Abstract
Mitochondrial dysfunction contributes to many human degenerative diseases but specific treatments are hampered by the difficulty of delivering bioactive molecules to mitochondria in vivo. To overcome this problem we developed a strategy to target bioactive molecules to mitochondria by attachment to the lipophilic triphenylphosphonium cation through an alkyl linker. These molecules rapidly permeate lipid bilayers and, because of the large mitochondrial membrane potential (negative inside), accumulate several hundredfold inside isolated mitochondria and within mitochondria in cultured cells. To determine whether this strategy could lead to the development of mitochondria-specific therapies, we investigated the administration and tissue distribution in mice of simple alkyltriphenylphosphonium cations and of mitochondria-targeted antioxidants comprising a triphenylphosphonium cation coupled to a coenzyme Q or vitamin E derivative. Significant doses of these compounds could be fed safely to mice over long periods, coming to steady-state distributions within the heart, brain, liver, and muscle. Therefore, mitochondria-targeted bioactive molecules can be administered orally, leading to their accumulation at potentially therapeutic concentrations in those tissues most affected by mitochondrial dysfunction. This finding opens the way to the testing of mitochondria-specific therapies in mouse models of human degenerative diseases.
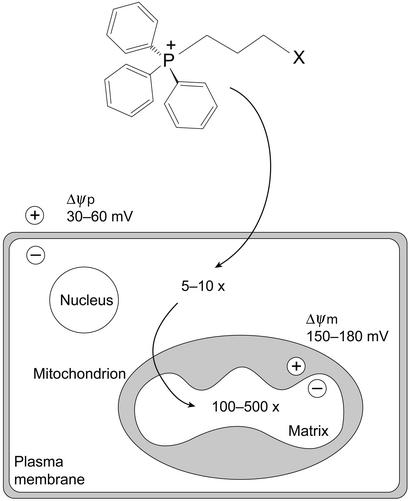
Mitochondria are central to cell life and death; therefore, it is unsurprising that mitochondrial damage contributes to a wide range of diseases including Friedreich's ataxia (1, 2), Parkinson's disease (3), diabetes (4), Huntington's disease (5), disorders associated with mitochondrial DNA mutations (6, 7), defective apoptosis in cancer and degenerative diseases (8), and the pathophysiology of aging (9–11). Despite the prevalence of mitochondrial dysfunction, mitochondria-specific therapies have not been developed, in part because of the difficulty of delivering therapeutic molecules to mitochondria in vivo. As a first step toward such therapies we developed a strategy to deliver bioactive molecules to mitochondria by covalent attachment to the triphenylphosphonium cation through an alkyl chain (Fig. 1; refs. 12–14). The delocalized positive charge of these lipophilic cations enables them to permeate lipid bilayers easily and to accumulate several hundredfold within mitochondria, because of the large membrane potential (−150 to −170 mV, negative inside; Fig. 1; refs. 12 and 15). The plasma membrane potential (−30 to −60 mV, negative inside; Fig. 1) also drives the accumulation of these molecules from the extracellular fluid into isolated cells, from where they are concentrated further within mitochondria, with >90% of intracellular lipophilic cations being present in mitochondria (16, 17). This selective uptake by mitochondria should greatly increase the efficacy and specificity of molecules designed to interact with mitochondria while also decreasing harmful side reactions (13).
Figure 1.
Uptake of alkyltriphenylphosphonium cations by mitochondria within cells. The lipophilic triphenylphosphonium cation is covalently attached to a biologically active molecule (X) such as an antioxidant or pharmacophore. The lipophilic cation is accumulated 5- to 10-fold into the cytoplasm from the extracellular space by the plasma membrane potential (Δψp) and then further accumulated 100- to 500-fold into the mitochondrial matrix by the mitochondrial membrane potential (Δψm). As these lipophilic cations pass directly through the lipid bilayer they do not utilize specific uptake systems and have the potential to distribute to mitochondria in all organs, including the brain.
If these mitochondria-targeted molecules are to have therapeutic potential then they must be taken up selectively by mitochondria in vivo. In particular, they should accumulate at therapeutically effective concentrations within mitochondria in the organs most affected by mitochondrial dysfunction, i.e., the heart, skeletal muscle, and brain (7, 18). Because alkyltriphenylphosphonium cations pass easily through lipid bilayers by non-carrier-mediated transport, they should be taken up by the mitochondria of all tissues, in contrast to hydrophilic compounds which rely on the tissue-specific expression of carriers for uptake. Supporting this concept, the methyltriphenylphosphonium cation (TPMP) is taken up by mitochondria within the perfused heart (19–21), liver (22), and skeletal muscle (23–25). Thus, once these mitochondria-targeted molecules enter the blood stream they should accumulate within mitochondria of all tissues. Of particular relevance for neurodegenerative diseases, the direct movement of lipophilic cations through lipid bilayers should enable these molecules to cross the blood–brain barrier and accumulate in brain mitochondria. However, little is known about the uptake, tissue distribution, and metabolism of alkyltriphenylphosphonium cations in vivo, or whether they can cross the blood–brain barrier. Furthermore, to test the efficacy of these compounds in mouse models of chronic degenerative diseases, convenient protocols for their long-term administration need to be established.
