Abstract
A cohort of 1022 consecutive singleton births was generated during 1987–1988 in the Faroe Islands, where increased methylmercury exposure occurs from traditional seafood diets that include pilot whale meat. The prenatal exposure level was determined from mercury analyses of cord blood, cord tissue, and maternal hair. At age 14 years, 878 of 1010 living cohort members underwent detailed neurobehavioral examination. Eighteen participants with neurological disorders were excluded. Blood and hair samples obtained from the participants were analyzed for mercury. The neuropsychological test battery was designed based on the same criteria as applied at the examination at age 7 years. Multiple regression analysis was carried out and included adjustment for confounders. Indicators of prenatal methylmercury exposure were significantly associated with deficits in finger tapping speed, reaction time on a continued performance task, and cued naming. Postnatal methylmercury exposure had no discernible effect. These findings are similar to those obtained at age 7 years, and the relative contribution of mercury exposure to the predictive power of the multiple regression models was also similar. An analysis of the test score difference between results at 7 and 14 years suggested that mercury-associated deficits had not changed between the two examinations. In structural equation model analyses, the neuropsychological tests were separated into five groups; methylmercury exposure was significantly associated with deficits in motor, attention, and verbal tests. These findings are supported by independent assessment of neurophysiological outcomes. The effects on brain function associated with prenatal methylmercury exposure therefore appear to be multi-focal and permanent.
Keywords: Adolescent, Food contamination, Methylmercury compounds, Neuropsychological tests, Neurotoxicity syndromes, Prenatal exposure delayed effects
1. Introduction
Methylmercury (MeHg) is a widespread contaminant of seafood and freshwater fish. The developing brain is considered the main target for MeHg toxicity, and the risk to consumers from MeHg exposure has been assessed by national and international agencies [11, 23, 28, 34]. Results from prospective epidemiological studies of birth cohorts from the Faroe Islands [18, 29] have contributed significantly to the data used for derivation of recommendations concerning human exposure limits, especially for pregnant women.
For the purposes of risk assessment, valid dose-effect relationships are essential, and the research in the Faroes has endeavored to represent both ends of the exposure-outcome relationship with the highest possible precision and sensitivity. Because of the need to obtain precise measures of the causative exposure [7, 14], the mercury concentration has been measured in cord blood and other biological samples from exposed participants. Detailed comparison of various prenatal exposure indices within the cohort has shown that the cord-blood concentration is consistently the most precise predictor of nervous system deficits determined during postnatal follow-up [7, 14].
At the same time, valid outcome variables must be sensitive to MeHg neurotoxicity and relatively robust to impacts of confounders. In choosing feasible effect parameters, an important consideration includes suitability for the age group and culture. As confounder-independent outcomes, neurophysiological measurements have provided evidence that developmental MeHg neurotoxicity is detectable through age 14 years [26]. Clinical neurological tests have shown mercury-related adverse effects neonatally [29], but such tests may not be sensitive enough at school age [18]. Standardized neuropsychological tests are useful to gain insight into functional domains and overall cognitive functioning and have been widely applied as sensitive indicators of central nervous system (CNS) dysfunction associated with exposure to neurotoxicants in both environmental and occupational exposure settings [9,39]. In choosing such measures for the study of children, it is important to consider the developmental stage of the child at both exposure and at the time of testing as well as cultural and psychometric parameters of the tests being administered [2,39].
Risk assessments have so far been based on functional outcomes up to early school age obtained in three prospective epidemiological studies [11, 23, 28, 34]. Data from older children and adolescents have been available only from less informative cross-sectional studies. Developmental exposure to inorganic lead is known to cause permanent CNS damage, thus suggesting that deficits attributable to early developmental neurotoxicant exposure may be irreversible [2, 40]. However, long-term effects of prenatal and early childhood exposures to neurotoxicants may become more difficult to demonstrate with time, because an increasing number of other factors play a role in test performance and it may be difficult, if not impossible, to define and measure these covariates. Thus, the increasing complexity of confounder adjustment may cause problems in the statistical analysis of cognitive test data. Despite these potential problems, prospective assessment of cohorts with well defined exposures occurring early in development is essential to understanding how neuropsychological outcome measures can be utilized as long-term indicators of early neurotoxicant insults.
We have prospectively followed a birth cohort from the Faroe Islands for 14 years. The Faroes are located in the North Atlantic between Norway, Shetland, and Iceland. In this Nordic fishing community, excess exposure to MeHg is mainly due to the traditional habit of eating meat from the pilot whale, while baseline exposures are due to frequent intake of other types of seafood that contains lower MeHg concentrations [38]. In a study conducted when the cohort children were 7 years of age, the main finding was that decrements in specific functional domains were associated with prenatal MeHg exposure [18]. Tests assessing the domains of attention, language, and verbal memory showed the most robust effects, while measures of motor speed and visuospatial function showed less consistent effects. Among several measures of prenatal and post-natal exposure, the strongest associations were obtained with the cord-blood mercury concentration as the exposure indicator [17–19]. These findings were robust in analyses controlled for age, sex and confounders, and they persisted after exclusion of high-exposure participants (i.e., maternal hair-mercury concentrations above 10 μg/g) [18]. Ingestion of whale blubber causes exposure to lipophilic contaminants, notably polychlorinated biphenyls (PCBs), but the possible neurotoxic influence of this exposure did not explain the MeHg-associated neurobehavioral deficits [6, 16, 29]. The examination at age 14 years that is described in this paper included a clinical test battery similar to the one applied at age 7 years.
2. Materials and methods
The cohort was assembled in the Faroe Islands during a 21-month period in 1986–1987 [13]. The primary indicator of intrauterine exposure to MeHg was the mercury concentration in cord blood, and concentrations in maternal hair at parturition were also determined. Subsequently, mercury concentrations were measured in stored cord tissue (dry weight) from about half of the cohort members examined [16]. MeHg exposure was found to vary considerably: 15% of the mothers had hair-mercury concentrations above 10 μg/g, while 4% were below 1 μg/g, a level that corresponds to the exposure limit recommended by the U.S. Environmental Protection Agency [28, 34]. Exposure to PCBs was assessed by analyzing cord tissue from 435 of the cohort subjects for major PCB congeners [16]. The frequency of maternal intake of fish and of pilot whale meat was available from a questionnaire administered shortly after parturition [13]. Cohort members were first invited for detailed examination at school age (7 years), when a total of 917 of eligible children (90.3%) participated [18].
At age 14 years, a total of 878 of 1,010 living cohort members (86.9%) were examined. The 438 boys and 440 girls examined had an average age of 13.83 (S.D., 0.32) years. Most examinations took place at the National Hospital in Tórshavn, the capital of the Faroe Islands, during April–June of 2000 and 2001. For families who had moved to Denmark, examinations were also offered in Odense in November, 2000. Transportation costs and other expenses were reimbursed in accordance with rates approved by the Faroese ethical review committee. Each day, four participants were examined during the morning and four during the afternoon. Each group of participants rotated between four stations staffed by health professionals, who administered the clinical tests, and who had no access to information on individual exposure levels. These stations each lasted approximately one hour each and covered physical examination, neurophysiological tests, and two series of neuropsychological tests, each of which was administered by the same examiner in the same sequence throughout.
The thorough pediatric examination included otoscopy and assessment of neurological function. None of the adolescents had current middle ear infection. A total of 18 participants examined had neurological disorders thought to be independent of MeHg exposure and were excluded from the data analysis. The MeHg exposure of these participants did not differ from that of other cohort members. Of 860 included participants that completed the 14-year examination schedule, 815 were examined also at age 7 years.
Blood specimens and hair samples were again obtained, and the proximal 2-cm hair segment was used for mercury analysis [26]. Detailed quality assurance data showed acceptable analyses for both exposure biomarkers. Results in μg may be converted to nmol by multiplying by 5.0.
The study protocol was approved by the ethical review committee for the Faroe Islands and the U.S. institutional review boards, and written informed consent was obtained from all parents.
