Abstract
Background
The National Wilms Tumor Study (NWTS) constitutes a unique resource for study of clinical, pathologic and epidemiologic features of Wilms tumor.
Procedure
Data from NWTS-3,4,5 were compiled for 7,455 patients with tumors of favorable (FH) or anaplastic (AH) histology. The associations of birth weight (BW) and age-at-onset with gender, intralobar (ILNR) and perilobar (PLNR) nephrogenic rests, tumor focality, congenital malformation syndromes and tumor histology were analyzed using descriptive statistics and linear regression.
Results
Mean BWs for male and female patients without PLNR were 3.52 and 3.36 kg, respectively, and for those with PLNR were 0.12 kg and 0.15 kg heavier. Mean age was 45 months for males with no rests whose tumors were unifocal and of triphasic favorable histology. ILNR or multifocality decreased the mean age by 18 and 10 months, respectively, whereas female gender, blastemal/FH or AH increased it by 3, 10 and 16 months. Over 90% of multifocal tumors occurred in the presence of demonstrated ILNR or PLNR or both. The apparent bimodality of the age distributions and later mean ages-at-onset for females with both unifocal and multifocal tumors were explained in part by the relative deficit in females of ILNR vs PLNR associated tumors.
Conclusions
These observations support the view that there are multiple pathways to Wilms tumorigenesis. They will facilitate selection of informative subgroups of patients for molecular analysis that may serve to identify the putative pathway for the majority of patients who cannot be classified provisionally on the basis of ILNR or PLNR.
Keywords: Epidemiology, molecular genetics, pathology, Wilms tumor
INTRODUCTION
Wilms Tumor (WT), whose pathogenesis was once thought to follow the same single-gene, two-hit model as retinoblastoma, has instead become a paradigm for genetic heterogeneity and complexity. [1-3] At least ten genes have been implicated in its development, most of which are associated with familial disease or malformation syndromes that affect only a small fraction of cases. [4, 5] Constitutional deletion of WT1 at 11p13 is well established as the genetic basis for the WT-aniridia-GU anomaly-retardation (WAGR) syndrome, while germline missense mutations in WT1 are responsible for most WTs that occur as part of the Denys-Drash syndrome. [6] Dysregulated expression of imprinted genes at 11p15.5, particularly loss-of-imprinting (LOI) of the insulin grown factor gene IGF2, occurs in a large fraction of WT cases. [7, 8]
Beckwith and colleagues [9] identified two distinct precursor lesions that distinguish WT according to pathogenesis. Intralobar nephrogrenic rests (ILNR), which occur in isolation within the renal lobe, the renal sinus or the calyceal walls, are associated with the WAGR and Denys-Drash syndromes and with GU anomalies.[10] Perilobar nephogenic rests (PLNR), which occur at the periphery of the renal lobe and are often found in multiplicity, are associated with Beckwith-Wiedemann syndrome (BWS) and hemihypertropy (HH). While most WT are triphasic, the stromal component tends to predominate in tumors associated with ILNR while the blastemal or embryonal epithelial components predominate in those associated with PLNR.
Epidemiologic criteria for distinguishing tumors according to pathogenesis include age-at-onset, gender, ethnicity and birth weight. The earlier onset for patients with bilateral disease was a key observation in Knudson's development of the 2-stage model. [1, 2] Further variations in age by gender and ethnicity were among the findings that led Breslow and Beckwith [11] to suggest the possibility of heterogeneity in WT pathogenesis. Early onset is a characteristic of ILNR associated tumors and those occurring in Asian populations. [9, 10] WT patients have higher mean birth weights in comparison with the general population, more so if the tumor is associated with PLNR. [12]
Recent work has combined molecular, microscopic and epidemiologic observations in an attempt to identify biological subgroups of WT that may present distinct targets for therapeutic intervention.[4] Ravenel and colleagues [13] proposed LOI of IGF2 as defining one major subgroup having late onset and a histologic pattern associated with PLNR. Schumacher and co-workers [14] proposed WT1 mutation as identifying a subgroup having early onset, the stromal predominant histology associated with ILNR and poor response to therapy. Since the majority of WT occur in the apparent absence of nephrogenic rests, it is hoped that such molecular criteria will facilitate their classification into subgroups having a distinct etiology, pathogenesis and prognosis. Such molecular classification will be aided by full understanding of the variation in and association between the microscopic characteristics of nephrogenic rests and tumor histology, on the one hand, and the epidemiologic characteristics on the other. The present investigation is intended to contribute to this understanding.