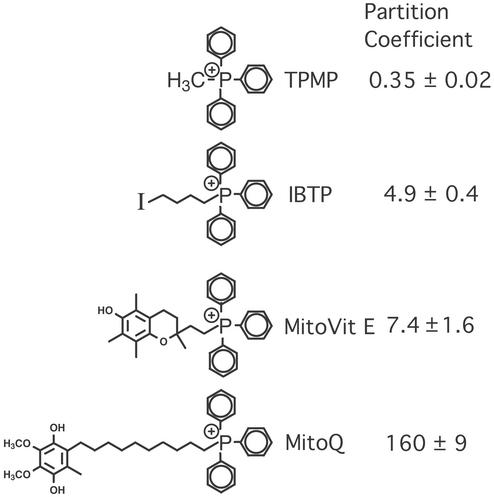
This procedure of targeting bioactive molecules to mitochondria can be adapted to any neutral, bioactive molecule. As a first step we chose to develop mitochondria-targeted antioxidants, because the respiratory chain is the major source of reactive oxygen species in vivo, and mitochondrial oxidative damage is thought to be a proximal cause of pathology in many of the diseases listed in the last section (7, 10, 11, 26). Because the natural antioxidants vitamin E and coenzyme Q are thought to protect mitochondria from oxidative damage in vivo (27–31), we developed mitochondria-targeted derivatives of these molecules (Fig. 2). Experiments in vitro showed that [2-(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)ethyl]triphenylphosphonium bromide (MitoVit E) and a mixture of mitoquinol [10-(3,6-dihydroxy-4,5-dimethoxy-2-methylphenyl)decyl]triphenylphosphonium bromide and mitoquinone [10-(4,5-dimethoxy-2-methyl-3,6-dioxo-1,4-cyclohexadien-1-yl)decyl]triphenylphosphonium bromide (MitoQ) were rapidly and selectively accumulated by isolated mitochondria and by mitochondria within isolated cells (14, 32). Importantly, the accumulation of these antioxidants by mitochondria protected them from oxidative damage far more effectively than untargeted antioxidants, suggesting that the accumulation of bioactive molecules within mitochondria does increase their efficacy.
Figure 2.
Mitochondria-targeted compounds and their octanol–PBS partition coefficients. The octanol–PBS partition coefficients were from this study or refs. 14 and 32.
To see whether conjugation to a triphenylphosphonium cation could deliver bioactive molecules to mitochondria in vivo, we investigated the mode of delivery, tissue distribution, and clearance within mice of the simple alkyltriphenylphosphonium cation TPMP, and the uptake and tissue distribution of the two mitochondria-targeted antioxidants MitoVit E and MitoQ (Fig. 2). We found that all three of these lipophilic cations could be fed safely to mice on a long-term basis, generating potentially therapeutically effective concentrations within the brain, heart, liver, and muscle. This finding opens the way to developing mitochondria-targeted compounds as potential therapies for diseases involving mitochondrial dysfunction.
Materials and Methods
Compounds.
TPMP bromide was from Sigma. MitoVit E, MitoQ, 4-iodobutyltriphenylphosphonium iodide (IBTP), and rabbit antiserum against triphenylphosphonium were prepared as described (14, 32, 33). [3H]TPMP (50–60 Ci/mmol; 1 Ci = 37 GBq) was from American Radiolabeled Chemicals. 3H-enriched MitoVit E (200–800 mCi/mol) and MitoQ (5.9 Ci/mol) were synthesized as described (14, 32, 33). Octanol–PBS partition coefficients were determined as described (14, 32).
Animals and Administration Protocols.
Female Swiss–Webster mice (8–10 weeks old; ≈20–30 g) were supplied by the Department of Laboratory Animal Sciences, University of Otago, and were maintained at 22–23°C on a 12-h light/12-h dark cycle and had free access to drinking water and standard chow, or to a complete liquid diet. All experiments were approved by the University of Otago animal ethics committee. Weight, appearance, and behavior were monitored continually to gauge gross effects of the administered compounds, and any mice displaying behavioral changes or weight loss consistent with toxicity were killed. For injections, ethanolic solutions of TPMP were diluted 1:100 in PBS, and MitoVit E, MitoQ, and IBTP were dissolved in PBS supplemented with sufficient DMSO to maintain solubility. Intraperitoneal or i.v. (tail vein) injections (100 μl) were given to pairs of mice and compared with vehicle-injected controls. Single i.v. injections of TPMP or MitoVit E up to 300 nmol were well tolerated with no overt toxicity observed over the subsequent 7 days. Toxicity was evident at 500 nmol and above within 12 h of injection. These correspond to maximum tolerated doses of 3.8 and 6 mg/kg of body weight with toxic effects evident at 6.4 and 10.2 mg/kg for TPMP and MitoVit E, respectively. Intravenous injection of 750 nmol MitoQ was well tolerated, with toxic effects evident at 1,000 nmol MitoQ, corresponding to a maximum tolerated dose of 20 mg/kg with toxicity evident at 27 mg/kg.
