Abstract
During development, excitatory synapses in the CA1 region of the hippocampus undergo activity-dependent and N-methyl-d-aspartate (NMDA) receptor-dependent long-lasting changes in synaptic efficacy. These bidirectional changes occur between limits that determine the dynamic range within which synapses operate. It is unknown whether the dynamic range itself is also activity-dependent and NMDA receptor-dependent. Here, we show that chronic blockade of NMDA receptors in hippocampal slice cultures during early postnatal development does not affect the dynamic range but results in a lower threshold for the induction of long-term potentiation. Thus, the dynamic range of CA3-CA1 synapses, unlike long-term potentiation threshold, is NMDA receptor-independent, thereby providing functional stability to the hippocampal network during development.
Hippocampal synapses are known to be highly plastic. Their efficacy undergoes activity-dependent changes, either as long-term potentiation (LTP; refs. 1 and 2) or long-term depression (LTD; refs. 3 and 4), which are thought to be important for the development of neuronal circuits and information storage. In area CA1, LTP and LTD are N-methyl-d-aspartate (NMDA) receptor-dependent phenomena (3–5). The level of NMDA receptor activation during conditioning determines the direction of change in synaptic efficacy by regulating the amount of calcium entry. Modest NMDA receptor activation induces LTD, whereas strong activation of NMDA receptors produces LTP (6, 7). Experimental evidence (8–11) and a theoretical study (12) suggest that synaptic plasticity thresholds are not fixed but vary according to the recent history of neuronal activity, thus modulating the capacity of synapses to undergo LTP and LTD. This higher order of synaptic plasticity or metaplasticity (13) may play an important role in stabilizing synaptic efficacy during development.
To define the state of a synapse with respect to plasticity, knowledge about two parameters is necessary, namely, its actual strength and the range within which the synaptic strength can be modified. The latter property is called dynamic range and is defined as the difference between the minimal and maximal levels of transmission. Whether the dynamic range, like synaptic plasticity thresholds, is subject to activity-dependent modulation, however, is still an open question. This issue is of fundamental importance because any modulation of dynamic range would affect the potential of synapses for plastic changes, thereby influencing information processing in the hippocampus.
In the present study, we determined the influence of NMDA receptor-mediated activity on synaptic plasticity in the developing hippocampus. For this purpose, NMDA receptors were blocked with the specific antagonist CPP [(R,S)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid; 100 μM] during the first 3 weeks of in vitro development, and the ability of CA3-CA1 synapses to undergo plastic changes was tested. We report that long-term blockade of NMDA receptor-dependent synaptic plasticity during hippocampal development reduces the threshold for induction of LTP but has no influence on the dynamic range of CA3-CA1 synapses.
Methods
Organotypic Slice Cultures.
Slice cultures were prepared from newborn (postnatal day 0) Wistar rat pups as described (14) and following a protocol approved by the Veterinary Department of the Canton of Zurich. Cultures were incubated in serum-based medium containing the NMDA receptor antagonist CPP (100 μM). Sister cultures that were exposed to medium served as controls. Medium was replaced daily in both conditions.
Electrophysiology.
After 3 weeks in vitro, cultures were transferred to a recording chamber mounted on the stage of an upright microscope (Axioscope FS, Zeiss) and superfused continuously with warmed (32°C) saline containing 137 mM NaCl, 2.7 mM KCl, 2.8 mM CaCl2, 2 mM MgCl2, 11.6 mM NaHCO3, 0.4 mM NaH2PO4, 5.6 mM glucose, and 10 mg/liter phenol red, pH 7.4. For all experiments, one recording was carried out per culture.
Intracellular recordings were obtained from CA1 pyramidal cells by using an Axoclamp-2A amplifier (Axon Instruments, Foster City, CA) and sharp microelectrodes filled with 1 M potassium methylsulfate. To evoke excitatory postsynaptic potentials (EPSPs), stimuli (0.1 ms, 5–15 μA) were delivered at 0.3 Hz by a monopolar glass stimulation electrode filled with extracellular solution and placed into the stratum radiatum close to the recording electrode. LTP was induced by synchronous pairing (SP) of synaptic stimulation with a postsynaptic burst of 10 action potentials induced by intracellular current injection (0.5–1.0 nA for 240 ms), whereas LTD was induced by asynchronous pairing (AP) in which the synaptic input was stimulated 800 ms after the postsynaptic burst (15). In both cases, pairing was repeated 100 times. Values of potentiation and depression were determined from the average slope of EPSPs over 5 min, taken 20 min after the end of the pairing procedure, and were expressed as a percentage of baseline slope, unless otherwise mentioned. Changes in EPSP slope of >10% were considered as LTP or LTD.