MATERIALS AND METHODS
Source of patients and data
During 1979-2002, 7,455 patients were enrolled on National Wilms Tumor Study (NWTS) 3, 4 or 5 with a central pathology diagnosis by one of us (JBB or EJP) of WT of favorable (FH) or anaplastic (AH) histology.[15] Patients with other renal neoplasms such as clear cell sarcoma or rhabdoid tumor of the kidney are not considered here. Tumors were classified according to the presence or absence of each of 30 distinct histologic subtypes or patterns, of which 10 were blastema, 10 epithelium and 10 stroma. For FH tumors, if any single pattern comprised two-thirds or more of the viable tumor sections examined then we classified the tumor as having the corresponding main type: blastemal, stromal or epithelial predominant. [15] The other FH tumors were classified as having mixed (triphasic) histology. A small number (n=168, 2%) with rare or indeterminate histologic patterns were omitted from analyses of histologic type. We ascertained the presence or absence of PLNR or ILNR or both in normal kidney adjacent to the tumor with reasonable certainty for 5,954 patients (80%). Birth weights were abstracted from clinical records for 4,486 patients (60%), more systematically for NWTS 4 and 5 (79% and 76%, respectively) than for NWTS 3 (15%). All tumors were classified as unifocal vs multifocal, the latter category comprising unilateral/multifocal, bilateral-at-onset and late (metachronous) bilateral tumors.
Statistical methods
Variations in birth weight and age-at-onset among different categories of patients were evaluated using summary statistics and multiple linear regression. Patient records with missing or indeterminate values for any variable in a particular regression equation were omitted. Regression results are stated as mean difference ± standard error of the difference between indicated categories. Frequency distributions for birth weight and age were estimated by nonparametric Gaussian kernel smoothing as implemented in the program S-Plus 6.2.1, with bandwidths selected using a normal reference density.[16] Chi-squared (χ2) and t-tests were used to evaluate the statistical significance (p–value) of differences in proportions and means. The influence of nephogenic rests on prognosis, with and without adjustment for the association between rests and other factors, was assessed using the Cox model with failure defined as relapse or progression of disease.[17] Death due to toxicity and the appearance of new disease in the contralateral kidney (late bilateral) were treated as competing risks leading to censorship of the record at that time. Results were stated in terms of relative risk of relapse, with 95% confidence intervals.
RESULTS
Nephrogenic rests
PLNR or ILNR or both were found in 2,494/5,954=42% of patients for whom their occurrence could be evaluated with some certainty. Their distribution varied markedly according to associated congenital anomalies (Table I). ILNR was reported in 35/45=78% of patients with WAGR syndrome and 78/152=51% of males with hypospadias and/or cryptorchism, but in only 24% of the general patient population. Patients with HH and especially BWS had more tumors occurring in association with PLNR and both PLNR and ILNR. The demonstrable occurrence of rests was also strongly associated with both gender and the multiplicity of tumor foci (Table II). Just over 90% of multifocal tumors occurred in the presence of PLNR or ILNR or both, whereas this was true for only 1/3 of unifocal tumors. Females had a relative excess of PLNR and a deficit of ILNR associated tumors in comparison with males, with the differences particularly striking for multifocal tumors. Finally, among tumors that were associated with at least one type of rest, those with stromal predominant FH were much more likely to occur in the presence of ILNR whereas those with blastemal or epithelial predominant FH or AH were more likely to occur in the presence of PLNR (Table III). These associations, most of which were highly statistically significant in view of the large numbers, are important to keep in mind when interpreting the results of the multiple regression analyses that follow.
Table I.
Distribution of nephrogenic rests in subgroups of patients having selected congenital malformation syndromes and anomalies
| Denys-Drash syndrome | Beckwith-Wiedemann syndrome | WAGR syndrome | Male GU anomaly (sporadic) | Hemihypertrophy (sporadic) | All patients | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Pct. | No. | Pct. | No. | Pct. | No. | Pct. | No. | Pct. | No. | Pct. | |
| None | 10 | 37% | 14 | 20% | 8 | 18% | 56 | 44% | 53 | 34% | 3460 | 58% |
| PLNR | 0 | 0% | 25 | 35% | 2 | 4% | 11 | 9% | 61 | 39% | 1184 | 20% |
| ILNR | 16 | 59% | 13 | 18% | 33 | 73% | 54 | 43% | 26 | 17% | 1062 | 18% |
| Both | 1 | 4% | 19 | 27% | 2 | 4% | 5 | 5% | 16 | 10% | 248 | 4% |
| Total | 27 | 100% | 71 | 100% | 45 | 100% | 126 | 100% | 156 | 100% | 5954 | 100% |
Table II.