TPMP, MitoVit E, and MitoQ were fed to mice in their drinking water. For some experiments, MitoQ was fed as part of a complete liquid diet that replaced solid food (34). Doses were determined by measuring water or liquid diet consumption and mouse weight. Drinking water supplemented with 500 μM TPMP was fed for at least 43 days without any gross signs of toxicity, corresponding to a maximum tolerated dose of 97 ± 13 μmol of TPMP/kg/day (34.6 ± 4.6 mg TPMP/kg/day). Toxicity was evident after 26 or 6 days when the drinking water was supplemented with 1 or 5 mM TPMP, respectively. Mice were fed 500 μM MitoVit E in their drinking water for at least 14 days without any gross signs of toxicity, corresponding to a maximum tolerated dose of 105 ± 9.2 μmol of MitoVit E/kg/day (60.4 ± 5.3 mg of MitoVit E/kg/day). Mice fed complete liquid diets supplemented with 500 μM or 1 mM MitoQ showed no toxic effects for at least 26 days, although food consumption decreased 20–35% compared with controls. The doses of MitoQ were 232 ± 4 and 346 ± 3.4 μmol/kg/day for the 500 μM and 1 mM diets, respectively (corresponding to 154 and 230 mg/kg/day). Mice fed a liquid diet supplemented with 2 or 5 mM MitoQ showed toxic effects after 10–11 days but at these concentrations food consumption was 70–80% lower than controls.
Analysis of Tissue Distribution of Compounds.
To determine the tissue distribution of 3H-labeled compounds, mice were killed by cervical dislocation and bled. The liver, brain (cerebrum, cerebellum, and brainstem), kidneys, heart, adipose tissue (i.p.), and skeletal muscle (gastrocnemius and quadriceps) were collected and weighed. For [3H]TPMP and [3H]MitoVit E, tissues were homogenized in 4 ml of 250 mM sucrose, 10 mM Tris⋅HCl (pH 7.4), and 1 mM EGTA by using an Ultraturrax homogenizer (IKA-Werke, Staufen, Germany; 2 × 30 s), and the 3H content was determined by scintillation counting. For [3H]MitoQ, tissues were homogenized in 4 ml of ice-cold methanol by using an Ultraturrax homogenizer (2 × 30 s) and then debris was pelleted by centrifugation (10,000 × g for 10 min). The methanolic supernatant was transferred to a scintillation vial, the solvent was evaporated under a stream of nitrogen, and scintillant was added to the residue. The specific activity of administered compound was used to calculate the tissue content in nanomoles per gram of wet weight tissue. For determination of fetal uptake, mice were fed 500 μM [3H]TPMP (0.25 Ci/mol) in their drinking water from 1 to 16 days after mating. Then day-16.5 embryos were isolated and their [3H]TPMP content was determined as above. Data are means ± range for pairs of litters. For determination of TPMP uptake by preweaning neonates, mice were fed 500 μM [3H]TPMP (0.25 Ci/mol) in their drinking water through gestation and lactation (26 days), then 7-day-old neonates were killed and the [3H]TPMP content of the brain, heart ,and liver was determined as above. Data are means ± range for pairs of litters, where like organs from each litter were pooled.
Preparation and Analysis of Mitochondria.
Mice were killed by cervical dislocation and bled. Liver mitochondria were prepared by homogenization in 250 mM sucrose, 10 mM Tris · HCl (pH 7.4), and 1 mM EGTA, followed by differential centrifugation (35). Heart mitochondria were prepared by homogenization in 210 mM mannitol, 70 mM sucrose, 10 mM Tris, and 0.1 mM EDTA (pH 7.4) by using an Ultraturrax followed by a Dounce homogenizer (Wheaton Scientific), and mitochondria were then isolated by differential centrifugation. The protein concentration was determined by the bicinchoninic acid assay using BSA as a standard (36). Mitochondrial protein samples were separated by SDS/PAGE and transferred to nitrocellulose, and butyltriphenylphosphonium-labeled proteins were visualized by immunoblotting with antitriphenylphosphonium antiserum in conjunction with appropriate secondary Abs and enhanced chemiluminescence, or a chromogenic substrate (33).
Extraction of Alkyltriphenylphosphonium Cations from Tissues.
MitoVit E was extracted from tissue homogenates in 4 ml of 250 mM sucrose, 10 mM Tris⋅HCl (pH 7.4), and 1 mM EGTA by vortexing with 1–2 vol of dichloromethane followed by separation of the organic and aqueous layers by centrifugation (≈1,000 × g for 2 min). This process was repeated (three times), the organic was layers pooled, the solvent was evaporated under a stream of nitrogen, and the residue was dissolved in 100 μl of ethanol. Spiking heart, liver, or brain homogenates with [3H]TPMP (250 nCi) showed that three such solvent extractions removed 93–95% of the [3H]TPMP.
Mass Spectrometry.
Analysis of MitoVit E accumulation by tissues was done using a Finnigan LTQ DECA (Thermo Finnigan MAT, Bremen, Germany) at the protein microchemistry facility (University of Otago). The flow rate was 5 μl/min and the m/z range was 100–2,000.
Results
Tissue Distribution of Alkyltriphenylphosphonium Cations After i.p., i.v., or Oral Delivery.
