mM) in sodium phosphate buffer (PB; 90 mM, pH 7.4) and monitored by EPR. To detect superoxide radicals, DMPO (100 mM) was used in the reaction mixture containing hypoxanthine (1 mM) and xanthine oxidase (0.1 units/ml) in PBS (50 mM, pH 7.4) in the presence of catalase (200 units/ml) and DTPA (0.1 mM). SQ radical of 4-OHE2 was generated and stabilized for detection by exposure to HRP (10 units/ml) in the presence of H2O2 (1 mM)/Zn-acetate (220 mM) in acetate buffer (50 mM, pH 5).
Cell Culture.
Human MCF7 cells were cultured in RPMI medium supplemented with 10% FCS and antibiotics (penicillin G potassium and streptomycin sulfate; 0.14 g/liter and 0.2 g/liter, respectively). Cell survival was assessed by a clonogenic assay (plating efficiency ranged between 80% and 90%). Stock cultures of exponentially growing cells were trypsinized, rinsed, and plated (5 × 105 cells per dish) into a number of 100-mm Petri dishes and incubated 16 h at 37°C before experimental protocols. Cells were exposed to various concentrations of 4-OHE2 for 2 h in the absence or presence of Tempol (1–5 mM), TPH (0.1–5 mM), ascorbate (1 mM), or cysteine (1 mM). Immediately after treatment, cells were washed twice with PBS (pH 7.4), trypsinized, counted, and plated into triplicate dishes. Each experiment was repeated a minimum of two times. Plates were incubated 7 days, and the colonies were fixed with methanol/acetic acid (3:1), stained with crystal violet, and counted. Only colonies containing >50 cells were scored. Error bars represent SD. For studies involving glutathione (GSH) depletion, cells were pretreated with 5 mM BSO for 22 h and then exposed to varying concentrations of 4-OHE2 for 2 h. After treatment cell survival was assessed as described previously. BSO treatment alone was not cytotoxic. For studies involving exposure of cells to 4-OHE2 under hypoxic conditions, cells were dispersed in 1.8 ml of medium, plated (2.5 × 105) into specially designed glass flasks, and incubated at 37°C overnight (21). 4-OHE2 (final concentration, 300 μM) was added to the cell monolayer immediately before the gassing procedure. For hypoxic conditions, cells were gassed with a humidified gas mixture of 95% nitrogen and 5% CO2 (Matheson Gas Products, Baltimore) at 37°C for 2 h. Flasks fitted with stoppers were connected in series and mounted on reciprocating platform and gassed. The gassing procedure yielded an effluent gas-phase oxygen concentration of <10 ppm as measured by a Thermox (Amtek, Pittsburgh) probe (21). Cell survival was assessed after the 2 h exposure as described previously. All statistical tests were done with the Student's t test with unequal variances.
Determination of 4-OHE2 Mutagenicity.
TK-6 cells (human B lymphoblast; TK, thymidine kinase) were grown in RPMI medium 1640 supplemented with 10% horse serum and antibiotics. Details for assessing survival and mutagenicity at the TK locus have been described (22, 23). TK-6 cells were treated with 0, 100, 120, and 140 μM of 4-OHE2 for 2 h in the absence or presence of 1 mM cysteine, 5 mM TPH, or 1 mM ascorbate. After treatment, cultures were grown in nonselective medium for 3 days to allow phenotypic expression before plating for determination of mutant fraction. Cells then were plated in microtiter plates in the presence of trifluorothymidine (TFT; 2 μg/ml). Cells from each culture also were plated at 1 cell per well in the absence of TFT to determine plating efficiency. All plates were incubated for 11 days before scoring colonies. Mutation plates were refed with fresh TFT medium and incubated for an additional 7 days to observe any late-appearing mutants.
GSH Determination.
For GSH measurements, cells were treated with 4-OHE2 (300 μM) in the presence or absence of Tempol (5 mM), TPH (5 mM), cysteine (1 mM), or ascorbate (1 mM) for 2 h. After treatment, the cells were rinsed, trypsinized, counted, and 106 cells (in cold PBS) were placed in duplicate tubes and centrifuged. After centrifugation (150 × g), the supernatant was removed, and 1 ml of cold 0.6% sulfosalicyclic acid was added to the cell pellet and the samples were stored at −20°C. GSH was measured by using the cyclic reductase assay (24), and protein was measured by using the Bradford method (25). GSH levels are expressed as the percentage of control values. The absolute GSH level for control MCF7 cells was 22.9 ± 2.4 μg of GSH per mg of protein.