Whole-cell voltage-clamp recordings of synaptic currents were obtained by using an Axopatch 200B amplifier (Axon Instruments) and pipettes (3–5 MΩ) containing 140 mM potassium or cesium gluconate, 10 mM KCl, 5 mM Hepes, 1.1 mM EGTA, 4 mM MgCl2, and 10 mM phosphocreatine (pH 7.5, 285 mOsM). Excitatory postsynaptic currents (EPSCs) were evoked in the presence of 50 μM picrotoxin and 40 μM bicuculline methochloride. A cut between CA1 and CA3 areas prevented the spread of epileptic activity into area CA1. (RS)-α-amino-3-hydroxy-5-methyl-4-isoxadepropionate (AMPA) receptor-mediated EPSCs were evoked at −70 mV in the presence of a subsaturating concentration of 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX, 0.3 μM) to avoid polysynaptic events, whereas NMDA receptor-mediated EPSCs were evoked at +30 mV after full blockade of AMPA receptors by application of 20 μM NBQX. Evoked currents were analyzed offline by using PCLAMP8 (Axon Instruments).
Miniature EPSCs (mEPSCs) were recorded at −70 mV in the presence of 0.5 μM tetrodotoxin/50 μM picrotoxin/40 μM bicuculline methochloride/100 μM CPP, and 200 events from each neuron were analyzed offline by the MINI ANALYSIS program (Synaptosoft, Decatur, GA). Dual-component mEPSCs were recorded in the absence of extracellular magnesium by using a potassium gluconate-based intracellular solution containing 10 mM EGTA to prevent induction of synaptic plasticity. The NMDA receptor-mediated component of dual-component mEPSCs was revealed by subtracting the scaled mean waveform of AMPA receptor-mediated mEPSCs (recorded in the presence of 100 μM CPP) from the averaged waveform of dual-component mEPSCs (recorded in the absence of receptor antagonists). The AMPA/NMDA ratio was calculated by using peak amplitudes of the AMPA and NMDA components, respectively.
Average values are expressed as means ± SEM. Statistical comparisons were made by using Student's t tests. Tetrodotoxin was obtained from Latoxan (Valence, France), and all other drugs were from either Tocris Cookson (Bristol, U.K.) or Sigma.
Results
Increased LTP and Decreased LTD upon Chronic Blockade of NMDA Receptors.
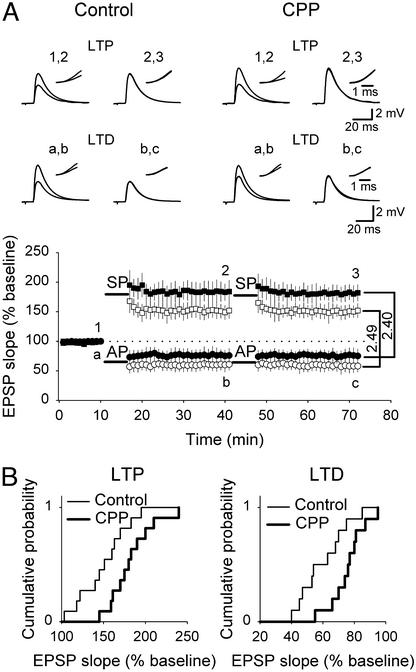
First, we investigated the ability of CA3-CA1 synapses in hippocampal organotypic slice cultures treated with CPP to undergo LTP and LTD. After washout of the NMDA receptor antagonist, intracellular recordings were obtained from CA1 pyramidal cells and LTP was induced by a SP protocol, i.e., an evoked EPSP was paired 100 times with a postsynaptic burst of action potentials induced by intracellular current injection (15). LTP was enhanced significantly in CPP-treated cultures compared with sister control cultures (control, 150 ± 8.5% of baseline EPSP slope; CPP, 183 ± 12.36% of baseline EPSP slope; n = 11 in each group, P < 0.02, Fig. 1). No additional potentiation could be induced by a second SP protocol, indicating that LTP was saturated (control, 98 ± 1.2% of potentiated EPSP slope; CPP, 99 ± 2.3% of potentiated EPSP slope; n = 11 in each group, Fig. 1A).