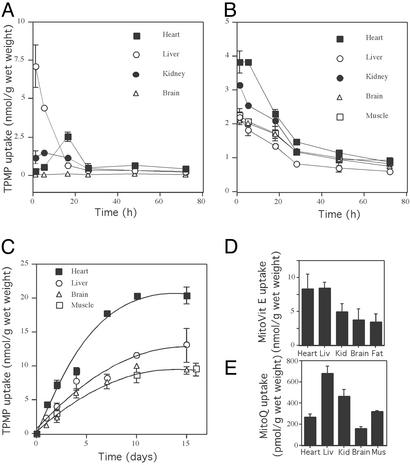
To determine how best to administer alkyltriphenylphosphonium cations to mice we first investigated the tissue distribution of the simple alkyltriphenylphosphonium cation TPMP. The uptake and distribution of TPMP in mitochondria and cells are similar to that of mitochondria-targeted bioactive molecules such as MitoVit E and MitoQ (Fig. 2) (14, 32). Mice injected i.p. with [3H]TPMP had substantial uptake of TPMP by the liver and kidneys within 1 h of injection but none by the brain (Fig. 3A). The TPMP content of the heart increased up to 16 h, because of redistribution from other organs (Fig. 3A). TPMP was rapidly cleared with >90% eliminated from all organs within 24 h, with substantial amounts of [3H]TPMP appearing in the urine (data not shown). The distribution of [3H]MitoVit E 4 h after an i.p. injection was similar to that of TPMP (data not shown). In contrast, when [3H]TPMP was added directly to the bloodstream by i.v. injection it was taken up rapidly by the brain as well as by the heart, muscle, and liver (Fig. 3B). Therefore, alkyltriphenylphosphonium cations can accumulate from the bloodstream into the major tissues of interest.
Figure 3.
Distribution of alkyltriphenylphosphonium cations within mice. (A) Intraperitoneal injection of TPMP. Mice were injected i.p. with 100 nmol of [3H]TPMP (10 Ci/mol; 1.2 mg of TPMP/kg), and the tissue content of [3H]TPMP was measured at various times after injection by scintillation counting. Data are means ± range for pairs of mice for each time point. (B) Intravenous injection of TPMP. Mice were injected i.v. with 100 nmol of [3H]TPMP (10 Ci/mol; 1.2 mg of TPMP/kg), and the tissue content of [3H]TPMP was determined by scintillation counting. Data are means ± range for pairs of mice for each time point. (C) Feeding TPMP in drinking water. Mice were fed 500 μM [3H]TPMP (0.25 Ci/mol) in their drinking water, and the tissue content of [3H]TPMP was measured at various times after starting feeding by scintillation counting. Data are means ± range for pairs of mice for each time point. (D) Feeding MitoVit E in drinking water. Mice were fed 500 μM [3H]MitoVit E (0.2 Ci/mol) in their drinking water, and the tissue contents of [3H]MitoVit E were measured after 4 days by scintillation counting. Data are means ± range for pairs of mice. (E) Feeding MitoQ in drinking water. Mice were fed 500 μM [3H]MitoQ (0.5 Ci/mol) in their drinking water, and the tissue contents of [3H]MitoQ were measured after 10 days by scintillation counting. Data are means ± range for pairs of mice. Liv, liver; Kid, kidney; Mus, muscle.
Although i.v. injection can deliver alkyltriphenylphosphonium cations to the target tissues, this procedure is cumbersome for long-term treatment. Therefore, we determined whether orally administered alkyltriphenylphosphonium cations would also accumulate in these tissues. Feeding mice 500 μM [3H]TPMP in their drinking water led to substantial TPMP accumulation by the heart, skeletal muscle, liver, and brain that came to a steady state after 7–10 days of feeding (Fig. 3C). Mice fed [3H]MitoVit E in their drinking water for 4 days had a similar tissue distribution as that found for TPMP (Fig. 3D). Furthermore, the amount of [3H]MitoVit E accumulated was comparable to the amount of TPMP accumulated after 4 days (Fig. 3C). When mice were fed [3H]MitoQ in their drinking water, they accumulated MitoQ in the same organs as accumulated TPMP and MitoVit E, namely the heart, liver, skeletal muscle, and brain (Fig. 3E). However, the amounts of MitoQ accumulated were lower than for TPMP and MitoVit E (Fig. 3E), presumably because of the greater hydrophobicity of MitoQ (Fig. 2). In summary, alkyltriphenylphosphonium cations are readily taken up by the heart, liver, brain, and skeletal muscle after oral administration.
Clearance of Orally Administered Alkyltriphenylphosphonium Cations.
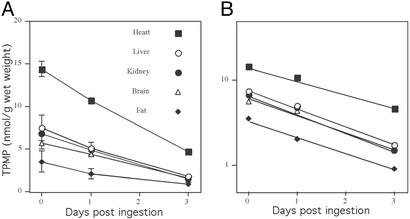
To be therapeutically useful, orally administered alkyltriphenylphosphonium cations should be cleared from the body at a reasonable rate, without accumulating irreversibly in any tissue. To investigate the rate of clearance of alkyltriphenylphosphonium cations, mice were fed [3H]TPMP in their drinking water for 7 days and then switched to water without TPMP, and the loss of [3H]TPMP from organs was measured (Fig. 4A). The first-order rates of clearance of TPMP were calculated from log plots of the washout data (Fig. 4B). These rates were similar for all organs investigated, giving a mean rate of 0.45 ± 0.07 day−1, which corresponds to a half life of 1.54 days ± 0.27 (means ± ranges). Hence, alkyltriphenylphosphonium cations are rapidly cleared from the body. Furthermore, the clearance rates from all organs studied here were similar, consistent with simple reequilibration of the lipophilic cation with the bloodstream by nonmediated movement through lipid bilayers.
