Abstract
In addition to their physiological function, metabotropic receptors for neurotransmitter γ-aminobutyric acid (GABA), the GABAB receptors, may play a role in the behavioral actions of addictive compounds. Recently, GABAB receptors were cloned in fruit flies (Drosophila melanogaster), indicating that the advantages of this experimental model could be applied to GABAB receptor research. RNA interference (RNAi) is an endogenous process triggered by double-stranded RNA and is being used as a tool for functional gene silencing and functional genomics. Here we show how cell-nonautonomous RNAi can be induced in adult fruit flies to silence a subtype of GABAB receptors, GABABR1, and how RNAi combined with pharmacobehavioral techniques (including intraabdominal injections of active compounds and a computer-assisted quantification of behavior) can be used to functionally characterize these receptors. We observed that injection of double-stranded RNA complementary to GABABR1 into adult Drosophila selectively destroys GABABR1 mRNA and attenuates the behavioral actions of the GABAB agonist, 3-aminopropyl-(methyl)phosphinic acid. Moreover, both GABABR1 RNAi and the GABAB antagonist CGP 54626 reduced the behavior-impairing effects of ethanol, suggesting a putative role for the Drosophila GABAB receptors in alcohol's mechanism of action. The Drosophila model we have developed can be used for further in vivo functional characterization of GABAB receptor subunits and their involvement in the molecular and systemic actions of addictive substances.
Keywords: fruit fly‖GABAB receptors‖ethanol‖CGP 54626‖3-APMPA
Fruit flies are used for research primarily because of the homology of Drosophila genes to those of mammals and because Drosophila is amenable to genetic manipulation including gene silencing through a process known as RNA interference (RNAi) (1–7). Typically, RNAi is triggered by double-stranded RNA (dsRNA), which is first processed by an RNase, Dicer (8), into 21- to 23-nt fragments. These fragments form a silencing complex that binds specifically to the dsRNA-complementary endogenous mRNA and leads to the destruction of the mRNA (1, 9). Injecting adult Drosophila intraabdominally with dsRNA results in the cell-nonautonomous silencing of the complementary endogenous mRNA throughout the body, including the CNS (10). Because this method does not interfere in the normal development of the animal but can replicate typical phenotypes produced by gene mutations (11), here we have used adult RNAi to silence γ-aminobutyric acid (GABA) B receptors.
The slow inhibitory GABA synaptic neurotransmission is mediated by the metabotropic G protein-coupled and cAMP-linked GABAB receptors (12, 13) that have been cloned in humans (14) and also recently in Drosophila (15). In addition to their physiological function, the GABAB receptors may play a role in the behavioral actions of addictive compounds such as ethanol (16–19). Although Drosophila has been used to study the mechanisms of action of alcohol (20–23), as yet, this model has not been used to characterize the involvement of GABAB receptors.
Mezler et al. (15) reported the cloning of three Drosophila GABAB receptor subunits: D-GABABR1, D-GABABR2, and D-GABABR3. D-GABABR1 and D-GABABR2 show high sequence homology to mammalian GABABR1 and GABABR2, respectively; the subunit D-GABABR3 seems to be an insect-specific subtype with no known mammalian counterpart and no known function. All three D-GABAB subunits are expressed in the CNS; in situ hybridization of Drosophila embryos showed that D-GABABR1 and D-GABABR2 are expressed in similar regions, whereas the D-GABABR3 displayed a unique expression pattern. Moreover, these receptors were functional only when D-GABABR1 and D-GABABR2 were coexpressed either in Xenopus laevis oocytes or mammalian cell lines, whereas D-GABAB-R3 was inactive in any combination. The in vitro pharmacology of the coexpressed D-GABABR1/R2 subunits was slightly different from the pharmacology of the mammalian GABAB receptors; e.g., baclofen was not effective as an agonist. However, similar to mammalian receptors, the Drosophila receptors were also activated by 3-aminopropyl-(methyl)phosphinic acid (3-APMPA) and inhibited by CGP 54626 (15).
The mammalian GABABR1 is responsible for GABA and ligand binding and mice lacking this subtype do not respond to typical GABAB agonists. Moreover, in the absence of drug treatment these mice also express numerous pathologic behaviors such as epilepsy, hyperalgesia, and impaired memory (24, 25). To circumvent these problems, which could be due to compensatory developmental responses of knockout mice, we planned our experiments such that RNAi was used for silencing of GABABR1 subunits only in adult Drosophila.
