Abstract
Proposing that a blend of the chemical diversity of small synthetic molecules with the immunological characteristics of the antibody molecule will lead to therapeutic agents with superior properties, we here present a device that equips small synthetic molecules with both effector function and long serum half-life of a generic antibody molecule. As a prototype, we developed a targeting device that is based on the formation of a covalent bond of defined stoichiometry between a 1,3-diketone derivative of an integrin αvβ3 and αvβ5 targeting Arg-Gly-Asp peptidomimetic and the reactive lysine of aldolase antibody 38C2. The resulting complex was shown to (i) spontaneously assemble in vitro and in vivo, (ii) selectively retarget antibody 38C2 to the surface of cells expressing integrins αvβ3 and αvβ5, (iii) dramatically increase the circulatory half-life of the Arg-Gly-Asp peptidomimetic, and (iv) effectively reduce tumor growth in animal models of human Kaposi's sarcoma and colon cancer. This immunotherapeutic has the potential to target a variety of human cancers, acting on both the vasculature that supports tumor growth as well as the tumor cells themselves. Further, by use of a generic antibody molecule that forms a covalent bond with a 1,3-diketone functionality, essentially any compound can be turned into an immunotherapeutic agent thereby not only increasing the diversity space that can be accessed but also multiplying the therapeutic effect.
Since Ehrlich's recognition of the potential of antibodies as therapeutic agents in the early 20th century (1), the development of monoclonal antibody (mAb) technology by Köhler and Milstein in the 1970s (2), and advances in antibody engineering since then (3), mAbs have gained importance for the treatment of a variety of diseases. In addition to a dozen mAbs approved by the U.S. Food and Drug Administration, a considerable number of biotechnology drugs in development are mAbs (4, 5). The mounting success of the antibody molecule as therapeutic agent is based on at least three properties; (i) a Fab moiety that permits antigen binding with high specificity and affinity, (ii) a Fc moiety that mediates effector functions, such as antibody-dependent cellular cytotoxicity (6), and (iii) a molecular mass of at least 150 kDa that permits a circulatory half-life of up to 21 days (7). By contrast, conventional therapeutic agents based on small synthetic molecules are clearly limited with respect to their short half-life in circulation, particularly in chronic treatment regimens like those needed in cancer therapy, and their inability to mediate effector functions. However, small synthetic molecules provide an unlimited chemical diversity provided through their isolation as natural products or de novo chemical synthesis and might be expected to eventually outperform mAbs in terms of specificity and affinity of antigen binding. It can further be anticipated that a blend of the unlimited chemical diversity of small synthetic molecules with the longer serum half-life and the effector function of an antibody molecule will lead to therapeutic agents with superior properties (Table 1).
Table 1.
Comparison of small synthetic molecules and monoclonal antibodies with respect to therapeutic applications
| Small synthetic molecules | Monoclonal antibodies |
|---|---|
| Unlimited structural diversity | Limited structural diversity |
| Easy to manufacture large diversity | Difficult to manufacture large diversity |
| Short half-life | Tunable half-life |
| Difficult to block protein–protein interactions | Adept at blocking protein–protein interactions |
| Reach recessed sites on macromolecules | Rarely reach recessed sites on macromolecules |
| Limited valency requires high affinity | Tunable valency provides activity even at low affinities |
| Limited control of biodistribution | Tunable biodistribution |
| Limited control of effector activities | Tunable effector activities |
| Cross-species activity is readily identified | Cross-species activity is difficult to find |
| Difficult to screen on complex targets | Easy to screen on complex targets |
| Binding screens are difficult and vary | Binding screens are standardized |
Boldface indicates advantages.
Here we present a conceptually new device that equips small synthetic molecules with both effector function and long serum half-life of a generic antibody molecule. Mabs have been suggested as carrier proteins of small synthetic molecules (8). In contrast to earlier studies (9–13), our approach is unique in that small synthetic molecules and mAb form a reversible covalent bond capable of reprogramming the specificity of the antibody both in vitro and in vivo, greatly expanding the scope of potential therapeutic applications of this approach.
