Abstract
Mating or vaginocervical stimulation [copulatory stimulus (CS)] induces two daily surges of the hormone prolactin (PRL) in rats. This unique secretory pattern of PRL surges is characteristic for the first half of pregnancy and is also present in ovariectomized (OVX) rats. Studies have shown that CS additionally provokes an acute release of the hormone oxytocin (OT). In this study, we tested whether a single injection of OT (iv) is sufficient to initiate the PRL secretion pattern of OVX/CS rats. Furthermore, we measured the 24-h profile of dopamine (DA) content in the anterior lobe of the pituitary gland, because DA is the major inhibitory factor of PRL secretion. The results indicated that a single injection of OT induces a PRL secretory rhythm and a DA release pattern similar to that initiated by CS. Immunocytochemical investigation showed that particular OTergic neurons in the hypothalamus express receptors for PRL, as well as for vasoactive intestinal polypeptide, which indicates an involvement in generating the PRL rhythm and entraining it to the ambient photoperiod. On the basis of this study, we suggest that the PRL-DA inhibitory feedback loop between lactotrophs and DAergic neurons plays a crucial role in generating the oscillatory PRL secretion pattern in CS rats. A timing signal, likely provided by the hypothalamic suprachiasmatic nucleus, entrains the autonomous PRL oscillation to a particular time of day. Mathematical modeling was used to illustrate the proposed network function. The experimental results further suggest an additional feedback mechanism in which certain hypothalamic OTergic neurons are influenced by PRL.
Keywords: dopamine, suprachiasmatic nucleus, mathematical modeling, lactotrophs
The early phase of pregnancy and pseudopregnancy in rats is characterized by two daily peaks of prolactin (PRL) secretion, referred to as nocturnal (at ∼0300) and diurnal (at ∼1700) surges (13, 15, 21). This unique secretory pattern is induced by the mating stimulus or vaginocervical stimulation [copulatory stimulus (CS)] and persists for ∼10 days in pregnant (45) animals and 12 days in pseudopregnant animals (44). The increased PRL output is important in maintaining the structural and functional integrity of the corpus luteum, which, in turn, is responsible for progesterone synthesis (44). The PRL secretory pattern in CS rats entrains to photoperiod and can be shifted within limits under various lighting conditions (53). Furthermore, this pattern becomes free running in constant darkness, which demonstrates that the PRL rhythm is a true circadian rhythm (6).
PRL secretion from lactotrophs, located in the anterior lobe of the pituitary gland, is controlled by a balance of PRL-releasing and -inhibiting hormones supplied to the pituitary gland from neurosecretory cells in the hypothalamus through the portal vasculature (1, 15, 28). Several in vivo and in vitro studies (12, 13, 15, 40) have indicated that oxytocin (OT) is a potential PRL-releasing hormone. Most recently, we (12) showed that bath application of OT to cultured lactotrophs elevates intracellular Ca2+ levels and induces PRL secretion. The PRL-inhibiting hormone is dopamine (DA), which is released primarily by tuberoinfundibular (TIDA) neurons of the arcuate nucleus (ARC) (3, 15, 28). Inhibition of lactotroph activity is mediated by the D2 subtype of G protein-coupled DA receptors (3). DA tonically inhibits the secretion of PRL, and PRL release occurs only when DA levels in the portal blood are reduced (7, 32). Whereas DA release by TIDA neurons inhibits lactotrophs, PRL secreted by the lactotrophs stimulates DA synthesis and metabolism in TIDA neurons (22, 35). Studies (10) further demonstrate a time delay between the subcutaneous injection of PRL and the increased DA release from TIDA neurons. Thus mutual interactions between TIDA neurons and lactotrophs form a short feedback loop (20, 34) that has been proposed to be largely responsible for PRL homeostasis (3).
The mating stimulus/CS in female rats serves as a trigger for the unique PRL rhythm, which lasts much longer than the stimulus. Thus there is some form of memory that persists throughout the duration of the rhythm (48, 49). Can other events trigger the PRL rhythm, and if so, will the rhythm persist for several days? Studies have shown that OT is released into the circulation as a direct response to CS. This has been demonstrated in sheep (26), rats (36), and humans (the Ferguson reflex) (14, 27, 30). The main goal of this study is to determine whether a sustained PRL rhythm can be triggered by a single injection of OT. To this end, we measure blood concentrations of PRL and DA concentrations in the anterior lobe of the pituitary gland after a single injection of OT to see whether they are rhythmic, and if so, whether they exhibit a phase relationship similar to that of CS-stimulated rats.
The PRL rhythm entrains to the photoperiod (6). One mechanism for entrainment is thought to be daily pulses of vasoactive intestinal polypeptide (VIP) from the hypothalamic suprachiasmatic nucleus (SCN) (12). It has been demonstrated (17) that VIP fibers originating in the SCN innervate DA neurons in the ARC. These neurons express VIP receptors of subtype 2 (VPAC2R) (17), and the application of oligonucleotide anti-sense to VIP mRNA into the SCN stimulates DA neuron activity (18), which suggests that the VIP connection from the SCN to TIDA neurons is inhibitory. We (12) have recently found that VIP fibers from the SCN also innervate OT neurons in the paraventricular nucleus (PVN). Therefore, it is likely that these OT neurons will be entrained by the VIP signal of SCN origin.
An earlier report (2) showed that OT is involved in the regulation of PRL surges of CS rats. Thus it is possible that OT neurons of the PVN form part of the neural circuitry responsible for the CS-induced PRL rhythm. If so, does PRL then feed back onto the OT neurons, as it does onto the DA neurons of the ARC? An additional goal of this study is to determine whether this feedback may occur, by performing an immunocytochemical (ICC) analysis of VPAC2R and PRL receptors (PRLR) in the PVN. In particular, we wish to determine whether OT neurons coexpress VPAC2R and PRLR, which would suggest that these neurons participate in both the PRL rhythm generation and its entrainment to the photoperiod.