Occurrence of nephrogenic rests by gender and multiplicity of tumor foci, with mean (± standard deviation) age-at-onset (mo) in each category
| Rest status | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unifocal | Multifocal | Unifocal | Multifocal | |||||||||
| No. | Pct. | Age | No. | Pct. | Age | No. | Pct. | Age | No. | Pct. | Age | |
| None | 1541 | 67% | 49.1±38 | 48 | 12% | 36.7±42 | 1828 | 68% | 54.4±38 | 43 | 8% | 60.3±51 |
| PLNR | 270 | 12% | 51.8±29 | 170 | 44% | 39.1±21 | 422 | 16% | 52.7±27 | 322 | 59% | 40.5±20 |
| ILNR | 445 | 19% | 28.7±24 | 102 | 26% | 20.3±17 | 402 | 15% | 30.4±24 | 113 | 21% | 18.7±15 |
| Both | 57 | 2% | 26.2±24 | 65 | 17% | 25.7±20 | 54 | 2% | 31.1±22 | 72 | 13% | 28.0±20 |
| Total | 2313 | 100% | 45.0±36 | 385 | 100% | 31.6±25 | 2706 | 100% | 50.1±35 | 550 | 100% | 36.0±27 |
Table III.
Occurrence of nephrogenic rests by histologic type
| Rest status | Mixed/FH | Blastemal/FH | Epithelial/FH | Stromal/FH | Anaplastic | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Pct. | No. | Pct. | No. | Pct. | No. | Pct. | No. | Pct. | No. | Pct. | |
| None | 1403 | 52% | 1221 | 66% | 380 | 65% | 92 | 55% | 305 | 58% | 3401 | 58% |
| PLNR | 389 | 14% | 490 | 26% | 140 | 24% | 13 | 8% | 144 | 28% | 1176 | 20% |
| ILNR | 772 | 28% | 97 | 5% | 44 | 8% | 56 | 34% | 47 | 9% | 1016 | 18% |
| Both | 148 | 6% | 46 | 3% | 17 | 3% | 6 | 4% | 26 | 5% | 243 | 4% |
| Total | 2712 | 100% | 1854 | 100% | 581 | 100% | 167 | 100% | 522 | 100% | 5836 | 100% |
Birth weight
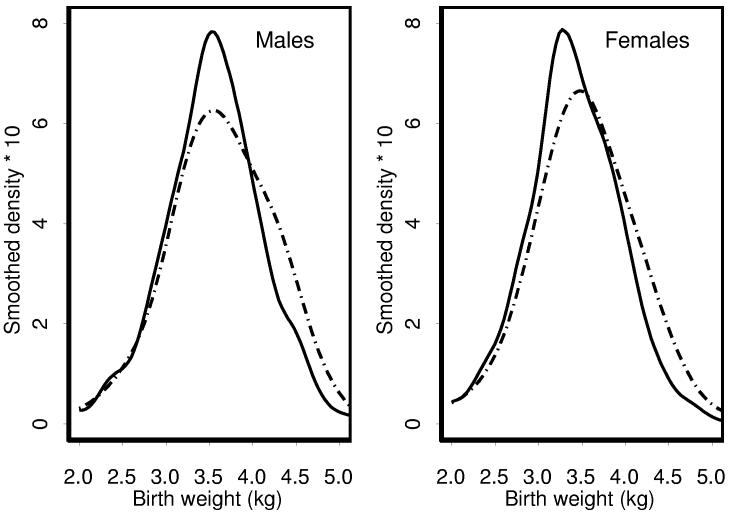
Patients known to have a twin or triplet (n=116, 1.6%) were excluded from the analyses of birth weight, whose principal determinants were gender and PLNR (Figure 1). Mean birth weights (± standard deviation) were 3.64±0.61 (n=312) and 3.52±0.61 (n=1292) kg for males with and without PLNR, respectively, whereas for females they were 3.51±0.62 (n=525) and 3.36±0.60 (n=1382) kg. Combining these results using linear regression, the birth weights for males were estimated to be an average of 0.150±0.021 and for patients with PLNR an average of 0.135±0.024 kg heavier when compared with females and patients without PLNR, respectively. The proportions of males with birth weight in excess of 4 kg were 83/312=27% for those with and 221/1292=17% for those without PLNR (X2=14.8, p=0.0001); for females they were 97/525=18% and 147/1382=11% (X2=21.0, p<0.0001).
Figure 1.