Figure 4.
Clearance of orally administered alkyltriphenylphosphonium cations. (A) Clearance of [3H]TPMP. Mice were fed 500 μM [3H]TPMP (0.25 Ci/mol) in their drinking water for 7 days, and were then switched to water without TPMP. The tissue content of [3H]TPMP was measured by scintillation counting at various times after withdrawing TPMP. Data are means ± range for pairs of mice for each time point. (B) Log plot of clearance of TPMP from mice. The data shown in A were replotted to determine the first-order rate constant for TPMP clearance.
Fetal and Neonatal Uptake of Alkyltriphenylphosphonium Cations.
Many mouse models of mitochondrial dysfunction are fatal in utero or early after birth (1, 37, 38). Consequently, to be of use in these models, alkyltriphenylphosphonium cations must be delivered to fetuses and to neonates before weaning. The easiest way to do this is by feeding the lipophilic cations to the dam, provided the compound is transmitted to the fetus through the placenta and to the neonate through the milk. To see whether alkyltriphenylphosphonium cations could cross the placenta, mice were fed 500 μM [3H]TPMP in their drinking water from days 1 to 17 of pregnancy. Fetuses were then isolated, and the [3H]TPMP uptake was found to be substantial (56 ± 23 nmol TPMP/g of wet weight). To determine whether this level of [3H]TPMP could be maintained in neonates between birth and weaning, mice were fed 500 μM [3H]TPMP in their drinking water from 1 day after mating until the neonates were 7 days old, when the tissue distribution of [3H]TPMP in the neonates was determined. There was substantial uptake of [3H]TPMP by the neonatal liver (53 ± 21), heart (168 ± 114), and brain (53 ± 30) (all nmol TPMP/g of wet weight). Therefore, alkyltriphenylphosphonium cations can be delivered to fetuses and neonates by oral administration to pregnant or lactating dams.
Alkyltriphenylphosphonium Cations Localize to Mitochondria Within Tissues.
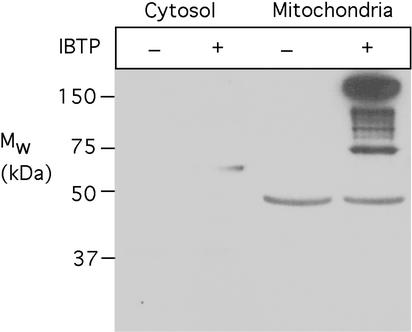
Once TPMP, MitoQ, and MitoVit E are taken up by cells they are predominantly localized to the mitochondria (14, 16, 32), and TPMP is taken up by the mitochondria of perfused organs (19–25). Therefore, the alkyltriphenylphosphonium cations accumulated by tissues in vivo are likely to be predominantly present in mitochondria. However, confirming the mitochondrial location of alkyltriphenylphosphonium cations within tissues in vivo is difficult, because on cell subfractionation these compounds will be rapidly lost from the mitochondria on depolarization. To circumvent this difficulty, we used IBTP, an alkyltriphenylphosphonium cation that is taken up by mitochondria in the same way as TPMP, MitoVit E, and MitoQ (33). Within mitochondria the iodo moiety of IBTP is slowly displaced by protein thiols, covalently binding the butyltriphenylphosphonium cation to mitochondrial proteins by a stable thioether linkage (33). This bond survives tissue homogenization and enables IBTP-labeled proteins to be visualized by immunoblotting (33). Therefore, IBTP labeling of proteins should indicate the intracellular location of alkyltriphenylphosphonium cations within tissues in vivo.
Mice were injected i.v. with IBTP and 4 h later heart mitochondrial and cytosolic fractions were isolated, their proteins were separated by SDS/PAGE, and they were analyzed for IBTP labeling by immunoblotting (Fig. 5). This procedure showed extensive IBTP labeling of mitochondrial proteins from the hearts of IBTP-treated mice but not of proteins in heart mitochondria from control mice (Fig. 5). There was negligible labeling of the heart cytosolic proteins from IBTP-treated mice (Fig. 5), indicating that the IBTP taken up by the heart in vivo was predominantly present within mitochondria. This experiment suggests that alkyltriphenylphosphonium cations taken up by tissues in vivo are predominantly localized within mitochondria.
Figure 5.
Accumulation of alkyltriphenylphosphonium cations within mitochondria in vivo. Mice were injected i.v. with 1 μmol IBTP or vehicle and 4 h later heart mitochondria and cytoplasm were prepared. Protein (25 μg) from control and IBTP-treated mitochondria and cytosolic fractions were separated by SDS/PAGE and then immunoblotted with antitriphenylphosphonium antiserum. The mitochondrial band at ≈45 kDa was a nonspecific binding artifact. These data are typical of three independent experiments. Analysis of mitochondrial and cytosolic marker enzymes during our mitochondrial preparations routinely shows negligible cross-contamination of the fractions.