2.1 Neuropsychological examination
The test battery was designed based on the criteria previously described [39] as applied at the 7-year examinations [18]. Because some of the tests used at age 7 were less feasible than desired and because other tests were thought to be appropriate for 14-year-old adolescents, some changes in the battery were made. Table 1 shows the tests used at the two examinations. Two Faroese-speaking board-certified psychologists administered the tests, each administering the same tests in the same sequence. Because test descriptions have been previously published, only the changes from the previous battery [18] will be outlined here.
Table 1.
Neuropsychological tests administered to cohort members at ages 7 and 14
| Main functional domain | Test name | 7 years | 14 years |
|---|---|---|---|
| Motor | NES2 Finger tapping, both hands | * | * |
| NES2 Finger tapping, preferred hand | * | * | |
| NES2 Finger tapping, non-preferred hand | * | * | |
| NES2 Hand-eye coordination | * | ||
| CATSYS mean finger tapping speed | * | ||
| CATSYS supination-pronation speed | * | ||
| Attention | NES2 CPT average reaction time# | * | * |
| NES2 CPT number of false negatives | * | * | |
| NES2 CPT number of false positives | * | ||
| WISC-R Digit Span | * | * | |
| WMS-III Spatial Span | * | ||
| CATSYS mean simple reaction time | * | ||
| Working Memory/Executive Function | WISC-R Similarities | * | * |
| Children’s Category Test | * | ||
| Language | Boston Naming Test: | ||
| Number correct without cues | * | * | |
| Number correct with cues | * | * | |
| Visuospatial | Block Design,WISC-R only | * | * |
| Block Design, WISC+WAIS | * | ||
| Bender Gestalt Test | * | ||
| Tactual Performance Test | * | ||
| Stanford-Binet Copying Test | |||
| Total score | * | ||
| Score for most difficult five designs | * | ||
| Memory | California Verbal Learning Test | * | * |
| Tactual Performance Test, recall | * | ||
| Stanford-Binet Copying Test, recall | * | ||
| Bender Gestalt Test, recall | * | ||
| Mood States | Nonverbal Analogue Profile of Mood States | * |
Duration 4 minutes at 7 years and 10 minutes at 14 years, with reaction times averaged over the last 3 and last 6 minutes, respectively.
Neuropsychological Examination System (NES2) Continuous Performance Test (CPT) [25]: The total duration of the sustained attention test was increased to 10 minutes, and the inter-stimulus interval was reduced to 1000 ms as age-appropriate adjustments.
The Catsys® equipment (Danish Product Development, 3070 Snekkersten, Denmark) allows electronic assessment of simple reaction time using joint auditory and visual stimuli, as well as tapping speed, and speed of pronation-supination of the forearm [10].
Wechsler Intelligence Scale for Children – Revised (WISC-R) Block Designs: This visuospatial test has been standardized in the US up to age 17 years [35] and a translated Norwegian version up to 16 years [33]. However, inspection of our data from the first year of testing showed a noticeable ceiling effect. To overcome this problem, we added the last three and most difficult test items from the Wechsler Adult Intelligence Scale – Revised (WAIS-R) version of the test [36]. The usual discontinuation criteria and scoring rules for bonus points were applied.
Stanford-Binet Copying [31]: Because this test had been successfully used in the field as an outcome parameter in MeHg-exposed children [20] and the scoring system is simpler, it replaced the Bender Visual Motor Gestalt Test, which was used as a measure of visual constructional abilities in the 7-year examinations. Administration of all 16 designs allowed equal numbers of test trials for all participants. Based on the manual [31], the last five (most difficult) items were identified as the most applicable to this group of participants given their age. Using these five items eliminated the noise from random errors on easier items. The outcome measures therefore included the total score for the full set items (16 designs) and the score for the last five items. As in our previous research involving other cohorts [20], an immediate memory condition was added as a measure of short-term memory for visuospatial material. This condition was administered immediately after the subject drew the 16 designs, with the instruction to draw all of the designs the subject could recall. The score for the number of correct designs drawn from memory was based on more lenient criteria, in which each drawing was scored as correct if the design was clearly recognizable, with no omissions of figure elements or gross distortions of proportions that would alter the perception of the gestalt [30].
Children’s Category Test [3]: Level 2 of this test consists of 6 subtests and 83 items. The nonverbal stimulus material is designed to assess components of working memory, including concept formation, capacity to adopt strategies and flexibly invoke new strategies in problem solving, and the ability to benefit from experience. The subject receives immediate feedback as to the correctness of each response. The task does not require verbal or complex motor responses and is untimed. Score is total number of errors.
Wechsler Memory Scale - III (WMS-III) Spatial Span [37]: For this task, the examiner taps with a finger a sequence on the blue blocks on a white form board. The subject has to repeat the demonstrated sequence. Series of increasing length are presented until the subject fails on both trials in a series of the same length. The series are administered first in forward and then in backward order. Score is the number of correct series repeated forward and backward. This test provides a visual analogue for WISC-R Digit Span. Both tests assess attention (forward spans) and working memory/executive function (backward spans).
2.2 Data analysis
Following descriptive analyses of the data, two approaches were taken to data analysis. First, multiple regression analysis was carried out as in the initial analyses of the 7-year data [18], and structural equation models were then developed, where test results were analyzed in larger, multi-test groupings [6].
The regression analysis was used to determine the associations between individual test outcomes, the cord-blood mercury concentrations and potential covariates. The covariates were selected to approximate the regression model used for analysis of the 7-year data set [8, 18]. Major obligatory confounders were therefore age, sex, maternal Raven score, and residence in town/village. Likewise, both maternal and paternal employment was again included, as was the time of the day of the examination (morning/afternoon), the language used (Faroese/Danish), and computer game experience (little/some/much; for computer-assisted tests only). Instead of the 7-year variable representing childcare before school entry, we used the participant’s grade in school.
An extended set of covariates was also examined, although some variables were available only for cohort members participating in the 7-year examinations (i.e., medical risk factors, maternal and paternal professional training, all of them included at age 7 years). These covariates also included family structure (whether the participant was living with both parents) and history of ever having tried tobacco smoking or alcoholic beverages. The participant’s school was rated as primary (up to 7th grade) or secondary (grades beyond 7th). The average Tanner score was obtained from the pediatric examination. All of these parameters showed only weak associations with MeHg exposures. A disadvantage of these supplementary analyses was that information was missing from several participants, thus affecting the statistical power of regression analyses that rely on complete cases. Additional analyses also incorporated the PCB exposure [16] and postnatal MeHg exposure parameters [26] as explanatory variables.
Contaminant concentrations entered the model after logarithmic transformation and the mercury regression coefficients were calculated to correspond to the change in the dependent variable associated with a doubling in MeHg exposure. Thus, these models assume a linear effect of log transformed mercury concentration on the neurobehavioral outcomes. We used generalized additive models to assess whether that assumption was appropriate, because these models do not require linearity assumptions while providing a smooth nonparametric dose-response curve [21]. The regression coefficients were standardized by expressing them as percent of the standard deviation of the unadjusted outcome parameter. Some outcomes were transformed to obtain normally distributed residuals, but these transformations did not influence the significance of the mercury effect, and results of the regression analysis are therefore presented for the non-transformed data. However, transformed data were used for structural equation models (see below). The p values reported are two-sided, and for one-sided hypothesis testing these results should be divided by two.
The results from participants who were examined both at age 7 years and 14 years were further examined using summary statistics to determine whether the mercury effect had changed between the two examinations. In one approach, the difference between the two scores was used as the dependent variable and related to the prenatal MeHg exposure after confounder correction. As the test score difference may depend on the initial test performance level, a supplementary analysis was conducted with the log-transformed relative increase as the dependent variable. In these analyses, the set of covariates were supplemented so that it also included all potential confounders previously considered in the analysis of the 7 year data [18].
Because multiple regression analyses may not adequately address problems with exposure imprecision, multiple testing and missing data, structural equation models were applied in order to address these issues [6]. These models incorporate two parts – measurement and structure. In the measurement part, the observed variables are considered to be manifestations of a smaller number of latent variables. The structural model then describes the relationship between the latent variables, possibly after adjustment for the effects of covariates. By including three independent measures of exposure (mercury in cord blood, mercury in maternal hair, and frequency of whale meat dinners during pregnancy), a latent exposure variable is generated that is likely to provide a closer approximation of true exposure than individual exposure measures considered alone [6].