MATERIALS AND METHODS
General Experimental Techniques
Animals
Adult female Spargue-Dawley rats (200–250 g; Charles River, Raleigh, NC) were kept in standard rat cages under a 12:12-h light-dark cycle (lights on at 0600), with water and rat chow available ad libitum. All animals were bilaterally ovariectomized (OVX) under halothane anesthesia. Animal procedures were approved by the Florida State University Animal Care and Use Committee.
CS
The uterine cervix was stimulated with an electrode constructed from a Teflon rod (diameter, 5 mm), with two platinum wires protruding from the tip. Each rat was stimulated twice, the first time at 1700 and the second time on the following morning at 0900. Stimulations were applied as two consecutive trains of electric current of 10-s duration (rectangular pulses, 1 ms of 40 V at 200 Hz). This procedure has been shown (19) to yield the highest success rate in initiating two daily PRL surges that are characteristic of mated rats.
Jugular vein catheter implantation
Polyurethane catheter tubing (Micro-Renathane; Braintree Scientific, Braintree, MA), extended with Tygon tubing (TGY-040-100; Small Parts, Miami Lakes, FL), was inserted into the jugular vein as the rats were anesthetized with halothane. The tubing, filled with heparinized saline (100 U/ml), was fitted subcutaneously and exteriorized at the back of the animal's neck. A daily flush with heparinized saline kept the line open until the start of the blood collection, ≥3 days after surgery.
Extraction of the anterior lobe of the pituitary gland
Animals were briefly sedated by inducing hypercapnia and then decapitated. Pituitary glands were quickly removed and placed on ice, and lobes were carefully dissected under a microscope (OPMI 1; Zeiss, Oberkochen, Germany). Isolated anterior pituitary lobes were placed in homogenization buffer (0.2 N perchlorate with 50 μM EGTA) and rapidly frozen in an ArcticIce tube transport block (USA Scientific, Ocala, FL). Tissue samples were stored at −80°C until they were assayed for DA by high-performance liquid chromatography with electrochemical detection (HPLC-EC). The DA content of the anterior lobe is of particular interest because this is the location of the PRL-secreting lactotrophs. DA content of the anterior lobe varies in response to manipulations that affect catecholamine synthesis (37). We (11) have previously shown that the DA content of the anterior lobe is inversely proportional to the rate of PRL secretion.
Radioimmunoassay
Serum concentrations of PRL were estimated in duplicate by the rat PRL radioimmunoassay (RIA) kit, as previously described (16). Rat PRL RP-3 standard was supplied by Dr. Albert Parlow through the National Hormone and Pituitary Program. The measurement of serum OT concentration was performed with the Phoenix OT kit (Phoenix Pharmaceuticals, Belmont, CA), which was validated for the use of rat serum. The assay sensitivity was 1 pg/ml serum. OT could affect the secretion of PRL through three routes. It could arrive at the anterior lobe through the short portal vessels connecting the posterior with the anterior lobe. It could also arrive through the long portal vessels from the median eminence (41, 50). Finally, the lactotrophs would respond to OT released from the posterior lobe into peripheral plasma. It is with the last assumption that we use peripheral plasma levels of OT as a reflection of that which influences PRL secretion (12).
DA measurement by HPLC-EC
DA concentrations were measured in extracts of the anterior lobe of the pituitary gland, as previously described (42). The amount of catecholamine in each sample was estimated by direct comparison to the area under each peak for known amounts of catecholamine. The amount of 3,4-dihydroxybenzylamine (DHBA, RT = 6.5 min) recovered was compared with the amount of DHBA added as internal standard and corrected for sample loss (usually <5%). The assay detects 30 pg of DA.
Data analysis
All values are expressed as means ± SE. Two-way analysis of variance (treatment × time, with repeated measures for time) was used for the comparison of differences among treatment groups, followed by Bonferroni post hoc comparison. Statistical analyses were performed using GraphPad Prism 3.0 (GraphPad Software, San Diego, CA), and graphs were created with Microsoft Excel. Differences were considered significant at the level of P ≤ 0.05.
Experiments
Experiment 1: induction of PRL secretory rhythm by bolus injection of OT
OT (H-2510, Bachem) in the concentration of 25 μM was injected into OVX animals through the jugular vein catheter (0.1 ml/100 g body wt) under halothane anesthesia at 1600. For the control experiments, an equal amount of sterile saline was injected into animals in the same manner and at the same time of day that OT was given. Starting at 1800, 2 h after the OT injection, 200 μl of blood were collected at each time point (see Fig. 1) throughout days 1, 2, 4, and 6 after injection. Blood loss during sampling was compensated by sterile saline replacement. Serum samples were stored at −40°C until analysis for PRL and OT concentration by RIA.
Fig. 1.
Secretory profile of prolactin (PRL; A and B) and oxytocin (OT; C and D) of ovariectomized (OVX) rats ≤5 days after a single intravenous injection (black arrow) of either OT or saline. Injection of OT induced a rhythmic PRL secretion pattern of 2 surges/day (A and B, solid line) that is similar to OVX/vaginocervical stimulation [copulatory stimulus (CS)] rats (A, dashed line). By contrast, saline injection had no effect on PRL secretory profile (A and B, dotted line). Measurement of OT serum level showed high values for the first 20 h after OT injection (C, solid line; >80 pg/ml for the first 16 h). Thereafter, level fluctuations were similar to those of OVX/CS rats (C, dashed line). Injection of saline had no effect on OT secretory profile (C and D, dotted line). Values are expressed as mean ng/ml PRL ± SE (n = 3–10 serial samples) and mean pg/ml OT ± SE (n = 3–10 serial samples). *Significantly higher PRL levels for OT-injected animals compared with saline-injected animals (P < 0.05); #significantly higher PRL level for CS animals compared with saline-injected animals (P < 0.05).