As a prototype, we developed a targeting device that is based on the formation of a reversible covalent bond between a diketone derivative of an integrin targeting Arg-Gly-Asp (RGD) peptidomimetic and the reactive lysine of mAb 38C2. mAb 38C2 belongs to a group of catalytic antibodies that were generated by reactive immunization and mechanistically mimic natural aldolase enzymes (14, 15). Through a reactive lysine, these antibodies catalyze aldol and retro-aldol reactions using the enamine mechanism of natural aldolases (14–18). In addition to their remarkable versatility and efficacy in synthetic organic chemistry (reviewed in ref. 14), aldolase antibodies have been used for the activation of prodrugs in vitro and in vivo (19–22). Yet another feature of these antibodies, namely their ability to form a reversible covalent bond with 1,3-diketones by using an enamine docking mechanism (14–16) has remained largely unexplored in terms of potential applications.
Materials and Methods
Synthesis of SCS-873.
SCS-873 was synthesized in a sequence of 13 steps starting from the commercially available 3-methyl-4-bromo anisole. Methods used for the synthesis of the parent SmithKline Beecham compound (23) were modified to prepare the amine precursor of SCS-873. An activated N-hydroxysuccinimide ester of 4′-glutaramidophenyl hexane-3,5-dione was then reacted with the amine to provide the compound SCS-873 (S.C.S., C.R., C.F.B. III, and R.A.L., unpublished data). 1H NMR (500 MHz, CDCl3): d 8.96 (s, 1H), 7.90 (d, J = 4.1 Hz, 1H), 7.49 (m, 3H), 7.08 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.4 Hz, 2H), 6.75 (dd, J = 8.5, 2.6 Hz, 1H), 6.65 (d, J = 2.6 Hz, 1H), 6.57 (t, J = 5.9 Hz, 1H), 6.48 (d, J = 8.8 Hz, 1H), 5.46 (s, 1H), 5.05 (d, J = 16.1 Hz, 1H), 4.06 (m, 2H), 3.78 (d, J = 16.1 Hz, 1H), 3.62–3.30 (m, 20H), 2.86 (m, 5H), 2.54 (t, J = 8.1 Hz, 2H), 2.42 (m, 3H), 2.27 (t, J = 7.0 Hz, 2H), 2.09 (m, 2H), 2.03 (s, 3H), 2.00 (m, 2H), 1.75(m, 2H), 1.70 (m, 2H); MS: 874 (MH+), 896 (MNa+).
Cell Lines and Proteins.
Human melanoma cell line M21 was obtained from D. A. Cheresh (The Scripps Research Institute). Human Kaposi's sarcoma (KS) cell line SLK was provided by R. Pasqualini (University of Texas M. D. Anderson Cancer Center, Houston) with permission from S. Levinton-Kriss (Tel Aviv). Human colon cancer cell line SW1222 was provided by L. J. Old (Ludwig Institute for Cancer Research, New York). All human cell lines were maintained in RPMI medium 1640 containing 10% FCS and antibiotics. Mouse endothelial cell line MS1 was provided by W. B. Stallcup (Burnham Institute, La Jolla, CA). Mouse endothelial cell lines SVEC and MAEC were purchased from American Tissue Culture Collection. All mouse cell lines were maintained in DMEM supplemented with 4 mM l-glutamine/1.5 g/liter sodium bicarbonate/4.5 g/liter glucose/1 mM sodium pyruvate/10% FCS, and antibiotics. FITC-conjugated goat anti-mouse IgG (H+L) polyclonal antibodies were from Jackson ImmunoResearch. mAb 38C2 has been described (15) and is commercially available from Sigma.
Analysis of Complex Formation in Vitro.
The 38C2/SCS-873 complex was formed by incubating 3.3 μM (500 μg/ml) mAb 38C2 with 6.6 μM (5.8 μg/ml) SCS-873 in a volume of 50–200 μl for at least two hours at room temperature. Cells were detached by brief trypsinization with 0.25% (wt/vol) trypsin and 1 mM EDTA, washed with PBS, and resuspended at a concentration of 106 cells per ml in flow cytometry buffer [1% (wt/vol) BSA/25 mM Hepes in PBS, pH 7.4]. Aliquots of 100 μl containing 105 cells were distributed into wells of a V-bottom 96-well plate (Corning) for indirect immunofluorescence staining using a 1:20 dilution (25 μg/ml) of the preformed 38C2/SCS-873 complex in flow cytometry buffer and a 1:100 dilution of FITC-conjugated goat anti-mouse polyclonal antibodies in flow cytometry buffer. Incubation with 38C2/SCS-873 complex was for 1 h and with secondary antibodies for 45 min at room temperature. Flow cytometry was performed by using a FACScan instrument from Becton Dickinson.
Analysis of Complex Formation in Vivo.