Likewise, the neurobehavioral outcome variables were collected into a small number of latent variables determined from factor structure (Frodi Debes, unpublished results) and consistency with response modalities. According to this approach, test scores were assumed to reflect five broad functional groupings. These groupings are therefore empirically based and do not necessarily reflect functional domains typically used in neuropsychology. In order to include a sufficient number of tests in each group, outcomes were allowed to occur in more than one grouping. Motor outcomes included the three NES2 finger tapping scores, CATSYS supination/pronation score, CATSYS maximum finger tapping score. Attention outcomes were Digit Spans, Spatial Span, CATSYS mean reaction time, NES2-CPT mean reaction time, number of false responses, and number of missed responses. Spatial outcomes were the Children's category test, WISC-R Block Design, WISC+WAIS Block Designs, Copying total score, the Copying score for the last five designs, and number correctly recalled. Verbal outcomes comprised the two naming scores, the four California Verbal Learning Test (CVLT) scores, and the Similarities score. The memory outcomes were the four CVLT scores and the Copying recall score.
A separate model was developed for each of the neurobehavioral groupings considered as latent functions, and the mercury effect was expressed as percent of the standard deviation of the latent function result. The potential confounders previously considered in the primary regression model entered the structural equation model as covariates. Thus, we estimated the effect of a one-unit increase of the exposure after controlling for the potential confounders.
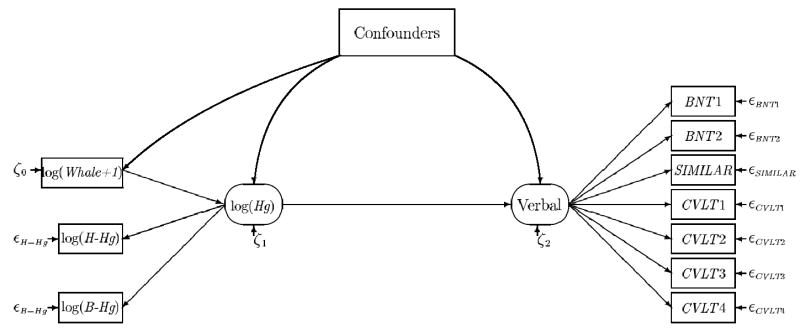
Because seafood also contains important nutrients, we conducted supplementary analyses, where maternal fish intake during pregnancy (number of fish dinners per week during pregnancy [13]) was included as a covariate that was allowed to affect both the MeHg exposure and the latent outcomes. Fig. 1 shows a path diagram that illustrates the initial model for verbal outcomes; similar model structures were developed for the four other sets of functional groupings.
Fig. 1.
Path diagram of the structural equation model that was used to determine the overall effect of prenatal methylmercury exposure on verbal functions, as expressed by a series of verbally mediated neuropsychological test results, and adjusted for confounders (H-Hg = maternal hair-mercury concentration; B-Hg = cord-blood mercury concentration; Whale = frequency of whale meat dinners during pregnancy; BNT = Boston Naming Test without and with cues; SIMILAR = WISC-R Similarities; and CVLT = California Verbal Learning Test scores for learning, short-term recall, long-term recall, and recognition).
The fit of these models was assessed in likelihood ratio testing against a more flexible model, which did not put any restrictions on the covariance matrix of the observed variables (known as the unrestricted model). If this test is rejected, then the proposed structural equation model does not fit the data as well as the unrestricted model. Models with a poor fit were modified until an acceptable fit was obtained by allowing for local dependence and differential item functioning [4,6]. Two outcomes are said to be locally dependent if the correlation between them cannot fully be explained by the underlying neurobehavioral function. Local dependence may arise because tasks resemble each other, and sub-scores on the same test may, therefore, show local dependence. In the present analysis, local dependence was anticipated for the two naming scores, the four verbal learning scores, the continuous performance scores, and the two copying scores, because tasks from the same test are interrelated, so that the scores are likely to be correlated even for participants with the same latent ability. Thus, by adjustment for local dependence, double-counting is avoided when highly correlated outcomes are included in the same model [6]. Furthermore, an initial model did not allow for so-called differential item function and therefore assumes that the covariates affect the observed outcomes only through the latent outcome. This means that, except for scale differences, a one-unit increase in a given covariate is assumed to lead to the same increase in all outcomes representing the same latent variable [6]. The appropriateness of this assumption was tested by also allowing covariates to affect the test scores directly.
3. Results
The prenatal MeHg exposures of the participants of the 14-year examinations (Table 2) were similar to those of the cohort as a whole [13]. Exposure levels at age 14 years averaged about one-fifth of those experienced prenatally, although exposures at age 7 years were slightly higher [26].
Table 2.
Results of mercury analyses for 860 members of a Faroese birth cohort examined at age 14 years
| Exposure biomarker | n | Geometric average | Interquartile range | Total range |
|---|---|---|---|---|
| Prenatal exposure | ||||
| Cord blood (μg/L) | 838 | 22.5 | 13.1-40.8 | 0.90-351 |
| Maternal hair (μg/g) | 858 | 4.21 | 2.53-7.66 | 0.17-39.1 |
| Exposure at age 7 years | ||||
| Blood (μg/L) | 606 | 9.00 | 5.00-18.4 | 0.30-63.0 |
| Hair (μg/g) | 803 | 2.99 | 1.71-6.20 | 0.04-37.6 |
| Exposure at age 14 years | ||||
| Blood (μg/L) | 779 | 4.08 | 2.29-7.46 | 0.26-39.9 |
| Hair (μg/g) | 842 | 0.96 | 0.45-2.29 | 0.02-9.65 |
Table 3 shows associations with potential confounders. Continuous variables were trichotomized for the purpose of this table only. The expected pattern of associations reflects dietary habits and local differences in availability of whale meat during 1986–1987. Accordingly, participants from fishing villages had higher prenatal exposures than those living in towns or in Denmark. Participants tested in Denmark or in the Danish language had lower exposure. Lower exposure was also associated with a higher maternal Raven score. Adolescents from smaller primary schools had higher exposure, as had participants examined in the afternoon, at an older age, and those living with both parents.
Table 3.