Experiment 2: determination of DA release pattern in the anterior lobe of the pituitary gland
A second series of animals was used to determine the daily DA profile in the anterior lobe of the pituitary gland of either OVX, OVX/CS, or OT-injected OVX animals. OT was injected at 1600 via the tail vein. A group of 4–5 animals was killed at 0000, 0200, 1200, 1400, 1600, 1800, and 2200 on the 2nd day after designated treatment (CS or OT injection). Collection of the pituitary glands was accomplished as described above.
Experiment 3: ICC analysis of VPAC2R and PRLR coexpression on OTergic neurons in PVN and periventricular nucleus. ICC labeling
Rats were transcardially perfused with 60 ml of cold perfusion rinse (0.5% NaNO2 in 0.9% saline solution) followed by 300 ml of cold 4% paraformaldehyde in 0.1 M PBS (pH 7.2). After the perfusion, the brains were removed, postfixed in 4% paraformaldehyde-PBS overnight, and placed in 20% sucrose-PBS for ≥12 h. Coronal sections (40 μm) of the brains [through the PVN and periventricular nucleus (PeVN)] were cut on a freezing microtome and stored in cryoprotectant (51). Before the incubation with the primary antibodies, the sections were rinsed three times in 0.1% Triton X-100 in PBS and exposed to 10% normal horse serum (Chemicon, Temecula, CA) in PBS for 1 h. The following primary antibody combinations were used for the triple-labeling study: OT (rabbit anti-OT, IHC-8152, dilution 1:5,000; Peninsula Laboratories, Belmont, CA), VPAC2R (goat anti-VPAC2R, SC-15961, dilution 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), and PRLR (mouse anti-PRLR, MA1-611, dilution 1:1,000; ABR Affinity BioReagents, Golden, CO). The binding specificity of the antibodies was verified earlier (12, 17). After the incubation period of 48 h at 4°C, secondary antibodies, conjugated to fluorescent probes (CY2, CY3, and CY5; Jackson Immunoresearch, West Grove, PA), were added in the concentration of 1:600 for another 24 h at 4°C. All primary and secondary antibodies were diluted in 0.4% Triton X-100-0.01% Na azide in PBS. Finally, sections were washed three times with PBS and mounted on glass slides, and coverslips were applied with Aqua-Poly/Mount (Poly-sciences, Warrington, PA) and sealed with nail polish.
image analysis and 3d reconstruction
High-resolution images of OT-, VPAC2R-, and PRLR-immunostained brain sections through PVN and PeVN were taken with a confocal laser-scanning microscope [laser wavelength combination: T1 = 633 nm, T2 = 543 nm, T3 = 488 nm on a Zeiss 510 microscope (Zeiss)], generating three channels. The 22 stacks of images (≤50 images/stack) recorded from ≥3 adjacent coronal brain sections of PVN and PeVN per animal (5 animals in total) were further processed by applying nonblind deconvolution, which increases the image quality and resolution by computationally removing artifacts of the recording process (AmiraDeconv Module 3.1 software, Mercury Computer Systems/TGS Unit, San Diego, CA). This process includes the application of the point spread function that describes the relationship between the ideal unblurred image of the specimen and the actual image generated by the microscope, which is based on iterative maximum-likelihood image restoration. After deconvolution, an isosurface was generated for each channel (isovalue/threshold = 5), and the photon mapping technique (24) was applied to the three-dimensional (3D) models to simulate global illumination with software implemented by Kevin Beason at Florida State University (http://www.csit.fsu.edu/∼beason/pane/), which generated photorealistic images with an improved 3D perception. The transparency of the green channel (OT immunolike staining) was increased so that the receptor staining (PRLR = blue, VPAC2R = red; see Fig. 3) could be seen within the soma.
Fig. 3.
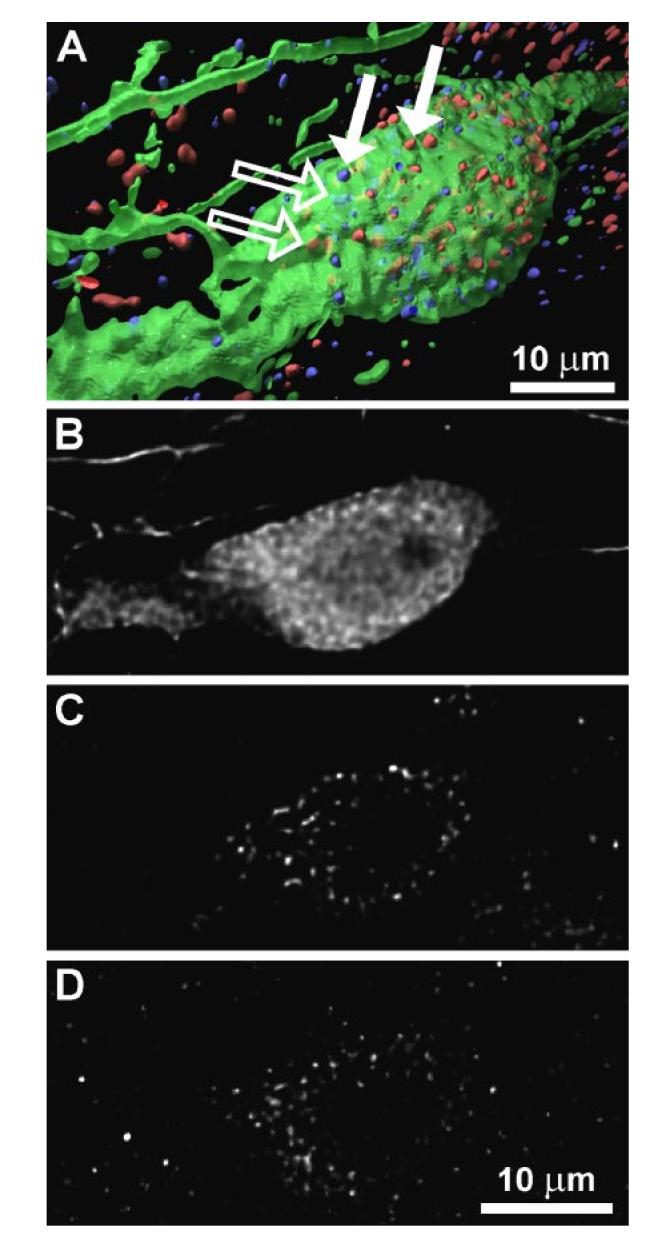
Immunocytochemical triple staining for OT (green), vasoactive intestinal polypeptide (VIP) receptor (red), and PRL receptor (blue) of a sample cell in paraventricular nucleus (PVN). A: 3-dimensional reconstruction of a PVN cell from a stack of optical sections taken by confocal laser-scanning microscope. In addition to receptors located on cell surface (filled arrows), there was also cytoplasmic immunopositve receptor staining (open arrows). B–D: display of a 2-dimensional scan from stack of optical sections on level of cell soma, divided into 3 recorded channels; B = OT (A, green), C = VIP receptor (A, red), D = PRL receptor (A, blue).