Results
Cytotoxicity of 4-OHE2.
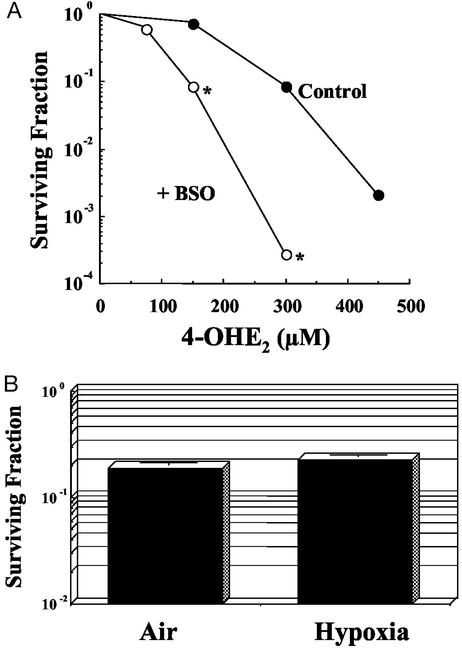
Based on the scheme for estradiol metabolism (Fig. 1), the effects of exposure to 4-OHE2 in MCF7 cells were evaluated to assess the role that SQ and Q play in the cytotoxicity. Potential cytotoxic candidates would include the 4E-SQ radical, superoxide, and downstream reactive oxygen species generated from redox cycling of 4E-SQ and/or 4-OHE2. Fig. 2A shows that 4-OHE2 reduced cell survival (filled circles) in a concentration-dependent manner. Because cellular defenses against oxidative damage rely, in part, on endogenous thiols, reduction in GSH should enhance oxidative stress-induced cytotoxicity. In addition to GSH detoxifying reactive oxygen species, we also assumed, based on Fig. 1, that 4-OHE2 cytotoxicity might be enhanced if GSH levels were lowered to the point that direct reduction by GSH of the SQ radical of 4-OHE2 to the hydroquinone would be ineffective. Lowered GSH levels may also impede removal of the Q by the conjugation reaction catalyzed by GST. Pretreatment of cells with BSO, which inhibits GSH biosynthesis (26), resulted in a GSH depletion to 11% of control values at the time of 4-OHE2 treatment and clearly increased 4-OHE2 cytotoxicity (Fig. 2A, open circles), which suggests that intracellular thiol concentration is a critical determinant of 4-OHE2-induced cytotoxicity. The result also raises the possibility of CE-induced free radical production. To determine whether superoxide generated by the oxidation of 4E-SQ is responsible for the cytotoxicity of 4-OHE2, cells were exposed to 4-OHE2 under aerobic or hypoxic conditions and the cytotoxicity was assessed. Fig. 2B illustrates that 4-OHE2 cytotoxicity for both aerobic and hypoxic treatments was not significantly different. Hence, 4-OHE2 cytotoxicity may not solely depend on oxygen-derived radicals but could also be mediated by other free radical species and/or the Q.
Figure 2.
(A) Survival of MCF7 cells exposed to various concentrations of 4-OHE2 for 2 h without or with pretreatment with BSO. GSH levels after BSO pretreatment were 11% of control values. *, P < 0.05. (B) Survival of MCF7 cells exposed to 300 μM 4-OHE2 for 2 h under either aerobic or hypoxic conditions (aerobic vs. hypoxic, not significant).
EPR Studies.
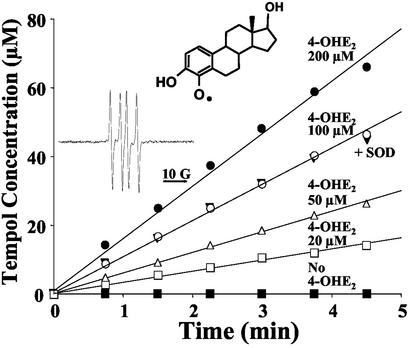
To determine the types of radicals formed from 4-OHE2 metabolism, chemical/EPR studies were conducted. Hydroxylated aromatic compounds such as the CEs are substrates of peroxidative enzymes such as HRP and produce the corresponding SQ radical (27). Inherently unstable SQ radicals of catecholamines can be detected by spin stabilization methods by using Zn2+ in an acidic environment (28). Therefore, EPR experiments were performed in the presence of Zn2+ to detect the estrogenic SQ radical. The EPR spectrum obtained from such experiments (Fig. 3 Inset) can be attributed to the SQ radical of 4-OHE2 (28). The half-life of the SQ radical stabilized by Zn2+ was determined by following the EPR signal intensity of the free radical as a function of time after removal of excess H2O2 by addition of catalase to the reaction mixture and was found to be >70 min (data not shown).