Frequency distributions of birth weight by gender. Solid line - no PLNR, dotted line - PLNR
Regression analysis showed that some of the excess birth weight in patients with PLNR was mediated through its association with overgrowth syndromes and multifocal tumors (Table IV). Mean birth weights were independently increased by 0.061±0.032, 0.147±0.060 and 0.244±0.096 kg for patients with multifocal tumors, HH and BWS, respectively, with these estimated effects additive in patients who had more than one such feature. The increase associated with PLNR per se, in the absence of multiple tumor foci or an overgrowth syndrome, was reduced from 0.135 to 0.096±0.027. The lowest birth weights, on average 0.449±0.124 kg lower, were found in the few patients with WAGR syndrome. ILNR had no apparent effect when added to the regression equation; its coefficient was −0.013±0.25 (p=0.60). Likewise, with the possible exception of a reduction in birth weight associated with stromal predominant as opposed to triphasic histology of 0.189±0.071 kg, which was of borderline statistical significance after accounting for the multiplicity of comparisons, tumor histology did not affect birth weight after accounting for these other factors.
Table IV.
Results of multiple linear regression analysis of birth weight on gender and presence of PLNR, multifocal tumor and selected congenital anomalies (n=3511 singletons)
| Factor | n | Coefficient | Std. Error | t-statistic | p-value |
|---|---|---|---|---|---|
| (Intercept) | 3.51 | 0.02 | 218.79 | 0.000 | |
| Female | 1907 | −0.15 | 0.02 | −7.14 | 0.000 |
| PLNR | 837 | 0.10 | 0.03 | 3.57 | 0.000 |
| Multifocal | 529 | 0.06 | 0.03 | 1.92 | 0.055 |
| HH | 112 | 0.15 | 0.06 | 2.46 | 0.014 |
| BWS | 43 | 0.24 | 0.10 | 2.55 | 0.011 |
| WAGR | 24 | −0.45 | 0.12 | −3.62 | 0.000 |
Baseline categories are Male gender, unilateral/unifocal tumor and without syndrome. HH=hemi-hypertropy; BWS=Beckwith-Wiedemann syndrome; WAGR=Wilms tumor/aniridia/GUanomaly/retardation symdorme
Age-at-onset
Age was strongly associated with gender, multiple tumor foci, ILNR and blastemal/FH or AH. Table II shows mean ages for patient subgroups defined by the first three of these factors. The overall mean ages for males and females were 42.5±34.4 and 47.5±35.0 months, respectively, with a mean difference between females and males of 5.0±0.8 months. Results of the regression analysis (Table V) summarize the differences in mean ages noted in Table II and the differences associated with histology. A rough summary of these results is the following. For males having unifocal tumors of mixed, epithelial or stromal predominant FH that are not associated with ILNR, the mean age at diagnosis was 45 months. This was reduced by 10±1 months if the tumor was multifocal and by 18±1 months if it was associated with ILNR. A favorable blastemal or anaplastic histology, however, increased the mean age by 10.5±1 or 16±1.5 months, respectively. These effects are additive so that, for example, the estimated mean age for females with multifocal tumors of mixed, epithelial or stromal predominant FH associated with ILNR is 45−10−18+3=20 months. The actual mean age for the 147 patients in this category was 19.3 months. After accounting for the association of gender with the other factors, the estimated conditional mean difference for females vs. males was reduced from 5 to 3±1 months. (Table V).
Table V.
Results of multiple linear regression analysis of age at diagnosis on gender, presence of ILNR, focality of tumor and histologic type of tumor (n=5836)
| Factor | n | Value | Std. Error | t value | Pr(>|t|) |
|---|---|---|---|---|---|
| (Intercept) | 44.86 | 0.78 | 57.30 | 0.000 | |
| Female | 3,206 | 3.02 | 0.85 | 3.56 | 0.000 |
| ILNR | 1,259 | −18.10 | 1.06 | −17.01 | 0.000 |
| Multifocal | 916 | −10.27 | 1.17 | −8.77 | 0.000 |
| Blastemal/FH | 1,854 | 10.54 | 0.96 | 10.99 | 0.000 |
| Anaplastic | 522 | 15.94 | 1.53 | 10.40 | 0.000 |
*Baseline categories are male gender, without ILNR, unifocal and other histologies.