Geometric averages and interquartile ranges (25th–75th percentiles) for cord-blood mercury concentrations (μg/l) in relation to potential predictors of neurobehavioral performance
| Covariate | N | Geometric Mean | Interquartile Range | P | |
|---|---|---|---|---|---|
| Sex | Boy | 417 | 23.5 | 13.9 - 41.5 | 0.14 |
| Girl | 421 | 21.6 | 12.6 - 39.4 | ||
| Age (years) | <13.7 | 324 | 20.7 | 13.0 - 35.5 | 0.0040 |
| 13.7-14.0 | 243 | 23.3 | 12.4 - 42.6 | ||
| >14.0 | 271 | 24.2 | 14.3 - 43.1 | ||
| Maternal Raven score | <44 | 318 | 25.1 | 13.3 – 47.0 | 0.0032 |
| 44-49 | 277 | 21.9 | 13.4 - 37.4 | ||
| >49 | 243 | 20.2 | 12.0 - 38.3 | ||
| Maternal fish dinners per week | 0-2 | 439 | 20.3 | 12.0 - 36.2 | <0.001 |
| >2 | 398 | 25.3 | 14.6 - 43.0 | ||
| Father employed | No | 55 | 24.8 | 13.9 – 51.0 | 0.39 |
| Yes | 768 | 22.4 | 13.2 - 40.5 | ||
| Mother employed | No | 162 | 23.0 | 13.8 – 38.8 | 0.69 |
| Yes | 671 | 22.4 | 13.1 – 41.0 | ||
| Mother professional training | No | 415 | 24.0 | 13.5 - 41.7 | 0.081 |
| Yes | 374 | 21.7 | 13.1 – 39.8 | ||
| Father professional training | No | 236 | 22.9 | 14.2 – 37.2 | 0.98 |
| Yes | 554 | 22.9 | 13.3 – 42.9 | ||
| Living with both parents | Yes | 692 | 23.3 | 13.8 - 41.4 | 0.012 |
| No | 134 | 19.0 | 11.2 - 35.9 | ||
| Tested in language | Faroese | 774 | 23.5 | 13.8 – 40.9 | <0.0001 |
| Danish | 64 | 14.1 | 6.7 - 36.7 | ||
| Residence | Villages | 386 | 27.6 | 17.7 - 48.8 | <0.0001 |
| Towns | 355 | 20.0 | 11.0 – 34.9 | ||
| Denmark | 96 | 15.5 | 7.7 - 34.5 | ||
| Time of day tested | Morning | 426 | 20.4 | 11.6 - 35.8 | 0.0003 |
| Afternoon | 410 | 25.1 | 14.2 - 45.5 | ||
| Smoking | No | 751 | 22.2 | 13.1 - 39.4 | 0.0409 |
| Yes | 73 | 27.4 | 13.9 - 52.8 | ||
| Alcohol | No | 789 | 22.4 | 13.1 - 40.5 | 0.22 |
| Yes | 35 | 26.8 | 16.8 – 47.7 | ||
| Computer game experience | No | 545 | 22.7 | 13.2 - 41.5 | 0.80 |
| Yes | 292 | 22.4 | 13.3 – 38.9 | ||
| Computer experience | Much | 742 | 22.5 | 13.0 - 40.8 | 0.78 |
| Some | 95 | 23.1 | 14.2 - 40.8 | ||
| School grade | 6th | 58 | 18.5 | 10.6 - 32.5 | 0.11 |
| 7th | 723 | 23.0 | 13.3 - 41.7 | ||
| 8th | 56 | 20.5 | 11.6 - 34.5 | ||
| Secondary school | No | 483 | 23.3 | 13.8 - 39.5 | 0.19 |
| Yes | 352 | 21.5 | 11.8- 43.0 | ||
| Tanner score | <2 | 148 | 22.6 | 14.2 - 40.2 | 0.78 |
| 2-2.5 | 298 | 22.2 | 12.0 - 41.5 | ||
| >2.5 | 342 | 23.3 | 14.0 - 41.7 |
The neuropsychological test results were generally within expected limits, based on Scandinavian and similar norms for this age range (Table 4). However, the Faroese adolescents performed above expectation given available norms for Block Designs, with a marked ceiling effect during the first year of examinations. Adding the three last designs from the adult version (WAIS-R) corrected for this imperfection in resolution at the high end, but was available for less than half of the participants.
Table 4.
Raw scores for neurobehavioral function tests administered at age 14 years.
| Test | N | Mean ± S.D. | Interquartile range | Total range |
|---|---|---|---|---|
| NES2 Finger Tapping | ||||
| preferred hand | 856 | 73.7 + 7.25 | 69 - 78 | 40 - 98 |
| Non-preferred hand | 856 | 66.1 + 7.17 | 62 - 71 | 43 - 92 |
| Both hands | 855 | 99.1 + 15.9 | 88 - 110 | 36 - 139 |
| NES2 Continuous Performance Test | ||||
| number of missed responses | 859 | 2.02 + 2.75 | 0 - 3 | 0 - 18 |
| number of false alarms | 859 | 3.93 + 3.21 | 2 – 5 | 0 - 28 |
| Average reaction time (ms) | 859 | 497 + 45.5 | 464 – 527 | 365 - 647 |
| CATSYS | ||||
| mean tapping maximum | 857 | 4.93 + 1.08 | 4.20 – 5.65 | 1.6 - 7.5 |
| supination-pronation | 856 | 4.96 + 1.11 | 4.15 - 5.80 | 2.15 - 7.50 |
| mean simple reaction time | 856 | 249 + 41.5 | 219 – 271 | 153 - 452 |
| WISC-R Digit Spans | 860 | 11.6 + 2.5 | 10 - 13 | 5 – 21 |
| Spatial Span | 860 | 16.8 + 2.8 | 15 - 19 | 6 – 25 |
| Children’s Category Test | 860 | 15.7 + 7.7 | 10 - 20 | 1 – 43 |
| Block Designs, WISC-R | 860 | 45.6 + 10.9 | 39 - 54 | 3 – 62 |
| Block Designs, WISC+WAIS | 309 | 51.6 + 16.3 | 41 - 64 | 6 – 83 |
| Stanford-Binet Copying test | ||||
| total copying score | 854 | 8.88 + 3.06 | 7 - 11 | 1 – 16 |
| score for five most difficult | 854 | 1.88 + 1.38 | 1 - 3 | 0 - 5 |
| Recalled number correct | 851 | 7.71 + 2.11 | 6 - 9 | 0 - 15 |
| WISC-R Similarities | 860 | 16.4 + 3.44 | 14 - 19 | 2 – 27 |
| Boston Naming Test | ||||
| number correct without cues | 859 | 40.6 + 5.6 | 37 - 44 | 21 - 58 |
| total number correct with cues | 859 | 44.9 + 5.2 | 42 - 48 | 22 - 60 |
| California Verbal Learning Test | ||||
| Learning, total correct | 859 | 50.6 + 7.8 | 45 - 56 | 25 - 68 |
| short delay, free recall | 859 | 10.9 + 2.4 | 9 - 13 | 1 - 15 |
| long delay, free recall | 858 | 11.2 + 2.2 | 10 - 13 | 3 - 15 |
| long delay, recognition | 857 | 14.3 + 1.0 | 14 - 15 | 7 - 15 |
All multiple regression analyses were based on complete cases (Table 5). Thus, the number of participants included depended upon the choice of exposure biomarker and confounders. Higher prenatal MeHg exposure was associated with lower finger tapping scores, increased reaction time, and lower cued naming scores; the cord-blood mercury concentration was a significant predictor of outcomes of all three types. The maternal hair mercury concentration showed significant or near-significant associations with deficits only on the three conditions of finger tapping, and the two measures of reaction time. The cord tissue mercury concentration showed no clear relation to finger tapping and reaction time results, but was associated with deficits on the naming and verbal learning results. One surprising find was that higher MeHg exposure was associated with better scores on WMS-III Spatial Span.
Table 5.
Test score change in percent of test score standard deviation associated with doubling in exposure, as indicated by multiple regression analysis with adjustment for covariates.a
|
Cord blood |
Maternal hair |
Cord tissue |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Test | N | Change | P | N | Change | P | N | Change | P |
| NES2 Finger Tapping | |||||||||
| Preferred hand | 758 | −4.18 | 0.16 | 776 | −6.01 | 0.052 | 379 | −5.43 | 0.19 |
| non-preferred hand | 758 | −4.15 | 0.16 | 776 | −7.15 | 0.020 | 380 | −5.36 | 0.23 |
| both hands | 757 | −6.41 | 0.033 | 775 | −7.22 | 0.022 | 379 | −6.42 | 0.17 |
| CATSYS | |||||||||
| mean tapping maximum | 759 | −1.59 | 0.61 | 777 | −0.21 | 0.95 | 380 | −8.35 | 0.08
7 |
| supination-pronation | 758 | −2.83 | 0.36 | 776 | −1.91 | 0.56 | 380 | −2.16 | 0.66 |
| mean reaction time (ms)b | 758 | 3.96 | 0.19 | 776 | 5.97 | 0.062 | 379 | 4.89 | 0.29 |
| NES2 Continuous Performance Test | |||||||||
| average reaction time (ms) b | 761 | 7.44 | 0.018 | 779 | 6.30 | 0.055 | 381 | 2.35 | 0.63 |
| number of false positives b | 761 | −0.97 | 0.75 | 779 | −1.18 | 0.71 | 381 | −5.98 | 0.20 |
| number of false negatives b | 761 | 0.72 | 0.82 | 779 | −1.95 | 0.55 | 381 | −6.66 | 0.16 |
| Digit Spans | 761 | 0.22 | 0.94 | 779 | −0.96 | 0.76 | 381 | 0.35 | 0.94 |
| Spatial Span | 761 | 7.25 | 0.019 | 779 | 7.51 | 0.021 | 381 | 4.48 | 0.37 |
| Stanford-Binet Copying | |||||||||
| total copying score | 757 | −2.99 | 0.33 | 775 | −4.12 | 0.20 | 378 | −0.62 | 0.90 |
| sum of most difficult 5 | 757 | −5.89 | 0.056 | 775 | −6.19 | 0.055 | 378 | −5.09 | 0.28 |
| recall number correct | 755 | 0.62 | 0.84 | 773 | 3.04 | 0.35 | 378 | −3.36 | 0.48 |
| Block Design, WISC-R | 761 | 3.65 | 0.22 | 779 | 2.96 | 0.38 | 381 | 6.94 | 0.15 |
| Block Design, WISC+WAIS | 276 | 3.46 | 0.48 | 283 | 6.29 | 0.24 | 214 | 9.76 | 0.11 |
| Children’s Category Test b | 761 | 0.49 | 0.87 | 779 | 1.98 | 0.53 | 381 | −0.70 | 0.88 |
| Similarities | 761 | −0.38 | 0.90 | 779 | 2.01 | 0.52 | 381 | 1.76 | 0.69 |
| Boston Naming Test | |||||||||
| correct without cue | 761 | −4.79 | 0.11 | 779 | −3.39 | 0.28 | 381 | −6.71 | 0.14 |
| total correct w/cues | 761 | −5.90 | 0.048 | 779 | −4.21 | 0.18 | 381 | −9.75 | 0.03
6 |
| California Verbal Learning Test | |||||||||
| learning, total correct, 5 trials | 761 | −2.84 | 0.34 | 779 | 0.61 | 0.84 | 381 | −9.01 | 0.04
4 |
| short delay, free recall | 761 | 1.45 | 0.63 | 779 | 4.79 | 0.13 | 381 | −4.33 | 0.35 |
| long delay, free recall | 760 | −0.42 | 0.89 | 778 | 2.42 | 0.45 | 380 | −7.43 | 0.11 |
| long delay, recognition | 759 | −2.94 | 0.35 | 777 | 0.08 | 0.98 | 379 | −6.01 | 0.19 |
Age, sex, maternal Raven score, residence in town/village, school grade, maternal and paternal employment, time of day, language, and computer experience (for computer tests only).