Materials and Methods
Drosophila and Injections.
Canton-S flies were cultured at 25°C and 50–60% humidity, on a 12 h light/12 h dark cycle, and on yeast, dark corn syrup, and agar food. Studies were performed with 5- to 7-day-old males. For injections, flies were anesthetized by CO2 (maximally for 5 min). Using custom-beveled glass pipettes (20 × 40 μm tip diameter) coupled to a cell injector and a micromanipulator, we injected a volume of 0.2 μl per fly by a pulse pressure of 300 kPa under a stereo microscope (10). Drugs were prepared as 10× stock solutions; 0.2 μl was injected per fly (the estimated volume of a fly is 2 μl). Then, 3-APMPA (also known as SKF-97541; Sigma), CGP 54626 (Tocris Cookson, Ellisville, MO), and ethanol (Sigma) were dissolved/diluted with Ringer's solution (7.5 g/liter NaCl/0.35 g/liter KCl/0.21 g/liter CaCl2; pH 7.6–7.8; sterile-filtered). The stock solutions of drugs were prepared in DMSO; DMSO concentration in final solutions was 1%. All control flies were injected with the corresponding vehicle.
Adult Drosophila RNAi.
In contrast to the cell-autonomous transgenic RNAi (26), the injectable RNAi appears to be effective throughout the cell body, i.e., cell-nonautonomous (10, 11), and is a preferable method for cell type-nonselective gene silencing. The preparation of dsRNA samples for injection proceeded as follows: an RT-PCR-amplified, 819-bp fragment of the Drosophila GABABR1 gene (1401–2220) was used for preparation of “long” dsRNA. The PCR primers used were direct 5′-taatacgactcactatattatttgtgtgcatgtgcacaa-3′ and reverse 5′-gtaatacgactcactatagggcatgacgtataacctcaa-3′. Experiments were also performed with “short” 21- to 22-bp dsRNA, which produced similar results (see Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org). Primers were designed with an attached T7 RNA polymerase promoter sequence. The specificity of the PCR product was verified with a restriction enzyme digestion (BglII site present at position 1811). This PCR product was directly used for in vitro transcription reaction with T7 RNA polymerase. RNA was heat-denatured at 80–85°C for 5 min and placed on ice. Gel electrophoresis was performed to assess the quality of dsRNA. The 750-bp GFP (Stratagene) dsRNA was used as a control (this gene is not normally expressed in Drosophila). Initially, two concentrations of “long” and “short” dsRNAs were used: 10 and 100 ng/μl. Because better results were obtained with a higher concentration, 100 ng/μl dsRNA was used in all subsequent experiments.
Assay of Endogenous mRNAs.
For assaying the endogenous contents of mRNAs, we used the RT-PCR assay (10). The total RNA from a single fly was extracted with TRIzol reagent (Invitrogen). The specific primers used in PCR amplification were direct 5′-ttatttgtgtgcatgtgcacaa-3′ and reverse 5′-gggatgacgtataacctcaa-3′ for GABABR1, direct 5′-gcctgggaaactcgacatggt-3′ and reverse 5′-ttgctccagttcgcacaccga-3′ for GABABR2, and direct 5′-atgaccatccgcccagcataca-3′ and reverse 5′-tgtgtattccgaccaggttac-3′ for RP49 (internal control). For the in situ PCR, heads of flies were manually dissected on dry ice, placed in 500-μl PCR tubes with a fix buffer (12% formaldehyde in PBS), rinsed in PBS, treated with proteinase K in PBS (20 μg/ml) for 5 min at room temperature, fixed again for 5 min, and rinsed several times in PBS. Reverse transcription was performed in the presence of oligo(dT) with MMLV RT (United States Biochemical) in 50 μl of a total reaction mix. PCR DIG Labeling Mix (Roche Applied Science) was used in the PCR amplification reaction (90°C for 15 s, 57°C for 20 s, and 72°C for 30 s; 15 cycles total). The PCR primers used were direct 5′-ttatttgtgtgcatgtgcacaa-3′ and reverse 5′-gggatgacgtataacctcaa-3′. Samples were washed in PBS three times (10 min per wash), incubated with anti-digoxigenin-AP Fab fragments (Roche Applied Science) at a dilution of 1:1,000 in PBS for 1 h at room temperature, and washed for 2 h in PBS (buffer changed four to five times). Color was developed with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP, Roche Applied Science). Samples were mounted in NaCl/glycerol solution.