Modeling
A phenomenological model for the TIDA neurons and lactotrophs was developed, incorporating known interaction pathways between these cell populations. We let PRL represent the activity level of lactotrophs and DA represent the activity level of TIDA neurons, and we begin with a description of the differential equation for the time dynamics of PRL.
Isolated lactotrophs are often tonically active (23). We represent this tonic (endogenous) drive by the factor Tp. In vivo, the lactotrophs are subject to inhibition from DA neurons, which we incorporate by dividing Tp by 1 + kdDA2. The parameter kd reflects the strength of the inhibition. The first term of the PRL differential equation is thus
| (1) |
This has a maximum value of Tp (no DA inhibition) and declines to 0 as DA concentration is increased. The second term in the PRL equation provides first-order decay, which reflects the fact that if PRL is elevated it will tend to return to a basal level over time. This term is −qPRL, where q is the decay rate. The PRL equation is then
| (2) |
The DA neurons of female rats are also tonically active (8). This tonic activation is represented by the factor Td. As described earlier (10, 20, 22, 35), PRL has been shown to stimulate DA activity. We expect that in vivo this stimulation would be delayed for up to several hours, according to previous reports (9, 10). However, more recent studies (31) have shown the possibility of a faster response to PRL, especially in cultured cells. Delayed stimulatory action of PRL is represented by multiplying Td by . The notation PRLτ represents the PRL concentration at time t − τ, where τ is a time delay. The factor kp is the strength of the PRL feedback. As with the PRL equation, we include a first-order decay term,−qDA. For simplicity, we use the same decay rate, q. As described earlier (17, 18), VIPergic neurons of the SCN innervate DA neurons of the ARC and likely provide time-of-day information. We add this pathway to the DA-PRL model through the parameter VIP, which represents the activity level of VIP neurons in the SCN. Because under normal lighting conditions the activity of these neurons is high during the morning, we model VIP as a square pulse that is elevated for 3 h in the morning, and 0 h at all other times. It has been shown (18) that the activity of DA neurons is inhibited by VIP. This inhibitory action is included in the differential equation for DA by adding the term −rvVIP·DA. The influence of VIP is incorporated in a multiplicative manner so that VIP inhibits only active cells, ensuring that DA does not become negative. Thus the DA differential equation is
| (3) |
Equations 2 and 3 were integrated numerically using a 4th-order Runge-Kutta algorithm in the C programming language. Results of this simulation were used in Fig. 4, with parameter values Tp = 6, kd = 1, q = 0.5, Td = 10, kp = 0.03, rv = 2, and τ = 3 h. The parameters were adjusted to produce a semicircadian PRL rhythm, illustrating that it is possible to produce such a rhythm solely from DA-PRL interactions, provided that the time delay, τ, is sufficiently long. To initiate and maintain the rhythm, a term −Td was added to Eq. 3 to counteract the tonic drive to the TIDA neurons after OT injection. A further description of the model and additional simulations are given in the companion paper (4).
Fig. 4.

A: model simulation of CS-induced PRL rhythm. B: oscillation in DA neuron activity is out of phase with PRL rhythm. Although mutual interaction between DA neurons and lactotrophs is sufficient to generate daily rhythm, VIP input from SCN (dashed line) entrains rhythm to photoperiod. All curves have been normalized.
RESULTS
Mating-Like PRL Secretory Pattern Induced by Bolus Injection of OT
Experiments were performed to investigate whether an OT surge is sufficient to induce the PRL secretory pattern that is characteristic of mated rats. OT was given as an intravenous bolus injection at 1600 to OVX rats through an implanted jugular vein cannula. As control, saline was injected at the same time of day in a different set of OVX animals (Fig.1, A and C, arrows). Two hours later, serial blood sampling was started and continued for ≤6 days. Pilot studies revealed that there is no response in PRL secretion to the OT injection within the first 2 h (M. Egli, unpublished data).
A first PRL surge appeared 6 h after the OT injection at 2200 in OT-injected animals (Fig. 1A, solid line). A PRL secretory rhythm with nocturnal and diurnal surges was fully established after another 23 h. This PRL secretory rhythm continued on days 4 and 5 (Fig. 1B, solid line) and through days 6 and 7 (M. Egli, unpublished data). Injection of the same amount of saline had no effect on PRL secretion (Fig. 1, A and B, dotted line). For the purposes of comparison, PRL levels on day 2 induced by CS are superimposed in Fig. 1 (dashed line). Although the PRL surges are somewhat larger in the OT-injected rats than in the CS rats, the pattern of PRL secretion is similar on day 2 (the only day measured for CS rats).