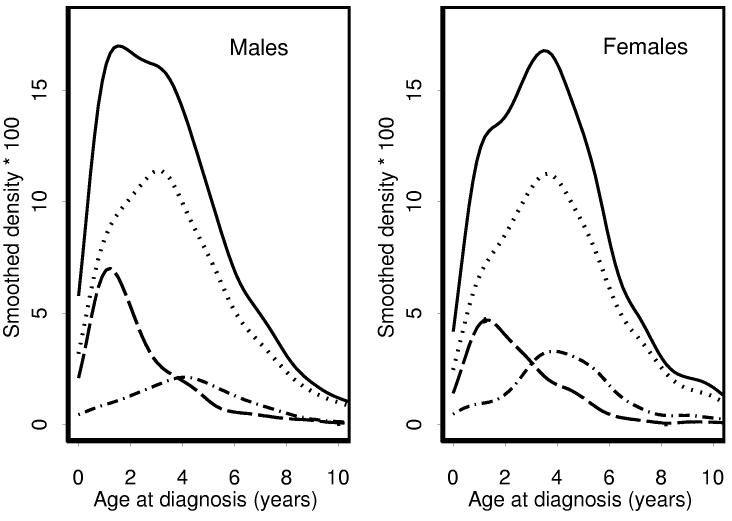
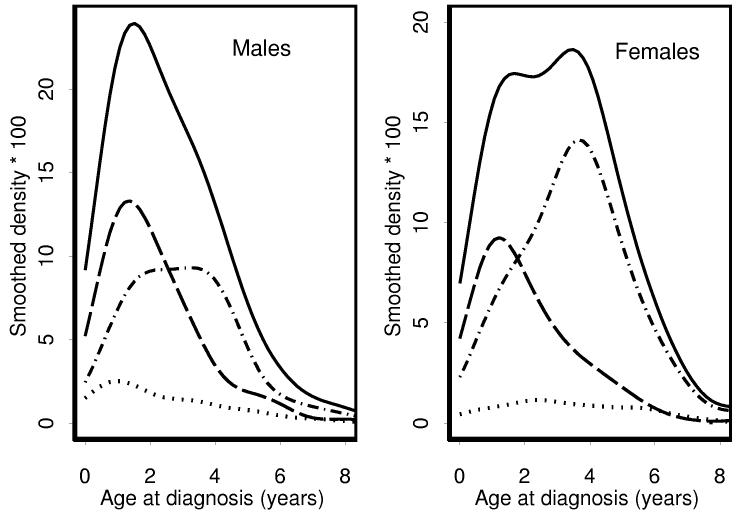
Further appreciation for the effect on age distributions of the relative predominance of ILNR vs. PLNR associated WT in males vs. females can be gleaned from Figures 2 and 3. Each age distribution was decomposed into three sub-distributions: tumors occurring in association with ILNR±PLNR, PLNR alone or neither rest. The near absence of multifocal tumors for which neither ILNR nor PLNR could be demonstrated is evident in Figure 3 from the fact that the area under the dotted line is quite small. The bimodality of the age distribution for females with multifocal tumors was due to the relative excess of PLNR associated tumors, especially those diagnosed at about four years of age. The early peak in the age distribution for males with unifocal tumors was due to a relative excess of ILNR; the later peak for females was due to a relative abundance of PLNR and the fact that the mean age for females whose tumor was associated with neither ILNR nor PLNR was about 5 months greater than for males (Table II).
Figure 2.
Frequency distributions and sub-distributions of age-at-onset by gender for patients with unifocal tumors. Solid line - all patients. Dotted line = rest negative. Dashed line = ILNR (+/− PLNR). Dashed and dotted line - PLNR only
Figure 3.
Frequency distributions and sub-distributions of age-at-onset by gender for patients with multifocal tumors. Solid line - all patients. Dotted line = rest negative. Dashed line = ILNR (+/− PLNR). Dashed and dotted line - PLNR only
Prognosis
When evaluated on their own, PLNR was associated with a poorer prognosis and ILNR with a better prognosis than for tumors that occurred in the absence of rests. Estimated percentages of patients remaining alive and free of disease at 10 years following diagnosis were 83±1% for no rests, 79±1% for PLNR alone, 89±1% for ILNR alone and 82±1% for both PLNR and ILNR. The relative risks of relapse for each of the latter three categories relative to no rests were 1.20 (1.03, 1.40) for PLNR alone, 0.62 (0.51, 0.76) for ILNR alone and 0.99 (0.73, 1.36) for both. After adjustment for the adverse prognosis associated with bilateral disease, AH and increasing age-at-onset, however, the statistical effects of nephrogenic rests per se on relapse were no longer significant. The adjusted relative risks were 1.02 (0.86, 1.22) for PLNR alone, 0.82 (0.66, 1.03) for ILNR alone and 0.97 (0.69, 1.36) for both.