Uptake of MitoVit E Assessed by Mass Spectrometry.
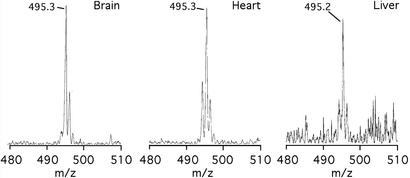
The mitochondria-targeted antioxidant MitoVit E was shown to be present within tissues in vivo by radioisotope distribution (Fig. 3D). To confirm that this compound was present in its active form and had not been metabolized or degraded, mice were fed MitoVit E for 14 days in their drinking water. The tissues were then isolated and MitoVit E was extracted from tissues by using organic solvent. Electrospray mass spectrometry was then used to confirm the identity of the intact MitoVit E cation, which had an m/z of 495.24470 by high-resolution mass spectrometry. This procedure showed that the intact MitoVit E cation (m/z 495.3 ± 0.1 Da) was present in the heart, liver, and brain of mice fed MitoVit E (Fig. 6).
Figure 6.
Analysis of accumulated MitoVit E by mass spectrometry. Mice were fed 500 μM MitoVit E in their drinking water for 14 days, then MitoVit E was extracted from tissues and analyzed by electrospray mass spectrometry. Similar analysis of control tissues gave no peaks in the region of interest (data not shown).
Discussion
We have shown that mitochondria-targeted compounds containing the lipophilic triphenylphosphonium cation accumulate within the heart, skeletal muscle, liver, and brain when administered orally to mice. Mice could be fed these compounds for several weeks, leading to stable steady-state concentrations within these tissues. Uptake was reversible, as shown by the rapid clearance of TPMP from all organs when oral administration stopped. That these compounds can enter the bloodstream and distribute to tissues in their intact, active form was shown by solvent extraction of the brain, heart, and liver of mice fed MitoVit E, followed by mass spectrometry. Experiments with the mitochondria-targeted thiol reagent IBTP suggested that these compounds were predominantly incorporated into mitochondria after their uptake by tissues. These data are consistent with the following pharmacokinetic model: after absorption from the gut into the bloodstream, orally administered triphenylphosphonium cations are taken up into all tissues by nonmediated movement through the lipid bilayer of the plasma membrane, assisted by the plasma membrane potential. From the cytosol most lipophilic cations are taken up into mitochondria, driven by the large membrane potential. After several days of feeding, the cation concentration within mitochondria comes to a steady-state distribution with circulating blood levels. At this point the mitochondrial concentration will be several hundredfold higher than that in the bloodstream, and the rate of oral absorption of the compounds will match the rates of excretion into the urine and bile. The mitochondrial pool of compound is in dynamic equilibrium and once feeding stops the accumulated cations will reequilibrate back into the bloodstream and be relatively rapidly excreted. That these compounds are taken up across the blood–brain barrier is of particular interest, because of the difficulties of delivering drugs to the brain and the number of neurodegenerative diseases involving mitochondrial dysfunction. The substantial uptake by the heart, presumably because of its high content of mitochondria, is also significant given the association of cardiomyopathy with mitochondrial dysfunction.
We have shown uptake of mitochondria-targeted compounds into mitochondria within tissues. This approach enables a range of small molecules to be targeted to mitochondria and extends and complements earlier approaches designed to target DNA and related molecules to mitochondria (39–41). For this strategy to have clinical potential, therapeutically effective concentrations of bioactive molecules must be delivered to mitochondria without toxicity. Lipophilic cations are toxic at high concentrations because their excessive uptake by mitochondria disrupts ATP synthesis. Even so, substantial doses of TPMP and MitoVit E were well tolerated by delivery from drinking water (30 and 60 mg/kg/day, respectively). The maximum tolerated acute doses by i.v. injection were 6, 10, and 20 mg/kg for TPMP, MitoVit E, and MitoQ, respectively. Interestingly, the maximum tolerated acute doses were broadly similar, despite the quite different side chains and hydrophobicities of the three compounds (Fig. 2). This result implies that the toxicity of these compounds is determined by the lipophilic cation, not the side chain. The well tolerated oral doses were ≈5-fold greater than the maximum acute i.v. doses, which may be due to differences in the bioavailability of these compounds by oral delivery but it is also likely that slow uptake and distribution after gradual oral administration will lead to lower toxicity. Further advantages of oral delivery are that it simplifies the testing of these compounds in mouse models of chronic diseases, and that this mode of delivery can be easily extended to patient studies. However, oral delivery of mitochondria-targeted compounds is of little use if the mitochondrial concentrations achieved by the maximum safe dose are too low to be therapeutically effective. The TPMP and MitoVit E concentrations that accumulated in tissues in vivo after feeding were ≈5–20 nmol/g of wet weight, which corresponds to >5–20 μM in the tissue as a whole. As these compounds will be accumulated within mitochondria the intramitochondrial concentration will be roughly millimolar. Although the therapeutic dose will vary considerably depending on the type of bioactive molecule targeted, high micromolar to millimolar mitochondrial concentrations are likely to be in the therapeutically effective range for many biologically active molecules. For example, mitochondria-targeted antioxidant concentrations of 1–2.5 μM were effective in preventing oxidative damage to isolated mitochondria (14, 32, 42). As these compounds will be accumulated further into cells, similar protective effects should be found by incubating cells with lower concentrations, and consistent with this 500 nM to 1 μM concentrations of mitochondria-targeted antioxidants are protective in cultured cells (43, 44). Although the organ distributions of all of the compounds investigated were similar, MitoQ was taken up to a lesser extent than TPMP or MitoVit E, presumably because of its greater hydrophobicity (Fig. 2). It is currently unclear whether this difference is due to decreased uptake from the bloodstream or to altered bioavailability from the gut, and ongoing work is characterizing the pharmacokinetics of all these compounds. If hydrophobicity does limit the bioavailability of some of these compounds, this is easily modulated by varying the length of the alkyl chain connecting the antioxidant and phosphonium moieties. For example, a derivative of MitoQ with a 3 carbon linker chain has been found to have an octanol–PBS partition coefficient of 2.8 (compare 160 for MitoQ). Thus, oral delivery of well tolerated doses of mitochondria-targeted compounds can deliver potentially therapeutic concentrations of compounds to mitochondria in vivo.