The measurement of OT in the same blood samples revealed that, after the OT injection, normal levels were reestablished after 22 h (Fig. 1C, solid line). An afternoon increase in circulating OT, which seems to be characteristic for OVX/CS rats (Fig. 1C, dashed line), occurred on the 2nd day after OT injection. However, this increase was not statistically significant. The afternoon elevation was diminished on days 4 and 5 (Fig. 1D). The injection of saline caused no fluctuation of the OT secretory profile (Fig. 1, C and D, dotted line).
DA Level Exhibits Daily Rhythm
Further investigations were carried out to determine whether the daily fluctuation of DA supply to the anterior lobe of the pituitary gland displays the same pattern in CS and in OT-injected OVX rats. The HPLC-EC analysis of the samples revealed a single peak of DA in the anterior lobe of the pituitary gland at 1200 in both the OT-injected and CS rats (Fig. 2, solid and dashed lines). Although the DA values from OVX/OT-injected animals were generally lower than those from OVX/CS rats, the peaks occurred at the same time in both sets of animals. No fluctuation of DA values was detected in the samples of control animals [Fig. 2, OVX (dotted line)], with DA remaining low throughout the day.
Fig. 2.

DA content in the anterior lobe of the pituitary gland of OVX/CS (dashed line), OVX/OT-injected (solid line), and OVX (dotted line) rats over a 24-h period. Peak values were measured at 1200 in both OVX/CS and OVX/OT-injected animals. Values are expressed as means of total dopamine (DA; ng/mg protein) ± SE (n = 3–5 anterior pituitary glands). *DA contents of CS animals were significantly higher than OVX baseline (P < 0.05); #significantly higher DA content for OT-injected animals compared with OVX baseline (P < 0.05).
OTergic Neurons in PVN Coexpress VPAC2R and PRLR
It has been shown (12) that OT stimulates PRL release from lactotrophs. Given the well-established DA-PRL feedback loop (20, 34), we sought to determine whether such a feedback loop might potentially exist between lactotrophs and OT neurons of the PVN and/or PeVN through verification of PRLR expression on OT neurons. Our laboratory has shown (12) that OT neurons in the PVN and PeVN express VPAC2R, which suggests that these neurons may be affected by the SCN. Thus we used a fluorescent triple-labeling approach, with antibodies against OT, VPAC2R, and PRLR to determine whether PRLR and VPAC2R were coexpressed on OTergic neurons. Analysis of the confocal microscopy-obtained images revealed cells with immunopositive staining for OT, VPAC2R, and PRLR in both PVN and PeVN (∼30% of all of the OT-immunolabeled cells). An example of such an OTergic neuron (green) in the magnocellular region of the PVN that coexpresses VPAC2R (red) and PRLR (blue) is shown in Fig. 3A. More than 50% of OT-immunolabeled cells showed immuoreactive staining for either VPAC2R or PRLR, especially in the magnocellular portion of the PVN, and few OT neurons expressed neither VPAC2R nor PRLR. By contrast, PRLR as well as VPAC2R immunostaining also appeared on non-OT-labeled cells. The 3D reconstruction of the image stacks shows the spatial distribution pattern of PRLR and VPAC2R within OT-immunopositive cells. Besides the numerous VPAC2 and PRL receptors on the OT cell surface (Fig. 3A, filled arrows), there were significant numbers of receptors of both types located inside the cell (Fig. 3A, open arrows). It is not possible with this method to determine whether, for example, the receptors are located on internal vesicles. More precise analytical techniques would be necessary to reveal such a relationship.
Short-Loop Feedback of PRL on TIDA Activity Participates in Regulation of Rhythmic Release of PRL in Response to CS
To investigate the plausibility of a PRL rhythm produced by mutual interaction between lactotrophs and DA neurons, we constructed a mathematical model that includes equations for lactotroph and DA neuron activity. We found that the short feedback loop is a viable rhythmic mechanism, provided that the stimulatory effect of PRL on TIDA neurons is delayed by 2 or more h (Fig. 4). In our model, PRL secretion from lactotrophs is high when DA neuron activity is low, because DA is an inhibiting factor. This results in an enhancement of DA neuron activity several hours later, because PRL is stimulatory to TIDA neurons. The resulting increase in DA secretion inhibits the lactotrophs, causing a fall in PRL secretion. Thus PRL secretion is low when the activity of DA neurons is high. The consequent removal of the stimulatory drive from the DA neurons causes DA activity to decline so that lactotroph activity can again rise and restart the cycle. One characteristic of this rhythm mechanism is that DA neuron activity peaks between the PRL surges (Fig. 4); that is, the PRL and DA peaks are out of phase.
DISCUSSION
Our study provides further insight into the neurosecretory network that governs rhythmic PRL secretion of CS rats. The results demonstrate that a single injection of OT (iv) in OVX animals causes an oscillatory secretion pattern of PRL, which resembles the typical PRL secretory profile of pregnant/pseudopregnant rats (15). Furthermore, this pattern was sustained for several days, which indicates that the oscillatory PRL secretion was not just a short-term temporal response to the OT injection. Although illustrated only on day 2 (Fig. 1A), the PRL surge amplitude of OVX/CS rats typically changes little during the 12-day period of pseudopregnancy (13, 19, 21). Therefore, the PRL surge amplitudes of OVX/OT-injected animals on days 4 and 5 are comparable with the surge magnitudes of OVX/CS rats. However, despite the initial PRL reaction to the OT injection, the semicircadian PRL secretory pattern was not established until day 2. The intravenous OT injection exerted a powerful stimulus to lactotrophs, which initiated PRL secretion (first secretory peak at 2200; Fig. 1). Nevertheless, there was a time lag of >2 h before this initial PRL response was detectable in the serum samples. This delay could be due to the time required for stimulatory OT to overcome the inhibitory DA tone. DAergic neurons will also be stimulated with a time delay because of the stimulatory action of PRL on DAergic neurons. Thus the initial PRL surge will be terminated by the associated increase in inhibitory DA tone.