Figure 3.
Oxidation of TPH in the reaction of 4-OHE2 + HRP (10 units/ml) + H2O2 (6 mM) in air-saturated PBS as a function of time. (Inset) EPR spectrum of the SQ radical of 4-OHE2 obtained by the reaction with HRP (10 units/ml), H2O2 (1 mM), and Zn2+ (220 mM) in air-saturated acetate buffer (50 mM, pH 5).
SQ radicals under aerobic conditions can either undergo oxidation to form the corresponding Q with concomitant generation of superoxide radical or be oxidized/reduced by suitable oxidizing/reducing agents. The ability of the SQ radical of 4-OHE2 to oxidize substrates was tested by monitoring the conversion of the hydroxylamine TPH to the EPR-observable, stable nitroxide Tempol (29). Fig. 3 shows Tempol accumulation as a function of time in reactions containing 4-OHE2 exposed to HRP (10 units/ml) + H2O2 (6 mM) in the presence of TPH (5 mM). Although the oxidation did not proceed in the absence of 4-OHE2, the oxidation was linear with time in the presence of 4-OHE2 and depended on [4-OHE2]. Hence, the SQ radical of 4-OHE2 is responsible for the oxidation of TPH. Because superoxide radicals can also oxidize TPH (30) and SQ radicals can, under aerobic conditions, generate superoxide radicals, the role of superoxide in this reaction was assessed. When CuZn superoxide dismutase (CuZn-SOD) was included in the reaction mixture with 4-OHE2 [100 μM] and the oxidation rate of TPH monitored, no difference in the rate of oxidation was noted (Fig. 3, filled triangle) when compared with the reaction in the absence of SOD (Fig. 3, open circles). We concluded that the oxidation of TPH is predominantly mediated by the SQ of 4-OHE2 rather than superoxide. The lack of superoxide formation as monitored by this assay is in agreement with the reports that o-SQs, unlike the p-SQs, do not reduce molecular oxygen (31) but participate in disproportionation reactions to produce the corresponding hydroquinone and the Q (32).
To test whether the SQ radical of 4-OHE2 reduces molecular oxygen and generates superoxide, independent EPR spin-trapping experiments were carried out with incubation mixtures of the Q form of 4-OHE2 (100 μM) with cytochrome P450 oxidoreductase in the presence of NADPH (1 mM) and DMPO (100 mM) in air-saturated phosphate buffer (50 mM, pH 7.4). Even after prolonged incubation, in contrast with the p-SQs (33), no detectable levels of the superoxide adduct of DMPO were observed, although the SQ radical was detected in similar incubations (34).
Modulation of 4-OHE2 Cytotoxicity.
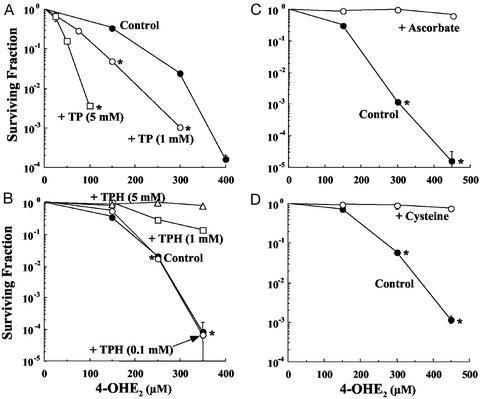
Because the proposed mechanism of mutagenicity and toxicity of estradiols involves the formation of Q formed from the SQ intermediate, any species that reduces the SQ back to the catechol should elicit protective effects. Conversely, any agent that facilitates the conversion of the SQ to the Q should exert sensitizing effects. Cell survival studies were carried out in MCF7 cells exposed to 4-OHE2 for 2 h in the absence and presence of the nitroxide Tempol and the hydroxylamine TPH as shown in Fig. 4 A and B. Coincubation of cells with 4-OHE2 with Tempol resulted in enhanced cytotoxicity (Fig. 4A), whereas TPH at concentrations of 1.0 and 5 mM provided near complete protection against 4-OHE2 cytotoxicity (Fig. 4B). In the EPR studies presented previously, TPH functioned as a reducing agent that inhibited the conversion of the SQ to the Q. This reductive reaction most likely underlies the protective effects of TPH against 4-OHE2-induced cytotoxicity. Conversely, Tempol functioning as a cooxidant can facilitate the formation of the Q from the SQ and enhance cytotoxicity. The results demonstrate that reducing agents provide cellular protection against 4-OHE2-induced cytotoxicity.