Mice were injected i.v. (tail vein) with 100 μl of 10 mg/ml mAb 38C2 in PBS or the same amount of an isotypic (mouse IgG2a) control antibody. SCS-873 was injected i.p. as 100 μl of 10 mg/ml in 50% PBS, 25% DMSO, and 25% ethanol. Sera were prepared by centrifuging eye bleeds taken 24, 48, 72, 96, and 168 h after the injections. We used a 1:100 dilution in flow cytometry buffer to analyze the prepared sera by flow cytometry as described above.
Mouse Tumor Models.
Tumor induction was performed by s.c. injection of 5 × 106 human KS SLK cells or 2 × 106 human colon cancer SW1222 cells in the right flank of nude mice. Four different groups of five animals were treated between days 1 and 20 after tumor induction. Treatment involved 200-μl i.v. injections of either PBS alone (groups 1 and 2) or 2.5 mg/ml mAb 38C2 in PBS (groups 3 and 4) once a week on days 1, 8, and 15. In addition, 50-μl i.p. injections of 10 mg/ml SCS-873 in 50% PBS, 25% DMSO, and 25% ethanol (groups 2 and 4) or solvent alone (groups 1 and 3) were given on days 2, 5, 8, 11, 14, 17, and 20. Tumor volumes of treated animals were measured every third day starting on day 9 by microcaliper measurements (volume = width × length × width/2). Toxicity was monitored by determining the body weight of mice once a week. As soon as the tumor volume reached 800 mm3 in the control groups (on day 45 for the KS model and on day 30 for the colon cancer model), euthanasia was performed and tumors were removed and weighed. Statistical significance between treatment groups was determined by two-tailed Student's t tests using Microsoft excel software.
In Vitro Proliferation Assays.
A total of 1 × 103 (SLK), 2.5 × 103 (SW1222 and SVEC), or 5 × 103 (MAEC and MS1) cells per well in a 96-well tissue culture plate were incubated with various concentrations of SCS-873 ranging from 50 nM to 100 μM in the presence or absence of 10 μM mAb 38C2 for 64 h at 37°C in a humidified CO2 incubator. [3H]thymidine (ICN Radiochemicals) was added to 0.5 μCi per well (1 Ci = 37 GBq) during the last 16 h of incubation. The cells were frozen at −80°C overnight and subsequently processed on a multichannel automated cell harvester (Cambridge Technology, Cambridge, MA) and counted in a liquid scintillation beta counter (Beckman Coulter). The background was defined by running the same assay in the absence of SCS-873. The inhibition in experiment E was calculated according to the following formula: (background − E)/background × 100%.
Results and Discussion
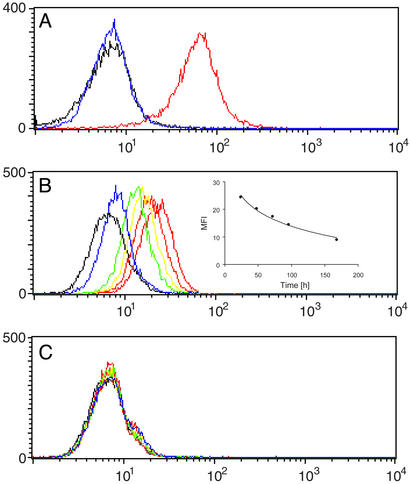
To show that a targeting module derivatized with a 1,3-diketone linker can reprogram the specificity of mAb 38C2 through reaction with its catalytic lysine residue (Fig. 1A), compound SCS-873 was synthesized (Fig. 1B). Applying the concept of reactive immunization (reviewed in ref. 14), we have shown that the immune system selects antibodies with reactive lysine residues based on their chemical reactivity with 1,3-diketone haptens. The pKa of the reactive lysine is perturbed by a hydrophobic microenvironment, rendering it unprotonated at neutral pH (pKa <7). For comparison, the pKa of the epsilon amino group of lysine in aqueous solution is 10.5. SCS-873 was based on an RGD peptidomimetic developed by SmithKline Beecham as an integrin antagonist with nanomolar affinity to human integrins αvβ3 and αvβ5 and 103- to 104-fold selectivity relative to human integrins α5β1 and αIIbβ3 (23). We first analyzed the binding of an equimolar mixture of SCS-873 and mAb 38C2 to cells from the human KS cell line SLK and human melanoma cell line M21. Both cell lines are known to express integrins αvβ3 and αvβ5. We found that