We (12) have previously shown that OT acts directly on lactotrophs to stimulate PRL secretion in a Ca2+ -dependent manner. Alternatively, the fact that rhythmic PRL secretion was maintained for several days after the OT injection suggests the involvement of an OT-sensitive “memory” that can be switched on by sufficient stimulation. OT may activate a population of interneurons that would act like a switch. After acute activation by OT, these interneurons could provide inhibitory input to DAergic neurons, allowing for a sustained PRL secretory rhythm. This model for an OT-sensitive memory is described in detail in Bertram et al. (companion article, Ref. 4). The mechanism for the rhythm is likely the delayed stimulatory effect of PRL on DA neurons, coupled with the inhibitory effect of DA on lactotrophs. The model prediction of an out-of-phase oscillation between PRL and DA (Fig. 4) is reflected in the experimental data shown in Figs. 1 and 2. It is therefore possible that the core oscillation of PRL secretion in response to CS in rats is driven by the feedback loop between PRL and DA.
The typical PRL secretory pattern of pregnant/pseudopregnant rats with surges at ∼0300 and 1700 appeared on day 2 and not on day 1. Because the system was still under the influence of a high level of OT on the morning of day 1 (Fig. 1), the timing signal may have been suppressed. This was not the case on subsequent days. The OT level returned to normal by 1600 of the 1st day. With the appearance of the new timing signal in the second night, the PRL secretory rhythm experiences an entrainment to the time of day when the usual PRL surges of pregnant/pseudopregnant rats occur (Fig. 1). The additional analysis of the DA content in the anterior lobe of the pituitary gland revealed that the 24-h profiles of CS and OT-injected animals are very similar (Fig. 2). Peak values of DA occur at ∼1200 in both OVX/CS and OVX/OT-injected animals. Increased activity of hypothalamic DA neurons at ∼1200 has also been shown in CS rats (29) and is consistent with our data. However, the values of OVX/OT-injected animals were generally lower than those of OVX/CS rats (Fig. 2, dashed line). These lower values could be the reason for the larger PRL surges of OVX/OT-injected animals on day 2 compared with those of OVX/CS rats (Fig. 1). In addition, it cannot be excluded that differences in the PRL surge magnitude between OVX/CS and OVX/OT-injected animals may also occur because of the absence of additional factors that are activated only by CS. Further experiments are needed to identify these extra factors and to demonstrate their involvement in shaping the PRL amplitudes of pregnant/pseudopregnant rats.
It is likely that the SCN, which is regarded as the mammalian clock that orchestrates circadian rhythms (39, 46, 52), entrains this PRL secretory rhythm to the ambient light-dark cycle (5). This entrainment may occur through VIPergic input onto DA neurons of the ARC and possibly OT neurons of the PVN and PeVN (12). Further evidence that VIP could affect the activity of OT neurons in the PVN and PeVN is shown in Fig. 3. This ICC study shows colocalized staining for OT, VPAC2R, and PRLR on neurons in the PVN and PeVN, which suggests that these OTergic neurons respond not only to VIP input but also to PRL. Our results are consistent with earlier reports of VPAC2R expression (25, 43) and PRLR expression (33, 38) in the PVN. The abundant non-OT-related receptor immunostaining within the PVN and PeVN demonstrates that other cell types could be influenced by PRL or VIP, as has been shown for vasopressinergic neurons of the magnocellular secretory system, which are regulated by PRL (33). By using the triple-labeling ICC technique, we demonstrate that the expression patterns of the different receptor types overlap in these brain nuclei. Because these nuclei are a major production site of OT (47), it is not surprising to find the two types of receptors coexpressed on a fraction of OTergic neurons. As with TIDA neurons (18), VIP of SCN origin may also mediate the timing signal to OT neurosecretory cells located in the PVN and PeVN. The observation of PRLR expression on OTergic neurons supports the hypothesis that PRL feeds back onto OTergic neurons, completing a two-way OT-PRL feedback loop.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical support of Cheryl Fitch-Pye, as well as the assistance of Kim Riddle and Jon Ekman from the Biological Sciences Imaging Resource at Florida State University (FSU). We also thank David Banks from the School of Computational Science at FSU for support in 3D reconstruction of confocal images and Dr. Albert Parlow for RIA reagents.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
GRANTS
This work was supported by National Institutes of Health Grants DA-19356 to R. Bertram, M. Egli, and M. E. Freeman and DK-43200 to M. E. Freeman.