To conclude, we have shown that mitochondria-targeted compounds can be delivered to the brain, heart, liver, and muscle by simple oral administration to mice. Mitochondria are a particularly important but neglected intracellular drug target because of their central position in energy and intermediate metabolism and their role in cell death (13, 45). This finding may help in developing novel compounds as potential therapies for diseases associated with mitochondrial dysfunction and as tools to unravel how mitochondrial damage contributes to human diseases.
Acknowledgments
This work was supported by a grant from the Health Research Council of New Zealand and by Antipodean Biotechnology, Ltd.
Abbreviations
- IBTP
4-iodobutyltriphenylphosphonium iodide
- MitoQ
mixture of mitoquinol [10-(3,6-dihydroxy-4,5-dimethoxy-2-methylphenyl)decyl]triphenylphosphonium bromide and mitoquinone [10-(4,5-dimethoxy-2-methyl-3,6-dioxo-1,4-cyclohexadien-1-yl)decyl]triphenylphosphonium bromide
- MitoVit E
[2-(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)ethyl]triphenylphosphonium bromide
- TPMP
methyltriphenylphosphonium cation
References
- 1.Puccio H, Simon D, Cossee M, Criqui-Filipe P, Tiziano F, Melki J, Hindelang C, Matyas R, Rustin P, Koenig M. Nat Genet. 2001;27:181–186. doi: 10.1038/84818. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan J. Proc Natl Acad Sci USA. 1999;96:10948–10949. doi: 10.1073/pnas.96.20.10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schapira A H, Mann V M, Cooper J M, Krige D, Jenner P J, Marsden C D. Ann Neurol. 1992;32:S116–S124. doi: 10.1002/ana.410320720. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa T, Edelstein D, Du X L, Yamagishi S-I, Matsumura T, Kaneda Y, Yorek M A, Beebe D, Oates P J, Hammes H-P, et al. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 5.Tabrizi S J, Workman J, Hart P E, Mangiarini L, Mahal A, Bates G, Cooper J M, Schapira A H. Ann Neurol. 2000;47:80–86. doi: 10.1002/1531-8249(200001)47:1<80::aid-ana13>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Pitkanen S, Robinson B H. J Clin Invest. 1996;98:345–351. doi: 10.1172/JCI118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace D C. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G, Dallaporta B, Resche-Rignon M. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 9.Beckman K B, Ames B N. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 10.Raha S, Robinson B H. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 11.Ames B N, Shigenaga M K, Hagen T M. Biochim Biophys Acta. 1995;1271:165–170. doi: 10.1016/0925-4439(95)00024-x. [DOI] [PubMed] [Google Scholar]
- 12.Murphy M P. Trends Biotechnol. 1997;15:326–330. doi: 10.1016/S0167-7799(97)01068-8. [DOI] [PubMed] [Google Scholar]
- 13.Murphy M P, Smith R A J. Adv Drug Delivery Rev. 2000;41:235–250. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 14.Kelso G F, Porteous C M, Coulter C V, Hughes G, Porteous W K, Ledgerwood E C, Smith R A J, Murphy M P. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 15.Liberman E A, Topali V P, Tsofina L M, Jasaitis A A, Skulachev V P. Nature. 1969;222:1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 16.Berry M N, Edwards A M, Barrit G J, Grivell M B, Halls H J, Gannon B J, Friend D S. In: Laboratory Techniques in Biochemistry and Molecular Biology. Burdon R H, van Knippenberg P H, editors. Vol. 21. Amsterdam: Elsevier; 1991. pp. 386–390. [Google Scholar]
- 17.Burns R J, Murphy M P. Arch Biochem Biophys. 1997;339:33–39. doi: 10.1006/abbi.1996.9861. [DOI] [PubMed] [Google Scholar]
- 18.Shigenaga M K, Hagen T M, Ames B N. Proc Natl Acad Sci USA. 1994;91:19771–19778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kauppinen R. Biochim Biophys Acta. 1983;725:131–137. doi: 10.1016/0005-2728(83)90232-3. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda H, Syrota A, Charbonneau P, Vallois J, Crouzel M, Prenant C, Sastre J, Crouzel C. Eur J Nucl Med. 1986;11:478–483. doi: 10.1007/BF00252793. [DOI] [PubMed] [Google Scholar]