Behavior.
The Drosophila Activity Monitoring System (Trikinetics, Waltham, MA), coupled to a computer, was used to monitor the locomotor activity of individual flies. After administration of vehicle or drugs, flies remain immobile (because of CO2 anesthesia and the actions of drugs) for a period. To quantify not only the total locomotor activity but also the duration of immobility, the system was slightly modified, i.e., the space in each individual recording tube was restricted to a length of 8 mm in the center of the photo beam. Flies were placed in the recording tubes within 2 min of injection and the sampling time was set at 1-min intervals. After flies recovered from the injection, they gradually resumed locomotor activity. In preliminary studies, we established that up to 15 min of CO2 anesthesia with or without injection does not result in any lasting effects on locomotor activity (e.g., >24 h). Nevertheless, different strains of flies express different degrees of basal locomotor activity; for Canton-S flies, we observed an average daytime activity >10 cpm (assayed over a period of several days). Thus, for this strain, we arbitrarily set the time of recovery from anesthesia as the first 1-min interval in which a fly produces 10 movements. Thereafter, the total locomotor activity was measured over the next 30-min period. The time to the first interval with more than 10 movements was used as the time of “awakening” and was analyzed with respect to the drug treatment variable. In addition, the level of locomotor activity was recorded and quantified for consecutive periods. Typically, 6–10 flies per experimental group were used and experiments were repeated two to three times.
Results
Localization of GABABR1 mRNA in Adult Flies and the Effect of dsRNA Injections.
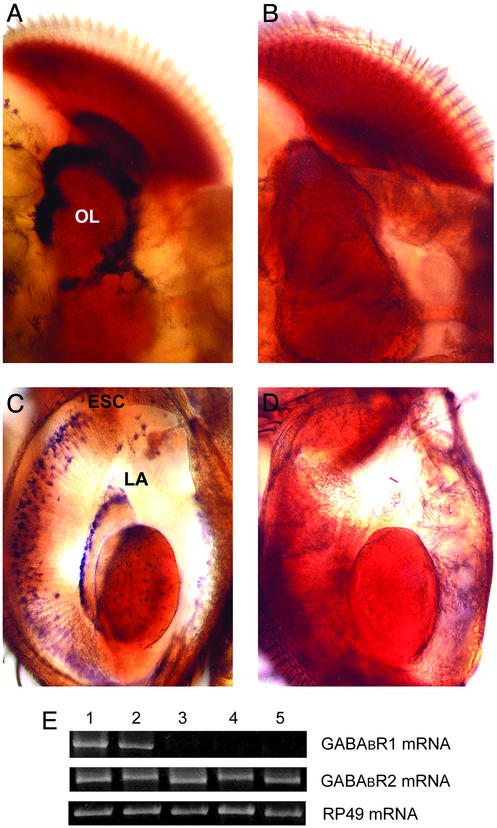
Previous studies on GABABR1 mRNA localization were performed in Drosophila embryos (15). Our studies localized GABABR1 mRNA in the CNS of adult Drosophila (Fig. 1). In addition to the localization shown in Fig. 1, we observed a GABABR1 mRNA signal in the giant fiber system, a pair of large bilaterally symmetrical interneurons that stimulate the visual jump–escape response and whose ventral and lateral dendrites receive mechanosensory and visual inputs (not shown). Injection of dsRNA complementary to endogenous GABABR1 mRNA led to the complete destruction of endogenous GABABR1 mRNA but not to the destruction of GABABR2 mRNA; this silencing of GABABR1 was evident 24 h after dsRNA injection and persisted for at least 3 days (Fig. 1).
Figure 1.
GABABR1 dsRNA destroys endogenous GABABR1 mRNA. Three days before the GABABR1 mRNA in situ PCR assay, the flies were injected with either GFP dsRNA (control; A and C) or GABABR1 dsRNA (RNAi; B and D). GABABR1 mRNA was found in A, the cortical layer of medulla and lobula neuropil of the optic lobe (OL; note the dark staining in A and its absence in B), and C [note the staining in the laminal outer ganglion cells (LA; the first neuropil of the optic lobe, which receives optic nerve fibers from compound eye photoreceptor cells) and the eye sensory cells (ESC)]. Note the absence of this staining in D. (E) RT-PCR assay of GABABR1, GABABR2, and RP49 (a ribosomal gene) mRNAs: lane 1, 24 h after vehicle; lane 2, 24 h after GFP dsRNA; lanes 3, 4, and 5, 24, 48, and 72 h after GABABR1 dsRNA, respectively. Note the absence of GABABR1 signals in lanes 3–5.