REFERENCES
- 1.Arey BJ, Freeman ME. Hypothalamic factors involved in the endogenous stimulatory rhythm regulating prolactin secretion. Endocrinology. 1989;124:878–883. doi: 10.1210/endo-124-2-878. [DOI] [PubMed] [Google Scholar]
- 2.Arey BJ, Freeman ME. Oxytocin, vasoactive-intestinal peptide, and serotonin regulate the mating-induced surges of prolactin secretion in the rat. Endocrinology. 1990;126:279–284. doi: 10.1210/endo-126-1-279. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–763. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- 4.Bertram R, Egli M, Toporikova N, Freeman ME. A mathematical model for the mating-induced prolactin rhythm of female rats. Am J Physiol Endocrinol Metab. 2006;290:E573–E582. doi: 10.1152/ajpendo.00428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bethea CL, Neill JD. Lesions of the suprachiasmatic nuclei abolish the cervically stimulated prolactin surges in the rat. Endocrinology. 1980;107:1–5. doi: 10.1210/endo-107-1-1. [DOI] [PubMed] [Google Scholar]
- 6.Bethea CL, Neill JD. Prolactin secretion after cervical stimulation of rats maintained in constant dark or constant light. Endocrinology. 1979;104:870–876. doi: 10.1210/endo-104-4-870. [DOI] [PubMed] [Google Scholar]
- 7.De Greef WJ, Neill JD. Dopamine levels in hypophysial stalk plasma of the rat during surges of prolactin secretion induced by cervical stimulation. Endocrinology. 1979;105:1093–1099. doi: 10.1210/endo-105-5-1093. [DOI] [PubMed] [Google Scholar]
- 8.Demarest KT, McKay DW, Riegle GD, Moore KE. Sexual differences in tuberoinfundibular dopamine nerve activity induced by neonatal androgen exposure. Neuroendocrinology. 1981;32:108–113. doi: 10.1159/000123140. [DOI] [PubMed] [Google Scholar]
- 9.Demarest KT, Riegle GD, Moore KE. The rapid “tonic” and the delayed “induction” components of the prolactin-induced activation of tuberoinfundibular dopaminergic neurons following the systemic administration of prolactin. Neuroendocrinology. 1986;43:291–299. doi: 10.1159/000124543. [DOI] [PubMed] [Google Scholar]
- 10.DeMaria JE, Lerant AA, Freeman ME. Prolactin activates all three populations of hypothalamic neuroendocrine dopaminergic neurons in ovariectomized rats. Brain Res. 1999;837:236–241. doi: 10.1016/s0006-8993(99)01667-4. [DOI] [PubMed] [Google Scholar]
- 11.DeMaria JE, Livingstone JD, Freeman ME. Characterization of the dopaminergic input to the pituitary gland throughout the estrous cycle of the rat. Neuroendocrinology. 1998;67:377–383. doi: 10.1159/000054336. [DOI] [PubMed] [Google Scholar]
- 12.Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology. 2004;145:3386–3394. doi: 10.1210/en.2003-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erskine MS. Prolactin release after mating and genitosensory stimulation in females. Endocr Rev. 1995;16:508–528. doi: 10.1210/edrv-16-4-508. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson J. A study of the motility of the intact uterus at term. Surg Gynecol Obstet. 1941;73:359–366. [Google Scholar]
- 15.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 16.Freeman ME, Sterman JR. Ovarian steroid modulation of prolactin surges in cervically stimulated ovariectomized rats. Endocrinology. 1978;102:1915–1920. doi: 10.1210/endo-102-6-1915. [DOI] [PubMed] [Google Scholar]
- 17.Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Res. 2001;919:48–56. doi: 10.1016/s0006-8993(01)02993-6. [DOI] [PubMed] [Google Scholar]
- 18.Gerhold LM, Sellix MT, Freeman ME. Antagonism of vasoactive intestinal peptide mRNA in the suprachiasmatic nucleus disrupts the rhythm of FRAs expression in neuroendocrine dopaminergic neurons. J Comp Neurol. 2002;450:135–143. doi: 10.1002/cne.10307. [DOI] [PubMed] [Google Scholar]
- 19.Gorospe WC, Freeman ME. The effects of various methods of cervical stimulation on continuation of prolactin surges in rats. Proc Soc Exp Biol Med. 1981;167:78–82. doi: 10.3181/00379727-167-41128. [DOI] [PubMed] [Google Scholar]
- 20.Grattan DR, Xu J, McLachlan MJ, Kokay IC, Bunn SJ, Hovey RC, Davey HW. Feedback regulation of PRL secretion is mediated by the transcription factor, signal transducer, and activator of transcription 5b. Endocrinology. 2001;142:3935–3940. doi: 10.1210/endo.142.9.8385. [DOI] [PubMed] [Google Scholar]
- 21.Gunnet JW, Freeman ME. The mating-induced release of prolactin: a unique neuroendocrine response. Endocr Rev. 1983;4:44–61. doi: 10.1210/edrv-4-1-44. [DOI] [PubMed] [Google Scholar]
- 22.Hentschel K, Moore KE, Lookingland KJ. Effects of prolactin on expression of Fos-related antigens in tyrosine hydroxylase-immunoreactive neurons in subdivisions of the arcuate nucleus. Brain Res. 2000;857:110–118. doi: 10.1016/s0006-8993(99)02362-8. [DOI] [PubMed] [Google Scholar]
- 23.Ho MY, Kao JP, Gregerson KA. Dopamine withdrawal elicits prolonged calcium rise to support prolactin rebound release. Endocrinology. 1996;137:3513–3521. doi: 10.1210/endo.137.8.8754781. [DOI] [PubMed] [Google Scholar]
- 24.Jensen HW. Realistic Image Synthesis Using Photon Mapping. AK Peters; Wellesley, MA: 2001. [Google Scholar]
- 25.Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- 26.Kendrick KM, Keverne EB, Hinton MR, Goode JA. Cerebrospinal fluid and plasma concentrations of oxytocin and vasopressin during parturition and vaginocervical stimulation in the sheep. Brain Res Bull. 1991;26:803–807. doi: 10.1016/0361-9230(91)90178-m. [DOI] [PubMed] [Google Scholar]