SCS-873 effectively mediated cell surface binding of mAb 38C2 (Fig. 2A). No binding of mAb 38C2 was detectable in the absence of SCS-873. The same results were obtained for other cells expressing either integrin αvβ3 or αvβ5, or both, including human and mouse endothelial cells. No binding to cells that do not express the target integrins, such as human colon cancer SW1222 cells, was observed (data not shown). Control experiments with derivatives of the RGD peptidomimetic that did not contain a 1,3-diketone confirmed that this moiety is required for binding of SCS-873 to mAb 38C2 (data not shown). We went on to show that, after independent i.p. and i.v. injections, respectively, SCS-873 and 38C2 form an integrin targeting conjugate in vivo, which was detectable for ≈1 week (Fig. 2B). Sera from control mice treated with an isotype matched mAb lacking the reactive lysine of 38C2 failed to show a cell binding complex (Fig. 2C). Further, no traces of SCS-873 could be observed by flow cytometry following attempted rescue of the small molecule by addition of mAb 38C2 to the collected sera (data not shown), suggesting that the serum half-life of SCS-873 in the absence of mAb 38C2 is similar to the serum half-life of its parental compound, which was determined to be ≈15 min (23). Based on catalytic activity, we had previously determined a mouse serum half-life of i.v. injected mAb 38C2 of ≈4 days showing an in vivo clearance rate with an exponential decay slope (19). The analysis of the decline of the mean fluorescence intensity over time revealed a similar clearance rate for the 38C2/SCS-873 complex with a half-life of ≈3 days (Fig. 2B). Thus, the circulatory half-life of SCS-873 was extended by more than two orders of magnitude through binding to mAb 38C2.
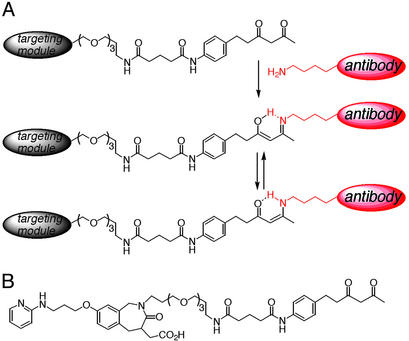
Figure 1.
(A) Reversible covalent bond formation between a targeting module derivatized with a 1,3-diketone and an antibody molecule with a reactive lysine. The 1,3-diketone interacts covalently with the reactive lysine residue of the antibody molecule. The resulting enamine is stabilized by tautomeric isomerization. (B) Structure of SCS-873, a 1,3-diketone derivative of an RGD peptidomimetic developed for high specificity and affinity to integrins αvβ3 and αvβ5. A long spacer between RGD peptidomimetic core and 1,3-diketone group was designed to allow simultaneous recognition of both moieties.
Figure 2.
SCS-873 directs mAb 38C2 to cells expressing integrin αvβ3 and αvβ5. (A) Flow cytometry histogram showing the binding of mAb 38C2 to human melanoma M21 cells in the presence of a twice equimolar concentration of SCS-873 (red). MAb 38C2 and SCS-873 were mixed before cell binding. FITC-conjugated goat anti-mouse polyclonal antibodies were used for detection. Like mAb 38C2 alone (blue), SCS-873 alone (data not shown) was indifferent from the background signal of secondary antibodies alone (black). The y axis gives the number of events in linear scale, the x axis the fluorescence intensity in logarithmic scale. (B and C) Flow cytometry histogram showing the binding of mouse sera to human melanoma M21 cells. Shown are 1:100 dilutions of sera from mice injected i.p. with 1 mg SCS-873 and i.v. with either 1 mg mAb 38C2 (B) or 1 mg isotypic control mAb (C). Sera were prepared from eye bleeds taken 24 h (red), 48 h (orange), 72 h (yellow), 96 h (green), and 168 h (blue) after the injections. FITC-conjugated goat anti-mouse polyclonal antibodies were used for detection. Typical results based on three individual mice in each treatment group are shown. Inset in B shows the decline of the mean fluorescence intensity (MFI) over time.