An increase in the test parameter denotes a deficit.
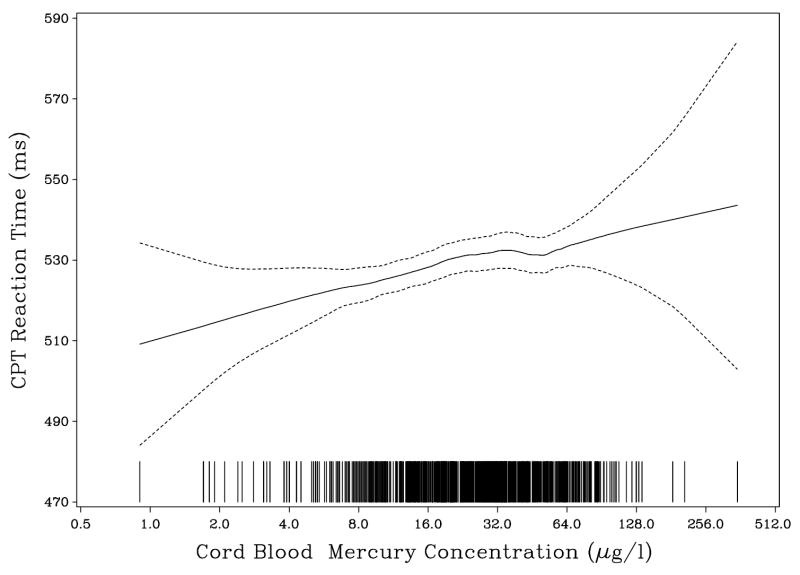
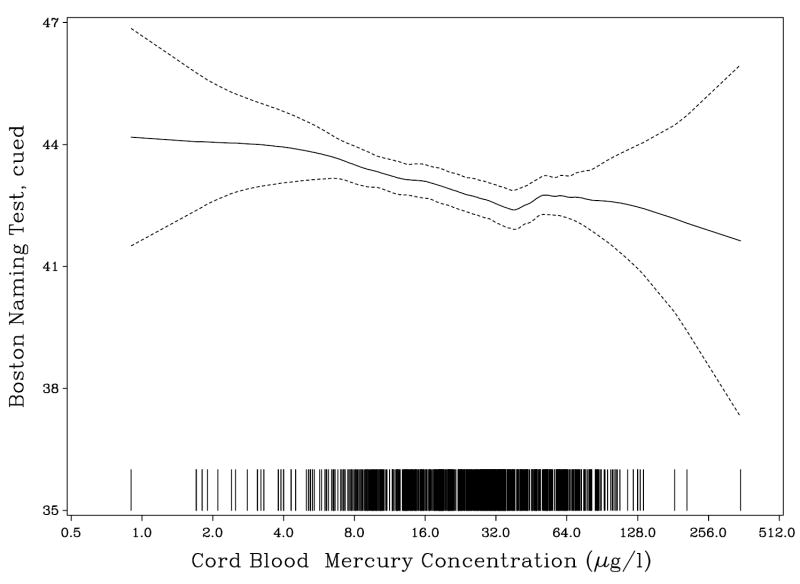
The estimated mercury effects remained virtually unchanged after adjustment for the extended set of confounders, except that, in this reduced data set, the p-value for the effect on the cued naming score was slightly above a 5% significance level. Some outcomes were transformed to obtain normally distributed residuals, but this change barely affected the mercury effects. Table 5 therefore shows the effect on the untransformed variables. The logarithmic transformation of the exposure variables assume a linear effect of each doubling of the exposure. The appropriateness of this assumption was confirmed in non-parametric models (Figs 2 and 3).
Fig. 2.
Association between prenatal methylmercury exposure and the average reaction time (ms) on the NES2 Continuous Performance Test administered to 859 participants from a Faroese birth cohort at age 14 years. Each line at the bottom represents one observation at the exposure level indicated.
Fig. 3.
Association between prenatal methylmercury exposure and the total correct score on the Boston Naming Test after cues administered to 859 participants from a Faroese birth cohort at age 14 years. Each line at the bottom represents one observation at the exposure level indicated.
In contrast to the prenatal exposure variables, markers of postnatal exposures were generally only weakly related to cognitive test scores at 14 years, with many coefficients suggesting effects in the direction opposite to expectation. Only one significant association was seen, i.e., for the hair mercury concentration at age 14 years on the NES2 finger tapping score on the preferred hand (effect, −5.18; p = 0.019). This effect appeared less clear when corrected for the full set of confounders (effect, −3.18; p = 0.17) and decreased further when adjusted for the prenatal exposure level (effect, −2.74; p = 0.26). On the other hand, prenatal effects changed only slightly after adjustments for postnatal exposure markers. However, due to limited power in a regression model with both prenatal and postnatal exposure variables, the p-values generally increased slightly. Thus, the p values for the three outcomes significantly associated with the cord blood mercury concentration without adjustment for postnatal exposure increased to levels ranging from 0.061 (CPT reaction time) to 0.11 (finger tapping), after adjustment for postnatal exposure. On the other hand, the Copying test (5 most difficult designs) now showed a significant p-value of 0.03 for the effect of prenatal exposure effects after adjustment for postnatal exposure.
Likewise, PCB exposure, although only available for half of the subjects, showed only weak associations with the outcomes, none of them reaching statistical significance. The p values for the three outcomes showing significant deficits associated with the cord blood mercury concentration (Table 5) varied from 0.15 (CPT reaction time) to 0.98 (finger tapping with both hands).
In general, a high percentage of the outcome variation could not be explained in the regression models and most covariate associations were weaker than at age 7 years [18]. Age was not a general predictor of test performance at this developmental stage and within the limited age range covered, and was only weakly related to scores on verbal abstraction, attention, and manual motor speed tasks. Sex was a stronger predictor of test performance, where boys performed faster on tasks assessing manual motor speed, while girls earned higher scores on most other tasks. No sex difference was seen in vocabulary, nonverbal abstraction, and visuospatial memory. The maternal Raven score was again an important predictor for most outcomes. Residence in towns was positively associated with naming ability and, to some degree, with other abilities. Computer experience was associated with better performance on the continuous performance tasks and finger tapping. However, the full complement of predictors explained only about 10% of the total variation (Table 6). Although prenatal MeHg exposure seemed a less important predictor of neuropsychological performance at age 14 than at age 7 years, this risk factor appeared to represent about the same proportion of the total variance explained by the regression model at the two occasions.