DISCUSSION
This study confirms several previously reported associations involving birth weight, age-at-onset, nephrogenic rests, histology and congenital malformation syndromes in patients with WT, many of which were discovered using the large and relatively unselected population of patients enrolled in the NWTS. Overall they provide support for the concept that WT develops along at least two biological pathways. Each is associated with a distinct set of molecular events, precursor lesions, histologic patterns and congenital malformations and with a distinct developmental time course. There are substantial biological variations within as well as between these pathways, however, and all of the mentioned features overlap to some extent. For example, patients with WT and sporadic GU anomalies often have no associated nephrogenic rests or no germline WT1 mutations. [18, 19] Many more molecular events remain to be discovered that can help explain these variations. Nonetheless, at present, they serve to define two “ideal types” of WT.
Ideal Type I WT is characterized by the presence of ILNR, stromal predominant FH, early onset and a higher likelihood of hypospadias or cryptorchism in males. Because these same features occur with the WAGR and Denys-Drash syndromes, the critical molecular event is presumed to involve WT1 mutation. Royer-Pokora et al. [6], who studied 117 WT patients with WT1 germline mutations, noted substantial further variation in age-at-onset, patterns of bilaterality and multifocality and associated congenital anomalies depending upon whether WT1 was deleted, subject to a missense mutation or subject to a gene truncation mutation. Germline WT1 mutations account for no more than 5% of WT and somatic WT1 mutations for perhaps another 10-15%.[4, 20] While most of these display the classic two-hit paradigm of mutation in one allele followed by loss (LOH) or a second mutation in the other, the association of WT1 and β-catenin mutations in the same cell may also play a key role in the pathway to malignancy. [20, 21] Sequencing of WT1 from tumors, precursor lesions and normal kidney in large numbers of patients will be needed to unravel the complex role of WT1 in tumor development. Selection and analysis of such patients according to the presence or absence of the phenotypic features associated with Type I WT will facilitate this process.
Ideal Type II WT is characterized by PLNR, AH or blastemal or epithelial predominant FH, and later age-at-onset. Because of the association with heavier birth weight and with overgrowth syndromes linked to chromosome 11p15, the molecular events leading to Type II WT were conjectured to involve dysregulated expression of genes in this region, particularly IGF2.[22] The finding that IGF2 LOI was indeed associated with late onset and tumors that are histologically “PLNR-like” supports its identification as the molecular criterion for Type II WT.[13] Fukuzawa and colleagues [23] demonstrated the absence of IGF2 LOI in 21 WT from Japanese children, the nearly complete absence of PLNR in Japanese and the much lower prevalence of PLNR in east-Asian American compared with white American patients from the NWTS. Absence of the Type II pathway nicely explains the lower incidence and earlier onset of WT long known to epidemiologists for east-Asian populations.[24, 25] Assay for IGF2 LOI in tumor, precursor and normal tissue for large numbers of patients will again be needed to sort out some of the variability within the Type II subgroup. For example, constitutional hypermethylation of H19 together with LOI of IGF2 are frequently found in WT that occur in conjunction with BWS though not in those that occur together with idiopathic HH. [26] Such assays will be most informative when tumors can be definitively classified as to the presence or absence of PLNR and the other features associated with Type II WT, and when other molecular evidence such as LOH at 11p loci and WT1 mutations can be examined at the same time.
NWTS investigators have previously noticed bimodality in WT age-at-onset distributions and remarked on the slight excess of females and their older ages.[25] These features may be explained, at least in part, by differences in age and gender of patients with PLNR vs. ILNR (Table II, Figures 2 and 3). Likewise, the relative absence of PLNR in east-Asian WT populations, plus possible gender bias in ascertainment, helps to explain the epidemiologic finding of an excess of males over females.[24, 25] However, NWTS females with no demonstrable rests and unifocal tumors are still more frequent and are diagnosed 5 months older, on average, than their male counterparts. This raises the intriguing question as to whether the molecular criteria of WT1 mutation and IGF2 LOI can be used to further classify the majority of patients with no discernible nephrogenic rests, no associated congenital malformations and typical triphasic FH, in such a way as to explain the gender differences. The WT in rest-negative kidneys are variable in appearance, some resembling the PLNR-derived tumor with others, actually a majority, having at least a tendency toward the appearance of the typical ILNR-derived tumor. It is likely that PLNR and ILNR represent two ends of a developmental spectrum, with intermediate stages being less obvious to the pathologist's eye. Systematic molecular study of large numbers of these patients, for whom all the relevant phenotypic data are available, will be needed to resolve the issue as to whether they arise through the pathways already described. Alternatively, at least some of these WT may arise from additional pathways having other molecular markers.