Behavioral GABAB Pharmacology in Flies.
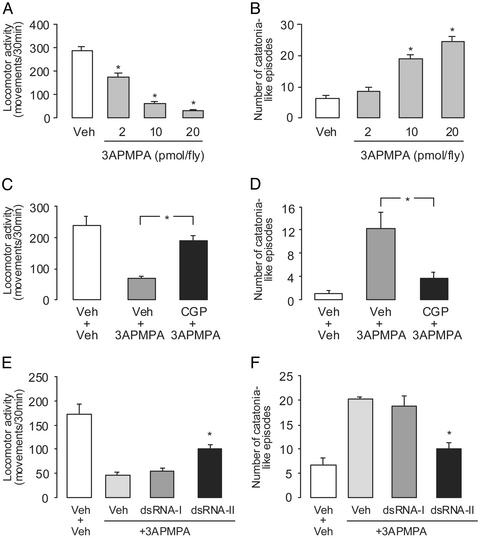
To evaluate the in vivo functioning of Drosophila GABAB receptors, we administered 3-APMPA (a GABAB agonist) and CGP 54626 (a GABAB antagonist) via intraabdominal injections and quantified the locomotor activity of injected flies. We observed that the injection of 3-APMPA into flies produces remarkable behavioral effects (see Movies 1–3, which are published as supporting information on the PNAS web site). After flies recovered from the injection (arbitrarily set in our system for monitoring the locomotor activity as the first 1-min interval in which a fly produces 10 movements; this time did not differ between vehicle- and drug-injected flies), 3-APMPA decreased locomotor activity measured over the subsequent 30-min period (Fig. 2). This GABAB agonist also caused intermittent jumps and periods of catatonia-like immobility that we also quantified. The behavioral effects of 3-APMPA, i.e., decreased locomotor activity and increased catatonic immobility, were prevented by injecting the flies 1 h before with the GABAB antagonist CGP 54626 (Fig. 2), suggesting that, indeed, the behavioral effects of 3-AMPA are mediated by GABAB receptors.
Figure 2.
Behavioral effects of the GABAB agonist 3-APMPA are attenuated by the GABAB antagonist CGP 54626 and by GABABR1 dsRNA. The GABAB agonist 3-APMPA triggered a dose-dependent reduction of locomotor activity in flies (A) and increased the number of catatonia-like episodes (B). These actions of the GABAB agonist were reduced by pretreatment with CGP 54626 (0.2 nmol per fly) 1 h before 3-APMPA (10 pmol per fly) (C and D). Similar results were obtained in experiments with 2 pmol of 3-APMPA per fly (not shown). In RNAi experiments, 3 days before GABAB agonist injection, the flies were injected with vehicle or dsRNA [control GFP dsRNA (dsRNA-I) or GABABR1 dsRNA (dsRNA-II)]. The behavioral effects of 10 pmol of 3-APMPA per fly were reduced by GABABR1 dsRNA (E and F). The bars represent mean values ± SEM. Significant differences (*, Scheffé's test) between drug-treated flies and corresponding controls are: A and B, P ≤ 0.001 vs. vehicle (Veh; n = 12 per group); C, P ≤ 0.05 (n = 6); D, P ≤ 0.001 (n = 6); and E and F, P ≤ 0.01 vs. other 3-APMPA-treated groups (n = 8).
GABABR1 RNAi Interferes with the Behavioral Actions of 3-APMPA.
Silencing of GABABR1 by RNAi in adult flies did not produce any marked effects on spontaneous locomotor behavior and recovery from anesthesia (data not shown). Also, the administration of GABAB antagonist CGP 54626 to adult flies did not significantly affect their locomotion. However, behavioral response to 3-APMPA (a GABAB agonist) was significantly reduced in GABABR1 dsRNA-injected flies (Fig. 2 and Fig. 5, which is published as supporting information on the PNAS web site). Our findings that endogenous GABABR1 mRNA was completely destroyed by RNAi (Fig. 1), whereas the behavioral actions produced by the GABAB agonist were reduced but not completely abolished (Fig. 2), suggest that the half-life of this receptor protein might be significantly longer than the half-life of the mRNA. Because the currently available antibodies against the GABABR1 subunit do not recognize Drosophila GABABR1, we cannot at this time examine the possibility that the protein may have a longer half-life than the mRNA.