Table 6.
Percent of variance explained by the multiple regression models for the outcome variables that showed statistically significant negative associations with cord blood mercury exposure at 14 years of age.a
|
Full model |
Mercury |
Percent of total |
||||
|---|---|---|---|---|---|---|
| Test | 7y | 14y | 7y | 14y | 7y | 14y |
| NES2 Finger Tapping | ||||||
| both hands | 9.6 | 8.2 | 0.3 | 0.6 | 3.4 | 7.7 |
| NES2 Continuous Performance Test | ||||||
| average reaction time (ms) | 16.2 | 5.6 | 3.0 | 0.6 | 18.7 | 11.6 |
| Boston Naming Test | ||||||
| total number correct with cues | 18.9 | 10.0 | 1.4 | 0.5 | 7.5 | 4.7 |
Results (R2) are indicated for the full regression model with all covariates, and the partial result for the cord-blood mercury concentration alone, and the latter as percent of the former, at age 7 and 14 years.
Assuming that prenatal MeHg neurotoxicity causes permanent changes, the mercury-associated effects observed at age 14 years could represent the deficits previously observed at age 7 years, as modified by learning and maturation. An analysis was therefore conducted based on outcomes available from both examinations and with a significant mercury effect at age 7 or 14 years (Table 7). For most outcomes, prenatal MeHg exposure showed only weak effects on the test score difference. The sign of the coefficients generally suggested that highly exposed participants may have been able to compensate in part for deficits seen at age 7 years. However, this effect was weak and only statistically significant for one of the CVLT scores. For two finger tapping scores (non-preferred hand and both hands), this analysis indicated that, between ages 7 and 14 years, highly exposed participants had fallen even further behind those less exposed, but again this tendency was not statistically significant. A supplementary analysis of the relative difference in test scores yielded similar results, but these models provided a poorer fit to the data.
Table 7.
Effect (beta) of 10-fold increase in the prenatal MeHg exposure on the change in scores of neuropsychological tests first applied at age 7 years and repeated at 14 years, after adjustment for covariates.
| Test | Beta | P |
|---|---|---|
| NES2 Finger Tapping | ||
| preferred hand | 0.342 | 0.66 |
| non-preferred | −0.308 | 0.64 |
| both hands | −2.35 | 0.16 |
| NES2 Continuous Performance Test | ||
| average reaction timea | −17.1 | 0.093 |
| number of false negativesa | −1.55 | 0.11 |
| Digit Span Forward | 0.078 | 0.60 |
| Boston Naming Test | ||
| number correct without cues | 0.621 | 0.20 |
| total correct with cues | 0.352 | 0.46 |
| California Verbal Learning Test | ||
| learning, total correct, 5 trials | 0.144 | 0.88 |
| short delay, free recall | 0.652 | 0.046 |
| long delay, free recall | 0.444 | 0.19 |
| long delay, recognition | 0.247 | 0.30 |
An increase in the test parameter denotes a deficit.
To take into account multiple statistical testing, exposure imprecision and missing data, we then applied structural equation models. The crude, initial models fitted the data poorly, but after adjustment for local dependence and differential item functioning, models with an acceptable fit were obtained for all five test groups (Table 8). The strongest mercury effect was found in regard to the motor and attention groupings. The effect for the verbal grouping is close to being significant at the 5% level, while the effect on spatial and memory groups is far from significant.
Table 8.
Mercury effects on five groups of neurobehavioral tests estimated in structural equation analysis with covariate adjustment with and without maternal fish intake during pregnancy.
|
No adjustment for fish intake |
Adjusted for fish intake |
|||||
|---|---|---|---|---|---|---|
| Test groupa | Goodness of fit (pb) | Effectc | p | Goodness of fit (p) | Effect | p |
| Motor | 0.062 | −7.41 | 0.034 | 0.070 | −9.37 | 0.0088 |
| Attention | 0.044 | −8.40 | 0.030 | 0.049 | −9.54 | 0.017 |
| Spatial | 0.0015 | 2.60 | 0.50 | 0.0013 | 1.04 | 0.80 |
| Verbal | 0.13 | −5.97 | 0.080 | 0.14 | −6.87 | 0.051 |
| Memory | 0.30 | −2.86 | 0.40 | 0.27 | −3.05 | 0.38 |
Motor (the three NES2 finger tapping scores, CATSYS supination/pronation score, CATSYS maximum finger tapping score), Attention (Digit Spans, Spatial Span, CATSYS mean reaction time, NES2-CPT mean reaction time, number of false responses, and number of missed responses), Spatial (Children's category test, WISC-R Block Design, WISC+WAIS Block Designs, Copying total score, the Copying score for the last five designs, and number correctly recalled), Verbal (the two Boston Naming Test scores, the four California Verbal Learning Test scores, and the Similarities score), and Memory (the four CVLT scores and the Copying recall score).
p-value in likelihood ratio test of the proposed model against the unrestricted model
Effect of true exposure doubling expressed in % of s.d. of latent response
Maternal fish intake during pregnancy was then added to the structural equation models (Table 8). Although fish intake appeared to exert a weak beneficial effect, it was not statistically significant. Inclusion of this adjustment changed the mercury coefficients in the direction anticipated.
Significant mercury effects corresponded to a decrease corresponding to 6.5–8.5% of the standard deviation for each doubling in the exposure (Table 8). These results are in accordance with the strongest regression coefficients in Table 5. For motor function, a doubling of the true MeHg exposure corresponded to 1.3 fewer taps on NES2 finger tapping with the preferred hand. As a result, at age 14 years, this effect is slightly stronger than at 7 years. Other effects were about the same or less. In the regression analysis, a strong positive mercury effect had been obtained for Spatial Span, and this tendency led to poorer fit of the structural equation for attention. A better fitting model with a stronger effect would be obtained by excluding this score, but this possibility was not further pursued.
As expected, local dependence were observed for the two naming scores, the four CVLT scores, the NES2 continuous performance scores, and the two Copying scores. Likewise, differential item functioning occurred for some outcomes, mostly because test scores depended on sex. Allowance for these factors improved the fit of the models, but affected the mercury coefficients only minimally. For the spatial outcomes the final model did not fit data as well as the unrestricted model and the results should be cautiously interpreted.
4. Discussion
This study presents results on neuropsychological performance of adolescents with widely differing degrees of prenatal exposure to MeHg from maternal seafood diets during pregnancy and lower postnatal exposures to this neurotoxicant. Adverse effects were identified in regard to motor speed, attention, and language. These effects were apparent both in multiple regression analyses and in structural equation models that take into account multiple testing, exposure imprecision, and incomplete data. Potential confounders were included in the analyses. These findings are in accordance with the results obtained from examination of the same cohort members at age 7 years and suggest that prenatal MeHg exposure is associated with enduring neurotoxic effects on CNS function [15]. Of particular interest, the Boston Naming Test results continued to show effects of prenatal MeHg exposure. At age 7, prenatal MeHg exposure was associated with the capacities to name pictured objects both under cued and uncued conditions, thus suggesting that lexical development was more limited at high exposures. The findings for age 14 further support an exposure-related limitation of lexical development, because participants with higher exposure had a poorer ability to recall names of objects, even when cues were provided, than did participants with lower exposures.
While the effect at age 14 years appeared to be somewhat weaker than seven years before, the overall explanatory power of the statistical models was also more limited than it was previously. A multitude of events and incidents, beneficial and adverse, may have occurred during the time between the two examinations and may have attenuated the correlation between two sets of tests and decreased the association with early-life covariates. For example, differences in time of onset of puberty, together with very high growth rates in this phase of development, may contribute unadjusted variability that overshadows the association with defined predictors, including MeHg exposure. In addition, the covariates and methylmercury exposure might particularly affect the rate at which skills are acquired at the younger age, while innate ability might be a more important predictor of performance at 14 years. Thus, although the neuropsychological performance results at age 14 were more affected by variability that could not be captured by adjustments using known covariates, the relative importance of MeHg exposure was about the same at the two examinations. In addition, analyses that incorporated test results at both examinations showed that the mercury-associated deficits had changed only little between age 7 and 14 years.