Figure 4.
Survival of MCF7 cells exposed to various concentrations of 4-OHE2 for 2 h in the absence or presence of (A) Tempol (1 and 5 mM), (B) TPH (0.1, 1.0 and 5.0 mM), (C) ascorbate (1 mM), and (D) cysteine (1 mM). *, P < 0.05.
To test the protective effects of other reducing agents against 4-OHE2-induced cytotoxicity, ascorbate (vitamin C) and cysteine (thiol) were evaluated as potential protective agents. From the cell survival data obtained after exposing cells to several concentrations of 4-OHE2 in the absence and presence of the reducing compounds, it can be seen that cysteine and ascorbate at a concentration of 1 mM completely protected against 4-OHE2 exposure (Fig. 4 C and D). The putative mechanism of protection of these agents would be reduction of the SQ radical and Q back to the catecholic state in the case of cysteine (2) and the SQ to the catecholic state with ascorbate (28).
GSH Depletion by 4-OHE2.
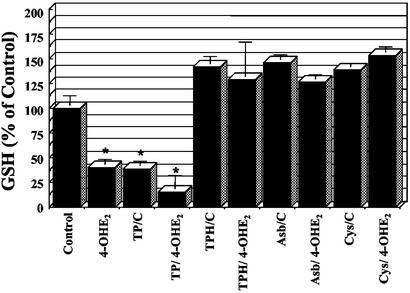
Cells challenged by oxidative stress often have decreased levels of GSH. Because GSH depletion by BSO pretreatment of cells was found to sensitize cells to 4-OHE2 exposure (Fig. 2), studies were conducted to determine whether 4-OHE2 treatment alone would decrease GSH levels. Fig. 5 shows that 4-OHE2 treatment resulted in a 60% decrease in GSH levels. The influence of Tempol, TPH, ascorbate, and cysteine on GSH levels in cells in the absence or presence of 4-OHE2 was also examined (Fig. 5). Exposure of cells to Tempol alone caused a similar reduction to that observed by 4-OHE2; however, GSH levels were decreased a further 85% by the combination of Tempol and 4-OHE2 treatment. On the other hand, cells incubated with TPH, ascorbate, or cysteine did not show a decrease in GSH levels in the absence or presence of 4-OHE2. The observed pattern of changes in GSH levels was similar to the trends in the cytotoxicity observed with 4-OHE2 in the absence and presence of the various agents tested. The sensitizing effect of Tempol in cells incubated with 4-OHE2 is consistent with the reduction in GSH of cells treated with both 4-OHE2 and Tempol. Likewise, the protective effect of TPH, ascorbate, and cysteine is consistent with the higher intracellular thiol content.
Figure 5.
GSH levels of MCF7 cells after exposure to 300 μM 4-OHE2 for 2 h in absence or presence of Tempol (5 mM), TPH (5 mM), ascorbate (1 mM), and cysteine (1 mM). *, P < 0.05.
4-OHE2-Mediated Mutagenicity.