Behavioral Effects of Ethanol Injections.
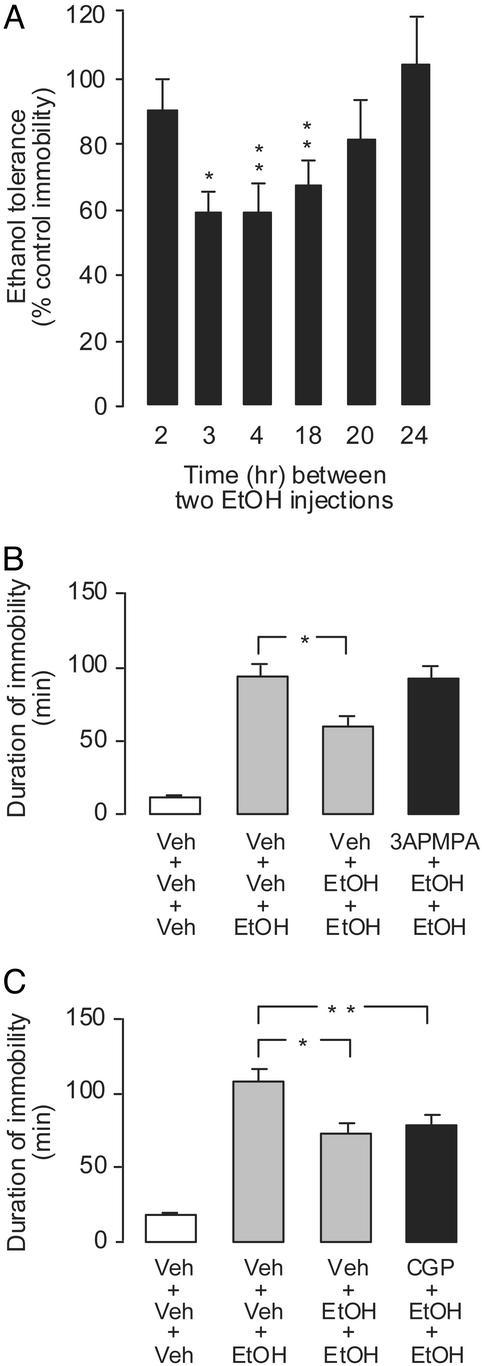
In our experimental model, ethanol was administered via injection and it caused a dose-dependent prolongation of immobility (e.g., anesthesia-like state; see Movies 1–3). The time from ethanol administration to the first interval with >10 movements was taken as the time of “awakening” (Fig. 3). The maximal effect of ethanol on awakening was observed with a dose of 0.8 μmol per fly. Assayed 24 h after injection, higher concentrations resulted in significant toxicity (assayed as the number of dead flies 24 h after injection; ≈20% with 1 μmol of ethanol per fly and 100% with 1.6 of μmol per fly; data not shown).
Figure 3.
CGP 54626 and GABABR1 RNAi attenuate motor impairment induced by ethanol. (A) Injection of ethanol (EtOH) caused a dose-dependent prolongation of immobility (*, P ≤ 0.001 vs. control, which is shown as 100%; Scheffé's test; n = 12 per group). (B) CGP 54626 (0.2 nmol per fly) 1 h before EtOH (400 nmol per fly) shortened EtOH immobility (*, P ≤ 0.001; n = 18–19). (C) dsRNA-II (as in Fig. 2), but not dsRNA-I, reduced motor impairment by EtOH (200 nmol per fly; *, P ≤ 0.05 vs. other EtOH-treated groups; n = 14–24). Results are expressed as a percentage of corresponding control immobility (11–15 min), and bars represent mean values ± SEM.
Behavior-Impairing Effects of Ethanol in Drosophila Involve GABAB Receptors.