The parental compound of SCS-873 binds to both integrins αvβ3 and αvβ5 with nanomolar affinity (23). Both integrins are expressed on the surface of a variety of tumor cells and are also up-regulated on angiogenic endothelial cells that infiltrate tumors in the course of neovascularization (24). Small molecule antagonists of integrin αvβ3 and αvβ5 (25), such as RGD peptidomimetics, interfere with the binding of the integrins to extracellular matrix proteins and, thereby, initiate endothelial cell apoptosis and inhibit angiogenesis (26). Consequently, integrin αvβ3 and αvβ5 antagonists are promising therapeutic agents in diseases involving neovascularization, such as cancer, diabetic retinopathy, and rheumatoid arthritis. It should be noted that study of small molecule antagonists, like the one we have studied here for modification, typically suffer from poor pharmacokinetics and are typically administered in animal models at very high doses or by continuous pump-based delivery strategies (27–32). Based on the cross-reactivity of the 38C2/SCS-873 complex with integrins αvβ3 and αvβ5 and the dual expression of integrins αvβ3 and αvβ5 on tumor cells and their supporting vasculature in some cancers, such as KS, melanoma, ovarian, and metastatic breast cancer, the 38C2/SCS-873 complex is expected to direct multiple therapeutic strikes against cancer, with respect to both target and mechanism, with a single drug. To study the 38C2/SCS-873 complex in a relevant animal model of cancer, we have used a xenograft of the human KS cell line SLK in nude mice. Our earlier studies of KS have used this model to examine the efficacy of integrin αvβ3 targeted therapy mediated by an in vitro evolved human antibody named JC-7U (33). Furthermore, to dissect out antitumor and antiangiogenic effects, the 38C2/SCS-873 complex was also evaluated in a xenograft model of human colon cancer. As noted above, both integrins αvβ3 and αvβ5 are highly expressed on the surface of SLK cells (ref. 33 and data not shown). Thus, in the KS model, both human tumor cells and mouse tumor endothelial cells are targeted by the 38C2/SCS-873 complex. It should be noted that application of the 38C2/SCS-873 complex in human therapy to AIDS-related KS would be expected to impact KS proliferation by the additional mechanism of blocking the interaction of the HIV type 1 Tat protein with αvβ3 and αvβ5 (34).
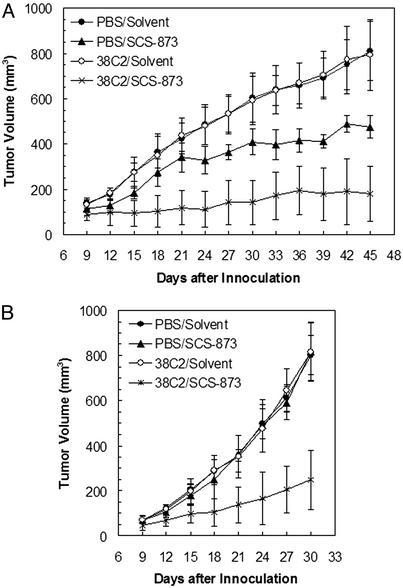
Treatment of the SLK xenograft in nude mice with the 38C2/SCS-873 complex formed in vivo revealed a significant decrease in tumor growth as compared with SCS-873 alone or mAb 38C2 alone (Fig. 3A). By using the same regimen, a SW1222 xenograft in nude mice was treated next. In contrast to human KS cell line SLK, the human colon cancer cell line SW1222 does not express integrin αvβ3 or αvβ5 (data not shown). However, tumor growth was drastically reduced in the presence of the 38C2/SCS-873 complex, whereas SCS-873 alone or mAb 38C2 alone had no effect (Fig. 3B). Thus, in the colon cancer model, the 38C2/SCS-873 complex is likely to mediate its tumor growth inhibition by an antiangiogenic effect directed against the mouse tumor endothelial cells. Most importantly, as both mouse tumor models clearly show, SCS-873 alone, which has a much shorter half-life and cannot trigger antibody-dependent cellular cytotoxicity, is significantly less effective than the 38C2/SCS-873 complex in inhibiting tumor growth. Furthermore, comparison of the therapeutic efficacy of the 38C2/SCS-873 complex in the KS model with our previously reported therapeutic studies involving the in vitro evolved human antibody JC-7U (33), indicates that it is superior. Thus, the chemically programmed antibody developed here outperforms both its small molecule and traditional monoclonal antibody counterparts. Additionally, our study revealed no obvious signs of toxicity for the 38C2/SCS-873 complex as indicated by no weight loss or behavioral change during the course of therapy.
Figure 3.