When considering the estimated effect size, comparison of regression coefficients with the standard deviations of the test scores is appropriate [19]. Because of the logarithmic transformation of the exposure scale, effects must be expressed in terms of log-scale increases. We found that a doubling of the prenatal MeHg exposure at age 7 years corresponded to a decrease in performance in the range of about 5–10% of the standard deviation [18,19]. The effect at age 14 years was similar in size (Table 8). The regression coefficients obtained for mercury may be compared to those obtained for age. At age 7 years, a doubling in MeHg exposure corresponded to a loss in development by about 1–2 months [18]. At age 14, age relationships for the neuropsychological tests are less steep, and a doubling in the MeHg exposure therefore corresponded to greater delays in development.
While most tests showed a mercury association in the direction predicted, Spatial Span showed an unanticipated positive effect of increased prenatal MeHg exposures. This tendency caused a poor fit of one of the structural equation models. Although a chance finding cannot be excluded, one must also consider the possibility that MeHg exposure may be associated with intake of essential nutrients from seafood, and that, for example, long-chain n-3 fatty acids may have beneficial effects on brain development [1,22]. Partial adjustment for this factor was provided by adding maternal fish intake during pregnancy as a covariate. This addition changed the mercury regression coefficients in the direction anticipated to better reflect the true adverse effect of methylmercury per se. This confounding had previously been considered as an explanation of the weak tendency of decreased (improved) visual evoked potential latencies at higher prenatal MeHg exposure levels [18]. It therefore seems likely that the outcomes of the present study are affected by both contaminant toxicity and beneficial nutrient effects in the opposite direction, although the different seafood components may not affect different brain functions to the same degree. Furthermore, it is apparent that any beneficial nutrients would have compensated only partially for the neurotoxic effects of MeHg in this cohort. However, adjustment for fish intake was based only on maternal recall of the frequency of fish dinners during pregnancy, thus not allowing any precise assessment of essential nutrient intakes. If more precise indicators of nutrient supply had been available, statistical control for their beneficial effects might have revealed an even stronger MeHg effect.
The results of the multiple regression analyses must be interpreted in light of various uncertainties, in particular exposure misclassification [7, 14]. Thus, while our previous studies have shown that the cord-blood mercury concentration is the exposure parameter that best predicts test performance, closely followed by the mercury concentration in cord tissue [17], both parameters are participant to substantial variability that exceeds the normal laboratory error. The mercury concentration in maternal hair is substantially more imprecise and may be severely affected by, e.g., permanent hair treatment [41]. Such exposure imprecision is likely to be random and would therefore bias the regression coefficients toward null. However, the imprecision of the exposure markers was taken into account in the structural equation models by viewing the observed parameters as reflections of a true underlying exposure variable. These more advanced statistical analyses revealed that the grouped test score outcomes, labeled as motor, attention, and verbal functions, had the clearest dose-effect relationships for developmental MeHg exposure.
In both the multiple regression and structural equation analyses, tests or groups of tests that showed the best dose-effect relationships with regard to prenatal MeHg exposure tended to be highly sensitive tests with wide score ranges or extremely accurate outcome measurements. For example, reaction time and manual motor speed as measured by computer are highly accurate. The Boston Naming Test has 60 items, allowing much better discrimination of the subject’s abilities than tests with fewer items. Therefore, the mercury-associations of the tests with superior psychometric properties strengthen the argument that MeHg has a long-term adverse effect on brain development and function. The tests assessing visuospatial skills appeared to have a low ceiling in this population at 14 years, while responses on tests of short-term memory generally had a narrow range, thereby limiting their ability to reveal exposure-effect relationships. Even given these provisos, the confluent finding of effects on tasks assessing attention, manual motor speed, and language/semantic memory suggests that the effects of MeHg at these levels of brain development are multi-focal or diffuse, affecting widely divergent brain structures. The existence of sustained neurotoxic effects in adolescents is also supported by the neurophysiological examinations that were carried out in parallel [26]. Thus, auditory evoked potential latencies at age 14 years increased at higher prenatal MeHg exposures, and the heart rate variability decreased. The notion of permanent effects is also consistent with the known neuropathological effects of prenatal MeHg exposure at much higher levels producing clinical Minamata disease [15,28], but clearly as a much more subtle picture.
Postnatal exposures were generally weakly related to cognitive test scores at 14 years, although postnatal exposures were poorly characterized and averaged only about one-fifth of the prenatal exposures. Significant effects were seen on the finger tapping scores, but these effects could be due to chance and were less certain, when corrected for the full set of confounders. Because the neuropsychological outcomes may be affected by both prenatal and postnatal exposures, and the exposures at different times were interrelated [26], these effects would be difficult to separate. In addition, using the postnatal exposure to adjust the regression coefficients for prenatal exposure is problematic, as this approach will involve two error-prone and associated predictors, thereby causing complex biases they may be difficult to predict [12]. Given these caveats, postnatal exposure in this population did not appear to affect the neurobehavioural outcomes to any appreciable degree. However, our results on peak V of the evoked potentials showed a possibly specific effect of postnatal (recent) MeHg exposures [26], thus supporting the finding that recent exposure could conceivably cause a decrease in motor speed. This effect is perhaps surprising, because the average MeHg exposure of the 14-year-olds was reflected by a hair-mercury concentration of about 1 μg/g, a level that corresponds to the Reference Dose used by the U.S. Environmental Protection Agency [34]. However, these findings are in accordance with several cross-sectional studies carried out in 7-to-12-year-old children, who also showed decreased motor speed associated with increased hair-mercury concentrations [15]. While the hair-mercury concentration in the latter studies reflected the child’s own recent exposure, an association with prenatal exposures could not be ascertained.
PCB is another neurotoxicant present in seafood, and increased exposures to methylmercury and PCBs are correlated [16]. At age 7 years, prenatal PCB exposure was associated with deficits on CPT reaction time, naming, and long-term verbal memory, although these tendencies all but disappeared after adjustment for mercury exposure [16,18]. This result was confirmed in a structural equation analysis that allowed for measurement errors in biomarkers of both MeHg and PCB [6]. No association with PCB exposure was seen at age 14 years.
The findings of the present study add to the substantial evidence on developmental methylmercury neurotoxicity [11,15,23,28]. One previous study carried out in the Seychelles [27] found no significant association between prenatal methylmercury and performance on neurobehavioral tests at age 8 years. However, the two studies are not contradictory, because the Seychelles study cannot exclude an effect at the magnitude observed at the Faroes [24]. In addition, the mercury effect was assessed only from analyses of maternal hair collected six months after parturition and after adjustment for the child’s postnatal mercury exposure [27]. Joint analyses based on three major prospective studies are in excellent agreement with the findings in the Faroes [23,28].
The long-term effects of developmental neurotoxicity are of substantial medical and societal importance. Significant exposure-associated deficits were observed at adolescence, although subtle neurotoxicity, e.g., due to lead exposure [2], may become more difficult to document in adolescents, who may have had a longer and partially unknown exposure record and who may also differ in regard to the timing and speed of puberty development and a variety of other cofactors. The present study differs from most previous studies by emphasizing domain-related brain functions, rather than formal IQ testing. However, some WISC-R subtests were included in this study, and MeHg neurotoxicity can be expressed in terms of approximate IQs [5]. Such IQ deficits are known to affect the likelihood that an adolescent completes high school and the chance of completing an academic or vocational education. The associated costs to society are therefore substantial [32].
Acknowledgments
This study was supported by grants from the US National Institute of Environmental Health Sciences (ES09797) and the Danish Medical Research Council. The contents of this paper are solely the responsibility of the authors and do not represent the official views of the NIEHS, NIH or any other funding agency. Advice on neurobehavioral test selection was contributed by the following members of the steering committee convened by NIEHS: David Bellinger, Kim Dietrich, Annette Kirshner, and David Otto. Arne Ludvig administered one of the test panels to all participants; Nicolina Sørensen and Flemming Juul Hansen conducted the pediatric examinations; Anne Sofie Veyhe administered the CATSYS tests; and Mari Ann Ellendersen was in charge of cohort maintenance and clinical scheduling.