- 27.Kruger TH, Haake P, Chereath D, Knapp W, Janssen OE, Exton MS, Schedlowski M, Hartmann U. Specificity of the neuroendocrine response to orgasm during sexual arousal in men. J Endocrinol. 2003;177:57–64. doi: 10.1677/joe.0.1770057. [DOI] [PubMed] [Google Scholar]
- 28.Lamberts SW, Macleod RM. Regulation of prolactin secretion at the level of the lactotroph. Physiol Rev. 1990;70:279–318. doi: 10.1152/physrev.1990.70.2.279. [DOI] [PubMed] [Google Scholar]
- 29.Lerant A, Herman ME, Freeman ME. Dopaminergic neurons of periventricular and arcuate nuclei of pseudopregnant rats: semicircadian rhythm in Fos-related antigens immunoreactivities and in dopamine concentration. Endocrinology. 1996;137:3621–3628. doi: 10.1210/endo.137.9.8756525. [DOI] [PubMed] [Google Scholar]
- 30.Lippert TH, Mueck AO, Seeger H, Pfaff A. Effects of oxytocin outside pregnancy. Horm Res. 2003;60:262–271. doi: 10.1159/000074243. [DOI] [PubMed] [Google Scholar]
- 31.Ma FY, Grattan DR, Goffin V, Bunn SJ. Prolactin-regulated tyrosine hydroxylase activity and messenger ribonucleic acid expression in mediobasal hypothalamic cultures: the differential role of specific protein kinases. Endocrinology. 2005;146:93–102. doi: 10.1210/en.2004-0800. [DOI] [PubMed] [Google Scholar]
- 32.McKay DW, Pasieka CA, Moore KE, Riegle GD, Demarest KT. Semicircadian rhythm of tuberoinfundibular dopamine neuronal activity during early pregnancy and pseudopregnancy in the rat. Neuroendocrinology. 1982;34:229–235. doi: 10.1159/000123304. [DOI] [PubMed] [Google Scholar]
- 33.Mejia S, Torner LM, Jeziorski MC, Gonzalez C, Morales MA, de la Escalera GM, Clapp C. Prolactin and 16K prolactin stimulate release of vasopressin by a direct effect on hypothalamo-neurohypophyseal system. Endocrine. 2003;20:155–162. doi: 10.1385/ENDO:20:1-2:155. [DOI] [PubMed] [Google Scholar]
- 34.Milenkovic L, Parlow AF, McCann SM. Physiological significance of the negative short-loop feedback of prolactin. Neuroendocrinology. 1990;52:389–392. doi: 10.1159/000125610. [DOI] [PubMed] [Google Scholar]
- 35.Moore KE, Demarest KT, Johnston CA. Influence of prolactin on dopaminergic neuronal systems in the hypothalamus. Fed Proc. 1980;39:2912–2916. [PubMed] [Google Scholar]
- 36.Moos F, Richard P. Level of oxytocin release induced by vaginal dilatation (Ferguson reflex) and vagal stimulation (vago-pituitary reflex) in lactating rats (author's transl) J Physiol (Paris) 1975;70:307–314. [PubMed] [Google Scholar]
- 37.Mulchahey JJ, Neill JD. Dopamine levels in the anterior pituitary gland monitored by in vivo electrochemistry. Brain Res. 1986;386:332–340. doi: 10.1016/0006-8993(86)90170-8. [DOI] [PubMed] [Google Scholar]
- 38.Pi XJ, Grattan DR. Distribution of prolactin receptor immunoreactivity in the brain of estrogen-treated, ovariectomized rats. J Comp Neurol. 1998;394:462–474. doi: 10.1002/(sici)1096-9861(19980518)394:4<462::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 40.Samson WK, Schell DA. Oxytocin and the anterior pituitary gland. Adv Exp Med Biol. 1995;395:355–364. [PubMed] [Google Scholar]
- 41.Sarkar DK, Gibbs DM. Cyclic variation of oxytocin in the blood of pituitary portal vessels of rats. Neuroendocrinology. 1984;39:481–483. doi: 10.1159/000124024. [DOI] [PubMed] [Google Scholar]
- 42.Sellix MT, Freeman ME. Circadian rhythms of neuroendocrine dopaminergic neuronal activity in ovariectomized rats. Neuroendocrinology. 2003;77:59–70. doi: 10.1159/000068334. [DOI] [PubMed] [Google Scholar]
- 43.Sheward WJ, Lutz EM, Harmar AJ. The distribution of vasoactive intestinal peptide2 receptor messenger RNA in the rat brain and pituitary gland as assessed by in situ hybridization. Neuroscience. 1995;67:409–418. doi: 10.1016/0306-4522(95)00048-n. [DOI] [PubMed] [Google Scholar]
- 44.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 45.Smith MS, Neill JD. Termination at midpregnancy of the two daily surges of plasma prolactin initiated by mating in the rat. Endocrinology. 1976;98:696–701. doi: 10.1210/endo-98-3-696. [DOI] [PubMed] [Google Scholar]
- 46.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 48.Terkel J. Neuroendocrinology of coitally and noncoitally induced pseudopregnancy. Ann NY Acad Sci. 1986;474:76–94. doi: 10.1111/j.1749-6632.1986.tb28000.x. [DOI] [PubMed] [Google Scholar]
- 49.Terkel J, Witcher JA, Adler NT. Evidence for “memory” of cervical stimulation for the promotion of pregnancy in rats. Horm Behav. 1990;24:40–49. doi: 10.1016/0018-506x(90)90025-s. [DOI] [PubMed] [Google Scholar]
- 50.Verbalis JG, Baldwin EF, Ronnekleiv OK, Robinson AG. In vitro release of vasopressin and oxytocin from rat median eminence tissue. Neuroendocrinology. 1986;42:481–488. doi: 10.1159/000124491. [DOI] [PubMed] [Google Scholar]
- 51.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 52.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 53.Yogev L, Terkel J. Effects of photoperiod, absence of photic cues, and suprachiasmatic nucleus lesions on nocturnal prolactin surges in pregnant and pseudopregnant rats. Neuroendocrinology. 1980;31:26–33. doi: 10.1159/000123046. [DOI] [PubMed] [Google Scholar]