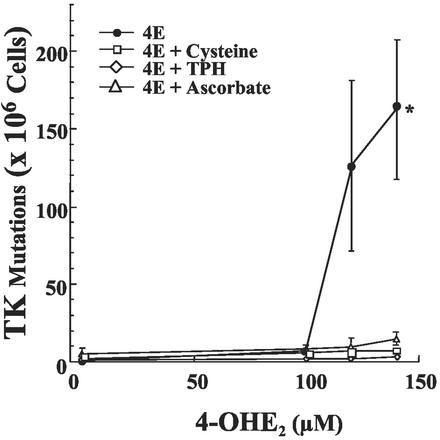
To test whether the compounds that exerted protective effects in MCF7 cells exposed to 4-OHE2 would also retain the protective effects against mutation induction, experiments were carried out with human lymphoblast TK-6 cells. These cells have been used to sensitively monitor mutation induction by a variety of putative mutagens at the autosomal TK locus (22, 23). Fig. 6 shows the mutation induction in TK-6 cells exposed to different concentrations of 4-OHE2 in the absence and presence of TPH, ascorbate, and cysteine. 4-OHE2 treatment enhanced TK mutation induction in a concentration-dependent manner. However, in the presence of TPH, ascorbate, or cysteine, mutation induction at the TK locus was essentially unaffected by the presence of 4-OHE2. The mutation induction in TK-6 cells by the presence of 4-OHE2 ± the reducing agents also paralleled the pattern observed in cell survival (data not shown).
Figure 6.
4-OHE2-induced mutations at the TK locus in TK-6 cells exposed to various concentrations of 4-OHE2 for 2 h in the absence or presence of TPH (5 mM), ascorbate (1 mM), or cysteine (1 mM). Each data point is the mean of two independent experiments with two replicates in each experiment. Bars represent SE; *, P < 0.05.
Discussion
Although it is well established that the Q analog of estrogen is involved in the mutagenic/carcinogenic effects of estrogen metabolism, the initiating events or species responsible for mutagenic/carcinogenic pathway are not well defined. We hypothesized that exposing cells to 4-OHE2 would result in cytotoxicity as a consequence of the formation of the SQ intermediate. Indeed, 4-OHE2 exerted cytotoxicity to MCF7 cells, and this cytotoxicity could be enhanced if GSH was lowered before 4-OHE2 treatment (Fig. 2A). Although intracellular GSH is necessary for Q detoxification by GST (2), it is also plausible that GSH protects as a result of reducing the SQ or the Q back to the catechol state (28).
Although our results are consistent with free radical-mediated cell killing, they do not define which radical(s) is responsible for the cytotoxicity. We questioned whether 4-OHE2 toxicity depended on oxygen because it is well known that most p-Qs undergo redox activation to the corresponding p-SQ. The p-SQ radical is reducing in nature and in aerobic conditions participates in futile redox cycling reactions to generate superoxide, hydrogen peroxide, and hydroxyl radicals and inflict cellular damage (33). o-SQs are generated either by a 1-electron reduction of o-Qs by enzymes such as cytochrome P450 reductase or by oxidation of the corresponding hydroquinone by peroxidases. However, unlike the p-SQ, o-SQs are oxidizing in nature and therefore do not reduce oxygen to superoxide (31). At pH <8.5, disproportionation of SQs to produce the corresponding hydroquinone and Q in an oxygen-independent manner is favored (32). Our EPR observations are consistent with these reports and point to the lack of superoxide generation by the SQ of 4-OHE2. No influence of oxygen on the 4-OHE2 cytotoxicity was noted (Fig. 2B) as cell survival was equivalent under both aerobic and hypoxic conditions. Because the Q of 4-OHE2 is the putative species that induces apurinic sites in DNA, which ultimately leads to mutagenicity and cytotoxicity (2), oxygen-independent SQ disproportionation reactions may be responsible for the observed adverse effects of estradiol exposure.
Tempol and TPH can modulate the conversion of the SQ in opposing directions and as such constitute a useful tool to study intracellular redox reactions (29, 35). The observed sensitization of the 4-OHE2 by Tempol and protection with TPH and other reducing agents (Fig. 4 A and B) is consistent with our hypothesis that conversion of the estrogen hydroquinone to the Q results from a series of 1-electron oxidations, and oxidation of SQ to the Q state either by disproportionation or direct 1-electron oxidation is a necessary step for the observed cytotoxicity. Therefore, the reason why Tempol enhances 4-OH CE-induced cytotoxicity is that Tempol supports the conversion of the SQ radical to the Q species. On the other hand, reducing agents decrease Q formation as well as convert the Qs back to hydroquinone by either a 1- or 2-electron process and hence protect against 4-OH CE-induced cytotoxicity. Protection by ascorbate and cysteine support the notion that reduction of the estrogen-derived SQ concentration and subsequent Q formation are important chemopreventive means to lessen oxidative activation of the CE to toxic/mutagenic Q metabolites. Consistent with these observations are the changes in the thiol status of cells treated with 4-OHE2 in the absence and presence of various protective agents and sensitizing agents. Although the thiol levels were maintained at control levels in the presence of all the protective agents, a significant reduction in the thiol levels was noted in the presence of Tempol and the decrease in intracellular thiols went along with the observed 4-OHE2 cytotoxicity.