Tumor growth inhibition mediated by mAb 38C2 in the presence of SCS-873. Tumor growth inhibition studies were based on mouse models of human KS (A) and human colon cancer (B). Tumor induction was performed by s.c. injection of 5 × 106 SLK cells or 2 × 106 SW1222 cells in the right flank of nude mice. Four different groups of five animals were treated between days 1 and 20 after tumor induction. Treatment involved 200-μl i.v. injections of either PBS alone (groups 1 and 2) or 2.5 mg/ml mAb 38C2 in PBS (groups 3 and 4) once a week on days 1, 8, and 15. In addition, 50-μl i.p. injections of 10 mg/ml SCS-873 in 50% PBS, 25% DMSO, and 25% ethanol (groups 2 and 4) or solvent alone (groups 1 and 3) were given on days 2, 5, 8, 11, 14, 17, and 20. Tumor volumes of treated animals were measured every third day starting on day 9. Shown are average tumor volumes ± SD. Statistical significant differences in tumor volume between group 4 (38C2/SCS-873) and group 2 (SCS-873 alone) were observed from day 15 (A) or day 12 (B) until the end of the experiment (P < 0.05 based on two-tailed Student's t tests).
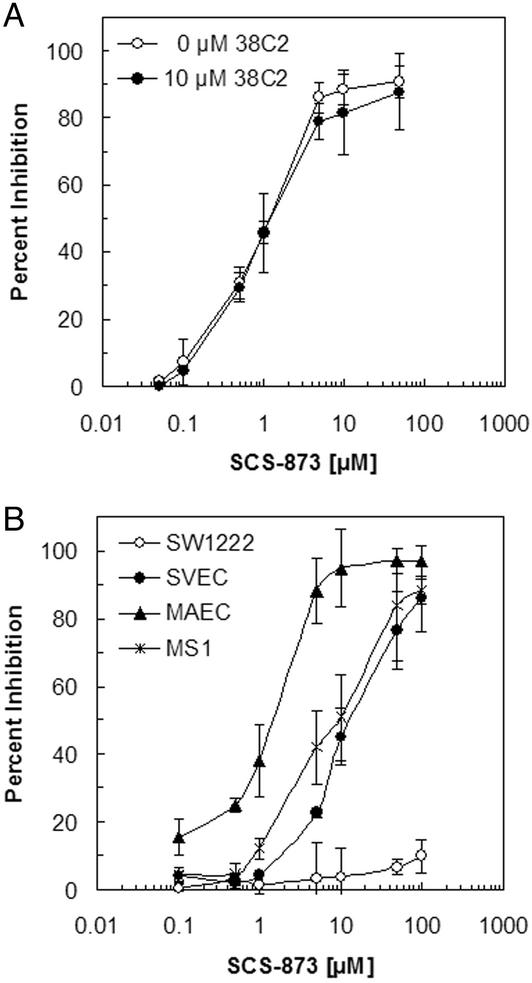
To further examine the mechanism by which the 38C2/SCS-873 complex inhibits tumor growth, we carried out a series of proliferation assays in vitro (Fig. 4). It was found that both free SCS-873 and 38C2/SCS-873 complex inhibit the proliferation of human KS SLK cells with an IC50 of ≈1 μM (Fig. 4A). This concentration is below the theoretical peak concentrations of 30 μM SCS-873 (0.5 mg per 20 ml mouse body volume) and 2 μM 38C2 (0.5 mg per 1.5 ml mouse blood volume) achieved after i.p. and i.v. injection, respectively. Thus, the prolonged serum half-life of cytotoxic SCS-873 in the presence of 38C2 sufficiently explains the tumor growth inhibition in the SLK xenograft, even though antibody-dependent cellular cytotoxicity is likely to be a contributing factor (6). A complete dose/response study may aid in unraveling these effects further. As expected from the SW1222 xenograft, SCS-873 also inhibits the proliferation of mouse endothelial cells but not human colon cancer SW1222 cells (Fig. 4B), suggesting an antiangiogenic effect in this model.
Figure 4.
SCS-873 inhibits the in vitro proliferation of cells expressing integrins αvβ3 and αvβ5. Shown are proliferation assays based on [3H]thymidine incorporation during DNA synthesis. (A) SCS-873 inhibits the in vitro proliferation of human KS cell line SLK with an IC50 of ≈1 μM. Note that the inhibitory activity of SCS-873 is neither diminished nor enhanced by mAb 38C2. (B) SCS-873 inhibits the in vitro proliferation of mouse endothelial cell lines SVEC, MAEC, and MS1 with an IC50 in the range of 1–10 μM. By contrast, the in vitro proliferation of human colon cancer cell line SW1222, which expresses neither integrin αvβ3 nor αvβ5, is not inhibited by SCS-873.