- 21.Wan B, Doumen C, Duszynski J, Salama G, LaNoue K F. Am J Physiol. 1993;265:H445–H452. doi: 10.1152/ajpheart.1993.265.2.H445. [DOI] [PubMed] [Google Scholar]
- 22.Steen H, Maring J G, Meijer D K. Biochem Pharmacol. 1993;45:809–818. doi: 10.1016/0006-2952(93)90163-q. [DOI] [PubMed] [Google Scholar]
- 23.Rolfe D F S, Newman J M, Buckingham J A, Clark M G, Brand M D. Am J Physiol. 1999;276:C692–C699. doi: 10.1152/ajpcell.1999.276.3.C692. [DOI] [PubMed] [Google Scholar]
- 24.Rolfe D F S, Brand M D. Am J Physiol. 1996;271:C1380–C1389. doi: 10.1152/ajpcell.1996.271.4.C1380. [DOI] [PubMed] [Google Scholar]
- 25.Rolfe D F S, Brand M D. Biochim Biophys Acta. 1996;1276:45–50. doi: 10.1016/0005-2728(96)00029-1. [DOI] [PubMed] [Google Scholar]
- 26.Ames B N, Shigenaga M K, Hagen T M. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews R T, Yang L, Browne S, Bail M, Beal M F. Proc Natl Acad Sci USA. 1998;95:8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lass A, Sohal R S. Arch Biochem Biophys. 1998;352:229–236. doi: 10.1006/abbi.1997.0606. [DOI] [PubMed] [Google Scholar]
- 29.Lass A, Forster M J, Sohal R S. Free Radical Biol Med. 1999;26:1357–1382. doi: 10.1016/s0891-5849(98)00330-x. [DOI] [PubMed] [Google Scholar]
- 30.Sokol R J, McKim J M, Goff M C, Ruyle S Z, Devereaux M W, Han D, Packer L, Everson G. Gastroenterology. 1998;114:164–174. doi: 10.1016/s0016-5085(98)70644-4. [DOI] [PubMed] [Google Scholar]
- 31.Lang J K, Gohil K, Packer L. Anal Biochem. 1986;157:106–116. doi: 10.1016/0003-2697(86)90203-4. [DOI] [PubMed] [Google Scholar]
- 32.Smith R A J, Porteous C M, Coulter C V, Murphy M P. Eur J Biochem. 1999;263:709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 33.Lin T K, Hughes G, Muratovska A, Blaikie F H, Brookes P S, Darley-Usmar V, Smith R A J, Murphy M P. J Biol Chem. 2002;277:17048–17056. doi: 10.1074/jbc.M110797200. [DOI] [PubMed] [Google Scholar]
- 34.Lieber C S, DeCarli L M. Alcohol Clin Exp Res. 1986;10:550–553. doi: 10.1111/j.1530-0277.1986.tb05140.x. [DOI] [PubMed] [Google Scholar]
- 35.Chappell J B, Hansford R G. In: Subcellular Components: Preparation and Fractionation. Birnie G D, editor. London: Butterworth; 1972. pp. 77–91. [Google Scholar]
- 36.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 37.Lebovitz R M, Zhang H, Vogel H, Cartwright J, Dionne L, Lu N, Huang S, Matzuk M M. Proc Natl Acad Sci USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Huang T-T, Carlson E J, Melov S, Ursell P C, Olson J L, Noble L J, Yoshimura M P, Berger C, Chan P H, et al. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 39.Seibel P E A, Trappe J, Villani G, Klopstock T, Papa S, Reichmann H. Nucleic Acids Res. 1995;23:10–17. doi: 10.1093/nar/23.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chrzanowska-Lightowlers Z M A, Lightowlers R N, Turnbull D M. Gene Ther. 1995;2:311–316. [PubMed] [Google Scholar]
- 41.Vestweber D, Schatz G. Nature. 1989;338:170–172. doi: 10.1038/338170a0. [DOI] [PubMed] [Google Scholar]
- 42.Echtay K S, Murphy M P, Smith R A J, Talbot D A, Brand M D. J Biol Chem. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- 43.Hwang P M, Bunz F, Yu J, Rago C, Chan T A, Murphy M P, Kelso G F, Smith R A J, Kinzler K W, Vogelstein B. Nat Med. 2001;7:1111–1117. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelso G F, Porteous C M, Hughes G, Ledgerwood E C, Gane A M, Smith R A J, Murphy M P. Ann NY Acad Sci. 2002;959:263–274. doi: 10.1111/j.1749-6632.2002.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 45.Schon E A. Trends Biochem Sci. 2000;25:555–560. doi: 10.1016/s0968-0004(00)01688-1. [DOI] [PubMed] [Google Scholar]