Ethanol immobility and 3-APMPA-induced immobility, determined by our computer-assisted monitoring of locomotion, appear to be caused by different factors. Thus, ethanol-injected flies lie down and are nonresponsive to external stimuli (except for occasional leg movements) until they recover, stand, and gradually start to move. On the other hand, 3-APMPA-injected flies recover from injection under the CO2 anesthesia almost as quickly as vehicle-injected flies; they stand up but do not move except for occasional jumps. Nevertheless, the behavior-impairing effect of ethanol was diminished by pretreatment with the GABAB antagonist CGP 54626 (Fig. 3). Surprisingly, this is similar to previous findings in mice; ethanol-induced motor impairment was investigated in mice by using their rotarod performance as the test response. Direct cerebellar microinfusion of a GABAB agonist and an antagonist produced dose-dependent accentuation and attenuation, respectively, of ethanol-induced acute motor impairment (16). Motor impairment in flies triggered by ethanol was also reduced by GABABR1 RNAi (Fig. 3). Thus, our data suggest that the behavioral actions of 3-APMPA, and also in part ethanol, are mediated by an activation of GABAB receptors.
In Drosophila, Rapid Tolerance to Ethanol Involves GABAB Receptors.
Recent studies in mice revealed that rapid tolerance to ethanol (measured as motor impairment on a rotarod apparatus) is also controlled by the GABAB receptors, but in a manner opposite from acute motor impairment. Thus, baclofen (a GABAB agonist) significantly blocked rapid tolerance to ethanol, and the blockade of rapid tolerance by baclofen was antagonized by previous administration of CGP 36742 or CGP 56433, which are GABAB antagonists (17). We conducted similar experiments with Drosophila. In these studies, motor impairment induced by ethanol injection was reduced by previous ethanol administration; this tolerance persisted for ≈18 h (Fig. 4). Similar to previous findings in mice, pretreatment with a GABAB agonist (i.e., 3-APMPA) blocked tolerance to ethanol in Drosophila, whereas CGP 54626 pretreatment did not significantly affect ethanol tolerance (Fig. 4).
Figure 4.
Repeated injections of ethanol led to ethanol tolerance that can be inhibited by pretreatment with 3-APMPA but not with CGP 54626. (A) Ethanol (EtOH; 800 nmol per fly) was injected twice (interval of 1–24 h), or flies were injected first with vehicle (Veh) and then with EtOH (nontolerant controls). The duration of immobility of flies treated with two EtOH is expressed as the percentage of immobility of the corresponding Veh + EtOH-treated controls (*, P ≤ 0.001; **, P ≤ 0.01; Student's t test; n = 7–16 flies per group and per time interval). The interval between the first injection (3-APMPA, 2 pmol per fly, or CGP 54626, 0.2 nmol per fly) and second injection (EtOH, 400 nmol per fly) (B and C) was 1 h, and the interval between the second and third injections was 4 h (to induce EtOH tolerance). All groups differed significantly from vehicle-only-injected flies; significant differences between EtOH-treated groups are indicated (*, P ≤ 0.05; **, P ≤ 0.01; Scheffé's test; n = 13).
Discussion
Previous work by others has established Drosophila as a useful model organism for studies of ethanol (20), including the mechanisms of ethanol tolerance (23). Exposure to ethanol vapors was used to trigger behavioral alterations in flies (20), whereas genetic mutations or feeding flies on drugs mixed with the food was used to investigate specific molecular mechanisms. These studies point to a pivotal role for cAMP and cAMP-related neurotransmitter systems in regulating the sensitivity of Drosophila to ethanol (21, 22). Our results clearly show an important role for the Drosophila GABA system, including the metabotropic cAMP-linked GABAB receptors, in the behavioral actions induced by ethanol treatment and indicate that Drosophila can be used to further characterize the mechanisms of the interactions between ethanol and GABAB receptors.
The inhibitory neurotransmitter GABA exerts its actions via two distinct receptor families: ionotropic receptors (GABAA and GABAC), which have been extensively studied, and metabotropic receptors (GABAB), which are less well characterized. The focus of research on the interactions of ethanol with the GABA system has been on GABAA receptors (27). However, it also has been shown that inwardly rectifying potassium channels coupled to GABAB receptors are targets of alcohol action in CNS neurons (28) and that GABAB receptors may participate in long-lasting potentiation of GABAergic synapses after a single in vivo ethanol exposure (29).