A key element of our approach is the reversible covalent bond between mAb and the small synthetic molecule. In general, the stronger the interaction, the longer is the circulatory half-life of the small synthetic molecule. Using surface plasmon resonance, the koff of the interaction of 38C2 and a 1,3-diketone attached to a linker similar to the linker used for SCS-873 was determined to be ≈1 × 10−4 s−1 (data not shown), which translates into a half-life (ln2/koff) of ≈2 h for the 38C2/SCS-873 complex. Although the interaction is strong, its reversibility warrants a slow drug release, resulting in a low free drug concentration that is maintained over a prolonged period. The in vivo circulatory half-life of the complex, however, was ≈3 days (Fig. 2B). The transient nature of the complex also likely suppresses its potential immunogenicity, although hapten-antibody complexes are not known to be particularly immunogenic. Generic design is another key element. In principle, 1,3-diketone derivatives of any small synthetic molecule can be synthesized. In addition, appropriate 1,3-diketone linkers can be covalently attached to a variety of therapeutically relevant macromolecules, such as proteins or peptides, RNA or DNA aptamers, or combinations therein. Most importantly, the antibody molecule in our two-component system is generic. In other words, the same mAb, preferentially a humanized derivative of mAb 38C2 (35), can be used for a multitude of therapeutic applications, ranging from a mere prolongation of the circulatory half-life to tumor targeting. Among the many therapeutic advantages small synthetic molecules gain through mAb docking (Table 1), blocking of extracellular protein–protein interactions should be pointed out. The development of small synthetic molecules that antagonize protein–protein interactions has proven to be very difficult (36). The much larger mAb molecule, on the other hand, is adept at blocking protein–protein interactions. Thus, the docking mechanism presented here may rescue a number of small synthetic molecules that despite high specificity failed as antagonists of extracellular protein–protein interactions.
Acknowledgments
We thank Larry Altobell for excellent technical assistance and Drs. L. J. Old, D. A. Cheresh, R. Pasqualini, S. Levinton-Kriss, and W. B. Stallcup for providing cell lines. C.R. gratefully acknowledges an Investigator Award from the Cancer Research Institute.
Abbreviations
- KS
Kaposi's sarcoma
- RGD
Arg-Gly-Asp
References
- 1.Ehrlich P. In: The Collected Papers of Paul Ehrlich. Himmelweit F, editor. London: Pergamon; 1960. [Google Scholar]
- 2.Köhler G, Milstein C. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 3.Carter P. Nat Rev Cancer. 2001;1:118–129. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- 4.Reichert J M. Nat Biotechnol. 2001;19:819–822. doi: 10.1038/nbt0901-819. [DOI] [PubMed] [Google Scholar]
- 5.Brekke O H, Sandlie I. Nat Rev Drug Discov. 2003;2:52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 6.Clynes R A, Towers T L, Presta L G, Ravetch J V. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk M A, van de Winkel J G J. Curr Opin Biotechnol. 2001;5:368–374. doi: 10.1016/s1367-5931(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 8.Rehlaender B N, Cho M J. Pharm Res. 1998;15:1652–1656. doi: 10.1023/a:1011936007457. [DOI] [PubMed] [Google Scholar]
- 9.Shokat K M, Schultz P G. J Am Chem Soc. 1991;113:1861–1862. [Google Scholar]
- 10.Lussow A R, Fanget L, Gao L, Block M, Buelow R, Pouletty P. Transplantation. 1996;62:1703–1708. doi: 10.1097/00007890-199612270-00001. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka F, Lerner R A, Barbas C F., III Chem Commun. 1999;12:1383–1384. [Google Scholar]
- 12.Chmura A J, Orton M S, Meares C F. Proc Natl Acad Sci USA. 2001;98:8480–8484. doi: 10.1073/pnas.151260298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Low P S. Cancer Immunol Immunother. 2002;51:153–162. doi: 10.1007/s00262-002-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka F, Barbas C F., III J Immunol Methods. 2002;269:67–79. doi: 10.1016/s0022-1759(02)00225-9. [DOI] [PubMed] [Google Scholar]