In summary, our results show that the cytotoxicity and mutagenicity exerted by 4-OHE2 is prevented by supplementation with commonly used antioxidants. The mechanism by which these antioxidants operate is likely by reducing the SQ back to the catecholic state and inhibiting the formation of the Q species. Thus, the SQ radical of the CE represents a molecular target susceptible to detoxification by dietary antioxidants such as ascorbate and might represent a defense against estrogen-mediated carcinogenesis. It is interesting to note that a study following dietary intake and breast cancer risk among 83,234 women who participated in the Nurse's Health Study (NHS) concluded that consumption of fruits and vegetables high in specific carotenoids and vitamins may reduce premenopausal breast cancer risk (36). This study also showed a substantial reduction in breast cancer risk among premenopausal women who had a positive family history of breast cancer with dietary intake of carotenes, lutein/zeaxanthin, total vitamin A, and total vitamin C from foods. Although considerable controversy exists in the literature linking breast cancer risk with dietary intake (37), the mechanistic implications of the present study provide a plausible explanation for the positive association of dietary supplements and decreased incidence of breast cancer found in the NHS and certainly lend credence for further research into the use of antioxidants as chemopreventive agents in breast cancer.
Acknowledgments
We thank Dr. John Cook for statistical analysis.
Abbreviations
- CE
catecholic estrogen
- SQ
semiquinone
- Q
quinone
- 4-OHE2
4-hydroxyestradiol
- HRP
horseradish peroxidase
- Tempol
4-OH-2,2,6,6-tetramethylpiperidine-1-oxyl
- TPH
1,4-dihydroxytetramethylpiperidine
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- BSO
l-buthionine sulfoximine
- GSH
glutathione
- TK
thymidine kinase
- EPR
electron paramagnetic resonance
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.DeMayo F J, Zhao B, Takamoto N, Tsai S Y. Ann NY Acad Sci. 2002;955:48–59. doi: 10.1111/j.1749-6632.2002.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 2.Cavalieri E L, Stack D E, Devanesan P D, Todorovic R, Dwivedy I, Higginbotham S, Johansson S L, Patil K D, Gross M L, Gooden J K, et al. Proc Natl Acad Sci USA. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsutsui T, Barrett J C. Environ Health Perspect. 1997;105, Suppl. 3:619–624. doi: 10.1289/ehp.97105s3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsutsui T, Tamura Y, Suzuki A, Hirose Y, Kobayashi M, Nishimura H, Metzler M, Barrett J C. Int J Cancer. 2000;86:151–154. doi: 10.1002/(sici)1097-0215(20000415)86:2<151::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Cavalieri E L, Rogan E G, Chakravarti D. Cell Mol Life Sci. 2002;59:665–681. doi: 10.1007/s00018-002-8456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberg R A. Sci Am. 1996;275:62–70. doi: 10.1038/scientificamerican0996-62. [DOI] [PubMed] [Google Scholar]
- 7.Fishman J, Osborne M P, Telang N T. Ann NY Acad Sci. 1995;768:91–100. doi: 10.1111/j.1749-6632.1995.tb12113.x. [DOI] [PubMed] [Google Scholar]
- 8.Liehr J G. Eur J Cancer Prev. 1997;6:3–10. doi: 10.1097/00008469-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Liehr J G, Ricci M J. Proc Natl Acad Sci USA. 1996;93:3294–3296. doi: 10.1073/pnas.93.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Key T J, Pike M C. Eur J Cancer Clin Oncol. 1988;24:29–43. doi: 10.1016/0277-5379(88)90173-3. [DOI] [PubMed] [Google Scholar]