References
- 1.Bakker EC, Ghys AJ, Kester AD, Vles JS, Dubas JS, Blanco CE, Hornstra G. Long-chain polyunsaturated fatty acids at birth and cognitive function at 7 y of age. Eur J Clin Nutr. 2003;57:89–95. doi: 10.1038/sj.ejcn.1601506. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC. Lead. Pediatrics. 2004;113(Suppl 4):1016–1022. [PubMed] [Google Scholar]
- 3.T. Boll, Children’s Category Test (Manual), Psychological Corp., San Antonio, TX, 1993.
- 4.K.A. Bollen, Structural Equations with Latent Variables, John Wiley, New York, 1989.
- 5.E. Budtz-Jørgensen, F. Debes, P. Weihe, P. Grandjean, Adverse mercury effects in 7-year-old children expressed as loss in ‘IQ’, University of Southern Denmark, Odense, 2004. URL www.chef-project.dk (accessed, 20 April, 2005).
- 6.Budtz-Jørgensen E, Keiding N, Grandjean P, Weihe P. Estimation of health effects of prenatal mercury exposure using structural equation models. Environ Health. 2002;1:2. doi: 10.1186/1476-069X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budtz-Jørgensen E, Keiding N, Grandjean P. Effects of exposure imprecision on estimation of the benchmark dose. Risk Anal. 2004;24:1689–1696. doi: 10.1111/j.0272-4332.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- 8.E. Budtz-Jørgensen, P. Grandjean, N. Keiding, Confounder selection in environmental epidemiology (submitted, 2005). [DOI] [PubMed]
- 9.L.J. Cronbach, Essentials of Psychological Testing. Harper & Row, New York, NY, 1970.
- 10.Despres C, Lamoureux D, Beuter A. Standardization of a neuromotor test battery: the CATSYS system. Neurotoxicology. 2000;21:725–735. [PubMed] [Google Scholar]
- 11.European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to mercury and methylmercury in food (EFSA-Q-2003-030). Brussels, 2004. http://www.efsa.eu.int/science/contam/contam_opinions/259_en.html (accessed 27 April, 2005).
- 12.W.A. Fuller, Measurement Error Models. Wiley & Sons, New York, NY, 1987.
- 13.Grandjean P, Weihe P, Jørgensen PJ, Clarkson T, Cernichiari E, Viderø T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch Environ Health. 1992;47:185–195. doi: 10.1080/00039896.1992.9938348. [DOI] [PubMed] [Google Scholar]
- 14.Grandjean P, Budtz-Jørgensen E, Keiding N, Weihe P. Underestimation of risk due to exposure misclassification. Eur J Oncol Suppl. 2003;2:165–172. [Google Scholar]
- 15.P. Grandjean, S. Cordier, T. Kjellström, P. Weihe, E. Budtz-Jørgensen, Health effects and risk assessments, in: N. Pirrone, KR Mahaffey KR (Ed.), Dynamics of mercury pollution on regional and global scales: atmospheric processes and human exposures around the world. Norwell, MA: Springer, 2005, p. 499–523.
- 16.Grandjean P, Weihe P, Burse VW, Needham LL, Storr-Hansen E, Heinzow B, Debes F, Murata K, Simonsen H, Ellefsen P, Budtz-Jørgensen E, Keiding N, White RF. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23:305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 17.Grandjean P, Budtz-Jørgensen E, Jørgensen PJ, Weihe P. Umbilical cord mercury concentration as biomarker of prenatal exposure to methylmercury. Environ Health Perspect. 2005;113:905–908. doi: 10.1289/ehp.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandjean P, Weihe P, White RF, Debes F, Araki S, Murata K, Sørensen N, Dahl D, Yokoyama K, Jørgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 19.Grandjean P, Budtz-Jørgensen E, White RF, Jørgensen PJ, Weihe P, Debes F, Keiding N. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am J Epidemiol. 1999;150:301–5. doi: 10.1093/oxfordjournals.aje.a010002. [DOI] [PubMed] [Google Scholar]
- 20.Grandjean P, White RF, Nielsen A, Cleary D, de Oliveira Santos EC. Mercury neurotoxicity in Amazonian children downstream from gold mining. Environ Health Perspect. 1999;107:587–591. doi: 10.1289/ehp.99107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.T.J. Hastie, R.J. Tibshirani, Generalized Additive Models, CRC Press, Boca Raton, FL, 1990.
- 22.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 23.JECFA (Joint FAO/WHO Expert Committee on Food Additives). Sixty-first meeting, Rome, 10–19 June 2003. Summary and conclusions. URL: ftp://ftp.fao.org/es/esn/jecfa/jecfa61sc.pdf (accessed, 19 April, 2005)
- 24.Keiding N, Budtz-Jørgensen E, Grandjean P. Prenatal methylmercury exposure in the Seychelles (letter) Lancet. 2003;362:664–5. doi: 10.1016/S0140-6736(03)14166-9. [DOI] [PubMed] [Google Scholar]
- 25.R. Letz, E.L.Baker, NES2 Neurobehavioral Evaluation System, Winchester, MA: Neurobehavioral Evaluation System, 1988.
- 26.Murata K, Weihe P, Budtz-Jørgensen E, Jørgensen PJ, Grandjean P. Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. J Pediatr. 2004;144:177–183. doi: 10.1016/j.jpeds.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 27.Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang LS, Clarkson TW. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–92. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- 28.National Research Council. Toxicological Effects of Methylmercury. National Academy Press, Washington, DC, 2000.
- 29.Steuerwald U, Weihe P, Jørgensen PJ, Bjerve K, Brock J, Heinzow B, Budtz-Jørgensen E, Grandjean P. Maternal seafood diet, methylmercury exposure, and neonatal neurological function. J Pediatr. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- 30.K. Sullivan, Neurodevelopmental aspects of methylmercury exposure: Neuropsychological consequences and cultural issues, PhD. Thesis in Behavioral Neuroscience, Boston University School of Medicine, 1999.
- 31.R.L. Thorndike, E.P. Hagen, J.M. Sattler, Stanford-Binet Intelligence Scale, 4th ed., Riverside, Chicago, IL, 1986.
- 32.Trasande L, Landrigan PJ, Schechter C. Public health and economic consequences of methyl mercury toxicity to the developing brain. Environ Health Perspect. 2005;113:590–596. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.J. O. Undheim, Håndbok for Wechsler Scale of Intelligence for Children – Revised (Manual). Norsk Utgave, Norsk Psykologforening, Oslo, Norway, 1978.
- 34.U.S. EPA (Environmental Protection Agency), Office of Science and Technology, Office of Water. Water Quality Criterion for the Protection of Human Health: Methylmercury, Final. EPA-823-R-01-001. Washington, 2001. URL: http://www.epa.gov/waterscience/criteria/methylmercury/document.html (accessed, 19 April, 2005).
- 35.D. Wechsler , Wechsler Intelligence Scale for Children – Revised, Psychological Corp., New York, NY, 1974.
- 36.D. Wechsler , Wechsler Adult Intelligence Scale – Revised, Psychological Corp., San Antonio, TX, 1981.
- 37.D. Wechsler, Wechsler Memory Scale – 3rd Edition (WMS-III). Psychological Corp., San Antonio, TX, 1997.
- 38.Weihe P, Grandjean P, Jørgensen PJ. Application of hair-mercury analysis to determine the impact of a seafood advisory. Environ Res. 2005;97:200–207. doi: 10.1016/j.envres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 39.R.F. White, F. Debes, R. Dahl, P. Grandjean, Development and field testing of a neuropsychological test battery to assess the effects of methylmercury exposure in the Faroe Islands, Proceedings of the international symposium on assessment of environmental pollutionand health effects of methylmercury, Kumamoto, Japan 1994, pp. 127–140.
- 40.White RF, Diamond R, Proctor S, Morey C, Hu H. Residual cognitive deficits 50 years after lead poisoning during childhood. Br J Ind Med. 1993;50:613–622. doi: 10.1136/oem.50.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasutake A, Matsumoto M, Yamaguchi M, Hachiya N. Current hair mercury levels in Japanese: survey in five districts. Tohoku J Exp Med. 2003;199:161–169. doi: 10.1620/tjem.199.161. [DOI] [PubMed] [Google Scholar]