Recently, it was observed that the GABAB receptor agonist baclofen reduces alcohol craving in alcohol-dependent patients (18). Moreover, baclofen blocks the development of rapid tolerance to ethanol in mice (17), whereas GABAB antagonists attenuate the acute behavior-impairing actions of ethanol (16) and produce more severe ethanol withdrawal syndrome symptoms (19). An earlier report also indicated that inhibition of GABAB receptors may antagonize acute behavioral effects of ethanol (30). Hence, the GABAB receptor antagonist phaclofen administered s.c. to mice reduced the ataxic and hypothermic effects of subsequent i.p. injections of ethanol; however, one may question the specificity of phaclofen for GABAB receptors. Our findings in Drosophila support the role of these receptors because we observed the attenuation of alcohol's effects not only with a different GABAB receptor antagonist (i.e., CGP 54626) but also with RNAi-mediated GABAB receptor silencing. Nevertheless, the rescue of the acute motor-impairing action of ethanol by GABABR1 antagonism was only partial, suggesting that the acute actions of ethanol are not mediated solely by GABAB receptors.
Similar to previous findings in mice (17), our experiments with Drosophila show that pretreatment with a GABAB receptor agonist also prevents the rapid tolerance to ethanol. In mice, certain doses of GABAB receptor antagonists (e.g., CGP 36742 and CGP 56433) facilitated ethanol tolerance. However, both in mice (17) and in flies, the doses of GABAB antagonists that antagonize the acute motor-impairing effects of ethanol did not facilitate rapid tolerance to ethanol. It has been proposed that a certain level of motor impairment on first ethanol exposure is required for the development of rapid tolerance (17). Thus, it is possible that a dose of CGP 54626 could be found that is ineffective in attenuating acute motor-impairing ethanol effects in flies and could facilitate rapid tolerance to ethanol.
We did not observe significant alterations of Drosophila locomotor activity after GABAB receptor antagonism or following GABAB receptor silencing in adult flies (i.e., by RNAi). This is different from major behavioral disturbances observed in GABAB receptor “knockout” mice (24, 25). It is possible that the pathologic behaviors of GABAB receptor-deficient mice are due in part to the developmental CNS alterations triggered by the absence of these receptors.
In insects, injections of dsRNA either into the pupae (31) or into adult organisms (10, 11, 32) lead to a cell-nonautonomous RNAi, which is preferable for studies of genes whose cell- or tissue-specificity of expression is unknown. It has been reported that functional GABAB receptors are present on insect motoneurons (33), in the CNS of Drosophila embryos (15), and our studies of localization of GABABR1 mRNA (Fig. 1) postulate the presence of these receptors in the neuropil of the optic lobe and also in the giant fiber system. Whether these or some other neurons are responsible for mediating the behavioral actions of ethanol and GABAB ligands is not clear at present. Additional work is also necessary to elucidate the exact mechanisms operative in GABAB receptor-mediated attenuation of behavioral responses to alcohol. We suggest that Drosophila may prove to be a good experimental model for these studies. For example, gene silencing in adult flies can be combined with pharmacological and biochemical tools to further characterize the functional role of individual subunits of GABAB receptors; i.e., D-GABABR1, D-GABABR2, and D-GABABR3. A better understanding of the molecular mechanisms linking GABAB receptors to the behavioral effects of ethanol may lead to novel concepts concerning the molecular biology of drug abuse.
Supplementary Material
Acknowledgments
We thank Tolga Uz for help in preparing the figures. This work was supported by National Institute on Drug Abuse Grant RO3DA14811 (to H.M.).