This study demonstrates the potential traps in applying the same diagnostic term to groups with inherent heterogeneity. “Wilms tumor” is a name applied to embryonal renal neoplasms that can arise from more than one developmental error (Knudson's “first hit”) and very possibly from more than one type of “second hit”. The location and appearance of ILNR clearly implies an error in early stages of nephrogenesis, whereas PLNR obviously arise late in nephrogenesis. It is simplistic, but not necessarily incorrect, to assume that PLNR is the result of excessive or prolonged exposure to IGF2 of nephrogenic blastema during the period of nephron formation. Two different patterns of kidney overgrowth occur in infants with BWS. One pattern has extreme overgrowth of the kidney, while retaining recognizable organizational characteristics. Neoformation of nephrons continues for weeks or even months into postnatal life (hyperplastic nephromegaly). Far more common in BWS is a kidney with the normal number of nephron generations and PLNR. Such kidneys obviously had normal cessation of nephron formation, which usually occurs around week 34. If a proliferative stimulus becomes active around that time, when only scattered responsive blastemal cells remain, scattered isolated PLNR will result. If the stimulus activates a few weeks earlier, a more continuous band of blastemal cells is present at the periphery of the developing cortex, which will produce the “diffuse” pattern of PLNR distribution. As long as the growth factor remains, the rests will continue to grow, resulting in scattered hyperplastic PLNR of diffuse “hyperplastic periolobar nephroblastomatosis”. Cessation of growth factor stimulation will result in maturation and obsolescence of the rests. The more blastemal cells are present, the greater the likelihood of “event 2”. The true oncogenic stimulus is not known, and need not be the same for all WT. Whether IGF2 plays a role in the post-induction phase of WT growth is not known.
This view of PLNR as a type of overgrowth seems reasonable. But ILNR may be fundamentally different, at least in some cases. We speculate that ILNR represents a localized developmental failure, an arrest of maturation in a small region of the developing metanephros. A cluster of immature cells remains while the remainder of the kidney continues to develop around it. Prior to the phase of nephron formation, the developing kidney is in what some have called the “stromagenic phase” of nephrogenesis-laying down the connective tissue framework within which the kidney will be constructed. Thus, it is not surprising that ILNR might have a predominantly stromal composition, and a different molcular pathogenesis. IGF2 can certainly still play a role in the subsequent life history of an ILNR. But this view permits consideration of ILNR pathogenesis as a form of undergrowth rather than overgrowth.
In conclusion, the strong correlations among birth weights, ages-at-onset, congenital anomalies, precursor lesions, histology and other clinicopathologic features of WT provide further evidence for heterogeneity in pathogenesis. They suggest a possible biological basis for the slight excess and older ages of girls compared to boys with WT and for other epidemiologic characteristics of the disease. A large, systematic molecular genetic study of WT1 mutation and IGF2 LOI or loss of heterozygosity (LOH) at 11p15 in patients already classified by the presence of ILNR and/or PLNR is required to confirm that these are indeed critical events that identify distinct types of WT. It will be of particular interest to determine whether the molecular events are associated with distinct phenotypes among the majority of patients who cannot now be classified on the basis of nephrogenic rests.
ACKNOWLEDGEMENTS
We thank investigators of the Children's Oncology Group and the health professionals who managed the care of children entered in the National Wilms Tumor Studies. This work was supported in part by grant R01 CA54998 from the National Cancer Institute.