- 11.Newbold R R, Liehr J G. Cancer Res. 2000;60:235–237. [PubMed] [Google Scholar]
- 12.Weisz J, Fritz-Wolz G, Clawson G A, Benedict C M, Abendroth C, Creveling C R. Carcinogenesis. 1998;19:1307–1312. doi: 10.1093/carcin/19.7.1307. [DOI] [PubMed] [Google Scholar]
- 13.Zhu B T, Conney A H. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Spink D C, Hayes C L, Young N R, Christou M, Sutter T R, Jefcoate C R, Gierthy J F. J Steroid Biochem Mol Biol. 1994;51:251–258. doi: 10.1016/0960-0760(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 15.Malins D C, Polissar N L, Gunselman S J. Proc Natl Acad Sci USA. 1996;93:2557–2563. doi: 10.1073/pnas.93.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halliwell B, Gutteridge J M C. Free Radicals in Biology and Medicine. Oxford: Oxford Univ. Press; 1999. pp. 246–350. [Google Scholar]
- 17.Han X, Liehr J G. Cancer Res. 1994;54:5515–5517. [PubMed] [Google Scholar]
- 18.Jefcoate C R, Liehr J G, Santen R J, Sutter T R, Yager J D, Yue W, Santner S J, Tekmal R, Demers L, Pauley R, et al. J Natl Cancer Inst Monogr. 2000;27:95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 19.Yager J D. J Natl Cancer Inst Monogr. 2000;27:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 20.Samuni A M, DeGraff W, Krishna M C, Mitchell J B. Mol Cell Biochem. 2002;234–235:327–333. [PubMed] [Google Scholar]
- 21.Russo A, Mitchell J B, Finkelstein E, DeGraff W G, Spiro I J, Gamson J. Radiat Res. 1985;103:232–239. [PubMed] [Google Scholar]
- 22.Furth E E, Thilly W G, Penman B W, Liber H L, Rand W M. Anal Biochem. 1981;110:1–8. doi: 10.1016/0003-2697(81)90103-2. [DOI] [PubMed] [Google Scholar]
- 23.Chuang Y E, Chen Q, Liber H L. Cancer Res. 1999;59:3073–3076. [PubMed] [Google Scholar]
- 24.Tietze F. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Griffith O W, Meister A. J Biol Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- 27.Liehr J G, Roy D. Free Radical Biol Med. 1990;8:415–423. doi: 10.1016/0891-5849(90)90108-u. [DOI] [PubMed] [Google Scholar]
- 28.Kalyanaraman B, Felix C C, Sealy R C. Environ Health Perspect. 1985;64:185–198. doi: 10.1289/ehp.8564185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuni A M, Afeworki M, Stein W, Yordanov A T, DeGraff W, Krishna M C, Mitchell J B, Brechbiel M W. Free Radical Biol Med. 2001;30:170–177. doi: 10.1016/s0891-5849(00)00459-7. [DOI] [PubMed] [Google Scholar]
- 30.Krishna M C, Grahame D A, Samuni A, Mitchell J B, Russo A. Proc Natl Acad Sci USA. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalyanaraman B, Korytowski W, Pilas B, Sarna T, Land E J, Truscott T G. Arch Biochem Biophys. 1988;266:277–284. doi: 10.1016/0003-9861(88)90259-7. [DOI] [PubMed] [Google Scholar]
- 32.Misra H P, Fridovich I. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 33.DeGraff W, Hahn S M, Mitchell J B, Krishna M C. Biochem Pharmacol. 1994;48:1427–1435. doi: 10.1016/0006-2952(94)90567-3. [DOI] [PubMed] [Google Scholar]
- 34.Nutter L M, Wu Y Y, Ngo E O, Sierra E E, Gutierrez P L, Abul-Hajj Y J. Chem Res Toxicol. 1994;7:23–28. doi: 10.1021/tx00037a004. [DOI] [PubMed] [Google Scholar]
- 35.Krishna C M, DeGraff W, Tamura S, Gonzalez F, Samuni A, Russo A, Mitchell J B. Cancer Res. 1991;51:6622–6628. [PubMed] [Google Scholar]
- 36.Zhang S, Hunter D J, Forman M R, Rosner B A, Speizer F E, Colditz G A, Manson J E, Hankinson S E, Willett W C. J Natl Cancer Inst. 1999;91:547–556. doi: 10.1093/jnci/91.6.547. [DOI] [PubMed] [Google Scholar]
- 37.Clemons M, Goss P. N Engl J Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]