Abbreviations
- GABA
γ-aminobutyric acid
- 3-APMPA
3-aminopropyl-(methyl)phosphinic acid
- dsRNA
double-stranded RNA
- GABABR
GABAB receptor
- D-GABABR
Drosophila GABABR
- RNAi
RNA interference
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fire A, Xu S, Montgomery M K, Kostas S A, Driver S E, Mello C C. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Kennerdell J R, Carthew R W. Nat Biotechnol. 2000;18:896–898. doi: 10.1038/78531. [DOI] [PubMed] [Google Scholar]
- 3.Fraser A, Kamath R S, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- 4.Piccin A, Salameh A, Benna C, Sandrelli F, Mazzotta G, Zordan M, Rosato E, Kyriacou C P, Costa R. Nucleic Acids Res. 2001;29:e55. doi: 10.1093/nar/29.12.e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalidas S, Smith D P. Neuron. 2002;33:177–184. doi: 10.1016/s0896-6273(02)00560-3. [DOI] [PubMed] [Google Scholar]
- 6.Schmid A, Schindelholz B, Zinn K. Trends Neurosci. 2002;25:71–74. doi: 10.1016/s0166-2236(02)02034-9. [DOI] [PubMed] [Google Scholar]
- 7.Hannon G J. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein E, Caudy A A, Hammond S M, Hannon G J. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 9.Zamore P D. Science. 2002;296:1265–1269. doi: 10.1126/science.1072457. [DOI] [PubMed] [Google Scholar]
- 10.Dzitoyeva S, Dimitrijevic N, Manev H. Mol Psychiatry. 2001;6:665–670. doi: 10.1038/sj.mp.4000955. [DOI] [PubMed] [Google Scholar]
- 11. Dzitoyeva, S., Dimitrijevic, N. & Manev, H. (2003) Dros. Inf. Serv., in press.
- 12.Bowery N G, Bettler B, Froestl W, Gallagher J P, Marshall F, Raiteri M, Bonner T I, Enna S J. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- 13.Duthey B, Caudron S, Perroy J, Bettler B, Fagni L, Pin J P, Prezeau L. J Biol Chem. 2002;277:3236–3241. doi: 10.1074/jbc.M108900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin S C, Russek S J, Farb D H. Gene. 2001;278:63–79. doi: 10.1016/s0378-1119(01)00678-3. [DOI] [PubMed] [Google Scholar]
- 15.Mezler M, Muller T, Raming K. Eur J Neurosci. 2001;13:477–486. doi: 10.1046/j.1460-9568.2001.01410.x. [DOI] [PubMed] [Google Scholar]
- 16.Dar M S. Brain Res Bull. 1996;41:53–59. [PubMed] [Google Scholar]
- 17.Zaleski M J, Nunes Filho J R, Lemos T, Morato G S. Psychopharmacology (Berlin) 2001;153:415–424. doi: 10.1007/s002130000581. [DOI] [PubMed] [Google Scholar]
- 18.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa G L, Gasbarrini G. Alcohol Alcohol. 2002;37:504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 19.Carai M A, Brunetti G, Lobina C, Serra S, Vacca G, Minardi G, Colombo G, Gessa G L. Eur J Pharmacol. 2002;445:195–199. doi: 10.1016/s0014-2999(02)01752-1. [DOI] [PubMed] [Google Scholar]
- 20.Weber K E, Diggins L T. Genetics. 1990;125:585–597. doi: 10.1093/genetics/125.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore M S, DeZazzo J, Luk A Y, Tully T, Singh C M, Heberlein U. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- 22.Bainton R J, Tsai L T, Singh C M, Moore M S, Neckameyer W S, Heberlein U. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 23.Scholz H, Ramond J, Singh C M, Heberlein U. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 24.Prosser H M, Gill C H, Hirst W D, Grau E, Robbins M, Calver A, Soffin E M, Farmer C E, Lanneau C, Gray J, et al. Mol Cell Neurosci. 2001;17:1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- 25.Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, et al. Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- 26.Van Roessel P, Hayward N M, Barros C S, Brand A H. Genesis. 2002;34:170–173. doi: 10.1002/gene.10146. [DOI] [PubMed] [Google Scholar]
- 27.Aguayo L G, Peoples R W, Yeh H H, Yevenes G E. Curr Top Med Chem. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- 28.Lewohl J M, Wilson W R, Mayfield R D, Brozowski S J, Morrisett R A, Harris R A. Nat Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- 29.Melis M, Camarini R, Ungless M A, Bonci A. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allan A M, Harris R A. Life Sci. 1989;45:1771–1779. doi: 10.1016/0024-3205(89)90516-x. [DOI] [PubMed] [Google Scholar]
- 31.Bettencourt R, Terenius O, Faye I. Insect Mol Biol. 2002;11:267–271. doi: 10.1046/j.1365-2583.2002.00334.x. [DOI] [PubMed] [Google Scholar]
- 32.Amdam G V, Simones Z L, Guidugli K R, Norberg K, Omholt S W. BMC Biotechnol. 2003;3:1. doi: 10.1186/1472-6750-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai D, Sattelle D. J Exp Biol. 1995;198:889–894. doi: 10.1242/jeb.198.4.889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.