References
- 1.Knudson AG. Mutation and cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudson AG, Jr., Strong LC. Mutation and cancer: a model for Wilms' tumor of the kidney. J Natl Cancer Inst. 1972;48:313–324. [PubMed] [Google Scholar]
- 3.Knudson AG. Intoduction to the genetics of primary renal tumors in children. Med Pediatr Oncol. 1993;21:193–198. doi: 10.1002/mpo.2950210308. [DOI] [PubMed] [Google Scholar]
- 4.Pritchard-Jones K. Molecular genetic pathways to Wilms tumor. Critical Review in Oncology/Hematology. 1997;8:1–27. doi: 10.1615/critrevoncog.v8.i1.10. [DOI] [PubMed] [Google Scholar]
- 5.Huff V, Amos CI, Douglass EC, et al. Evidence for genetic heterogeneity in familial Wilms' tumor. Cancer Res. 1997;57:1859–1862. [PubMed] [Google Scholar]
- 6.Royer-Pokora B, Beier M, Henzler M, et al. Twenty-four new cases of WT1 germline mutations and review of the literature: Genotype/phenotype correlations for Wilms tumor development. Am J Med Genet A. 2004;127A:249–257. doi: 10.1002/ajmg.a.30015. [DOI] [PubMed] [Google Scholar]
- 7.Rainier S, Johnson LA, Dobry CJ, et al. Relaxation of imprinted genes in human cancer. Nature. 1993;362:747–748. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa O, Eccles MR, Szeto J, et al. Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms' tumor. Nature. 1993;362:749–751. doi: 10.1038/362749a0. [DOI] [PubMed] [Google Scholar]
- 9.Beckwith JB, Kiviat NB, Bonadio JF. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms tumor. Pediatr Pathol. 1990;10:1–36. doi: 10.3109/15513819009067094. [DOI] [PubMed] [Google Scholar]
- 10.Beckwith JB. Nephrogenic rests and the pathogenesis of Wilms tunor: development and clinical considerations. Am J Med Genet. 1998;79:268–273. doi: 10.1002/(sici)1096-8628(19981002)79:4<268::aid-ajmg7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Breslow N, Beckwith JB. Epidemiological features of Wilms' tumor: Results of the National Wilms' Tumor Study. J Natl Cancer Inst. 1982;68:429–436. [PubMed] [Google Scholar]
- 12.Leisenring WM, Breslow NE, Evans IE, et al. Increased birth weights of National Wilms Tumor Study patients suggest a growth-factor excess. Cancer Res. 1994;54:4680–4683. [PubMed] [Google Scholar]
- 13.Ravenel JD, Broman KW, Perlman EJ, et al. Loss of imprinting of insulin-like growth factor-II (IGF2) gene in distinguishing specific biologic subtypes of Wilms tumor. J Natl Cancer Inst. 2001;93:1698–1703. doi: 10.1093/jnci/93.22.1698. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher V, Schuhen S, Sonner S, et al. Two molecular subgroups of Wilms' tumors with or without WT1 mutations. Clin Cancer Res. 2003;9:2005–2014. [PubMed] [Google Scholar]
- 15.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumor. Cancer. 1978;41:1937–1948. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Silverman BW. Density Estimation for Statistics and Data Analysis. Chapman and Hall; London: 1986. [Google Scholar]
- 17.Cox DR. Regression models and life-tables (with discussion) J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 18.Diller L, Ghahremani M, Morgan J, et al. Constitutional WT1 mutations in Wilms' tumor patients. J Clin Oncol. 1998;16:3634–3640. doi: 10.1200/JCO.1998.16.11.3634. [DOI] [PubMed] [Google Scholar]
- 19.Little SE, Hanks SP, Underwood LK, et al. Frequency and heritability of WT1 mutations in nonsyndromic Wilms' tumor patients: A UK Children's Cancer Study Group. J Clin Oncol. 2004;22:4140–4146. doi: 10.1200/JCO.2004.02.136. [DOI] [PubMed] [Google Scholar]
- 20.Huff V. Wilms tumor genetics. Am J Med Genet. 1998;79:260–267. doi: 10.1002/(sici)1096-8628(19981002)79:4<260::aid-ajmg6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Maiti S, Alam R, Amos CI, Huff V. Frequent association of beta-catenin and WT1 mutations in Wilms tumors. Cancer Res. 2000;60:6288–6292. [PubMed] [Google Scholar]
- 22.Olshan AF. Wilms' tumor, overgrowth, and fetal growth factors: A hypothesis. Cancer Genet Cytogenet. 1986;21:303–307. doi: 10.1016/0165-4608(86)90209-8. [DOI] [PubMed] [Google Scholar]
- 23.Fukuzawa R, Breslow NE, Morison IM, et al. Epigenetic differences between Wilms' tumours in white and east-Asian children. Lancet. 2004;363:446–451. doi: 10.1016/S0140-6736(04)15491-3. [DOI] [PubMed] [Google Scholar]
- 24.Stiller CA, Parkin DM. International variations in the incidence of childhood renal tumours. Br J Cancer. 1990;62:1026–1030. doi: 10.1038/bjc.1990.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow N, Olshan A, Beckwith B, Green DM. Epidemiology of Wilms tumor. Med Pediatr Oncol. 1993;21:172–181. doi: 10.1002/mpo.2950210305. [DOI] [PubMed] [Google Scholar]
- 26.Niemitz EL, Feinberg AP, Brandenburg SA, et al. Children with idiopathic hemihypertrophy and Beckwith-Wiedemann syndrome have different constitutional epigenotypes associated with Wilms tumor. Am J Hum Genet. 2005;77:887–891. doi: 10.1086/497540. [DOI] [PMC free article] [PubMed] [Google Scholar]