- 15.Wagner J, Lerner R A, Barbas C F., III Science. 1995;270:1797–1800. doi: 10.1126/science.270.5243.1797. [DOI] [PubMed] [Google Scholar]
- 16.Barbas C F, III, Heine A, Zhong G, Hoffmann T, Gramatikova S, Bjoernstedt R, List B, Anderson J, Stura A, Wilson I A, Lerner R A. Science. 1997;278:2085–2092. doi: 10.1126/science.278.5346.2085. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann T, Zhong G, List B, Shabat D, Anderson J, Gramatikova S, Lerner R A, Barbas C F., III J Am Chem Soc. 1998;120:2768–2779. [Google Scholar]
- 18.Zhong G, Lerner R A, Barbas C F., III Angew Chem Int Ed Engl. 1999;38:3738–3741. doi: 10.1002/(sici)1521-3773(19991216)38:24<3738::aid-anie3738>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Shabat D, Rader C, List B, Lerner R A, Barbas C F., III Proc Natl Acad Sci USA. 1999;96:6925–6930. doi: 10.1073/pnas.96.12.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rader C, List B. Chem Eur J. 2000;6:2091–2095. doi: 10.1002/1521-3765(20000616)6:12<2091::aid-chem2091>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Shabat D, Lode H N, Pertl U, Reisfeld R A, Rader C, Lerner R A, Barbas C F., III Proc Natl Acad Sci USA. 2001;98:7528–7533. doi: 10.1073/pnas.131187998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopin A, Pessah N, Shamis M, Rader C, Shabat D. Angew Chem Int Ed Engl. 2003;42:327–332. doi: 10.1002/anie.200390108. [DOI] [PubMed] [Google Scholar]
- 23.Miller W H, Alberts D P, Bhatnagar P K, Bondinell W E, Callahan J F, Calvo R R, Cousins R D, Erhard K F, Heerding D A, Keenan R M, et al. J Med Chem. 2000;43:22–26. doi: 10.1021/jm990446u. [DOI] [PubMed] [Google Scholar]
- 24.Brooks P C, Clark R A, Cheresh D A. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 25.Miller W H, Keenan R M, Willette R N, Lark M W. Drug Discov Today. 2000;5:397–408. doi: 10.1016/s1359-6446(00)01545-2. [DOI] [PubMed] [Google Scholar]
- 26.Stupack D G, Cheresh D A. J Cell Sci. 2002;115:3729–3738. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- 27.Engleman V W, Nickols G A, Ross F P, Horton M A, Griggs D W, Settle S L, Ruminski P G, Teitelbaum S L. J Clin Invest. 1997;99:2284–2292. doi: 10.1172/JCI119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto M, Fisher J E, Gentile M, Seedor J G, Leu C T, Rodan S B, Rodan G A. Endocrinology. 1998;139:1411–1419. doi: 10.1210/endo.139.3.5831. [DOI] [PubMed] [Google Scholar]
- 29.Lark M W, Stroup G B, Hwang S M, James I E, Rieman D J, Drake F H, Bradbeer J N, Mathur A, Erhard K F, Newlander K A, et al. J Pharmacol Exp Ther. 1999;291:612–617. [PubMed] [Google Scholar]
- 30.Miller W H, Bondinell W E, Cousins R D, Erhard K F, Jakas D R, Keenan R M, Ku T W, Newlander K A, Ross S T, Haltiwanger R C, et al. Bioorg Med Chem Lett. 1999;9:1807–1812. doi: 10.1016/s0960-894x(99)00283-8. [DOI] [PubMed] [Google Scholar]
- 31.Lode H N, Moehler T, Xiang R, Jonczyk A, Gillies S D, Cheresh D A, Reisfeld R A. Proc Natl Acad Sci USA. 1999;96:1591–1596. doi: 10.1073/pnas.96.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badger A M, Blake S, Kapadia R, Sarkar S, Levin J, Swift B A, Hoffman S J, Stroup G B, Miller W H, Gowen M, Lark M W. Arthritis Rheum. 2001;44:128–137. doi: 10.1002/1529-0131(200101)44:1<128::AID-ANR17>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 33.Rader C, Popkov M, Neves J A, Barbas C F., III FASEB J. 2002;16:2000–2002. doi: 10.1096/fj.02-0281fje. [DOI] [PubMed] [Google Scholar]
- 34.Ensoli B, Barillari C, Salahuddin S Z, Gallo R C, Wong-Staal F. Nature. 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka F, Lerner R A, Barbas C F., III J Am Chem Soc. 2000;122:4835–4836. [Google Scholar]
- 36.Cochran A G. Chem Biol. 2000;7:R85–R94. doi: 10.1016/s1074-5521(00)00106-x. [DOI] [PubMed] [Google Scholar]