Figure 1.
CA3-CA1 synapses express increased LTP and decreased LTD after chronic blockade of NMDA receptors during development. (A Lower) Summary graph showing that SP induced saturated LTP in both control (□) and CPP-treated (■) slices. Significantly higher potentiation was observed in CPP-treated slices compared with control slices. In a different set of control (○) and CPP-treated (●) slices, AP induced saturated LTD. The magnitude of depression was significantly higher in control than in CPP-treated slices. Numerical values marked on the right side of the graph, obtained by normalizing the mean value of saturated LTP to the mean value of saturated LTD, show that the dynamic range is equal in the two conditions. (Upper) Representative traces of EPSPs evoked at labeled time points are illustrated. (Insets) Superimposed rising phases of evoked EPSPs at a higher magnification. (B) Cumulative distribution plots of changes in baseline EPSP slope after induction of LTP (Left) and LTD (Right), showing increased LTP and decreased LTD in CPP-treated slices compared with control slices.
LTD was induced by an AP protocol, i.e., 100 times pairing of synaptic stimulation with a preceding burst of action potentials in the postsynaptic cell (15). The magnitude of depression was significantly lower in CPP-treated slices compared with control slices (control, 60 ± 8.2% of baseline EPSP slope; CPP, 76.2 ± 6.4% of baseline EPSP slope; n = 10 in each group, P < 0.02, Fig. 1). A second AP protocol failed to induce further depression, indicating that LTD was saturated (control, 98 ± 2% of depressed EPSP slope; CPP, 96 ± 2.6% of depressed EPSP slope; n = 10 in each group, Fig. 1A).
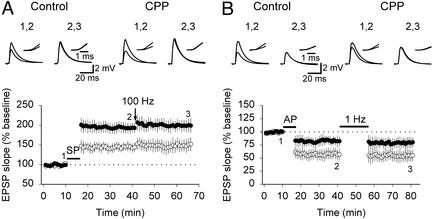
To determine whether the saturation of LTP and LTD was imposed by the stimulation protocols (SP and AP), we tested whether other established protocols for induction of synaptic plasticity could induce further changes in synaptic efficacy. LTP first was induced with SP (control, 144 ± 9.7% of baseline EPSP slope, n = 9; CPP, 193 ± 13.21% of baseline EPSP slope, n = 11; Fig. 2A), and, subsequently, three 100-Hz tetani lasting 1 s and separated by 10 s were delivered to the same excitatory afferents. Tetanic stimulation induced no additional potentiation, indicating that LTP already was saturated after SP (control, 106 ± 4.2% of potentiated EPSP slope, n = 9; CPP, 107 ± 3.89% of potentiated EPSP slope, n = 11; Fig. 2A). Next, LTD was induced with AP (control, 57 ± 11.2% of baseline EPSP slope, n = 9; CPP, 82 ± 9.4% of baseline EPSP slope, n = 10; Fig. 2B), and, subsequently, 900 stimuli were delivered to the same afferent pathway at 1 Hz (3). The latter LTD induction protocol failed to induce further depression, indicating that AP already had induced saturated LTD (control, 97 ± 2.7% of depressed EPSP slope, n = 9; CPP, 96 ± 3.6% of depressed EPSP slope, n = 10; Fig. 2B). These results suggest that LTP and LTD induced with pairing protocols reflect maximal potentiation and maximal depression of CA3-CA1 synapses, respectively.
Figure 2.
LTP and LTD induced by pairing protocols cannot be increased further by tetanic stimulation. (A Lower) Summary graph showing that SP induced saturated LTP in both control (○) and CPP-treated (●) slices, and, therefore, no further potentiation was induced with 100-Hz tetanic stimulation. (B Lower) Summary graph illustrating that 1-Hz stimulation failed to enhance LTD induced with AP in both control (○) and CPP-treated (●) slices, indicating that LTD already was saturated with AP. (A and B Upper) Representative traces of EPSPs evoked at marked time points are illustrated. (Insets) Superimposed rising phases of evoked EPSPs at a higher magnification.
Although it is well established that in the CA1 area of the hippocampus, induction of both LTP and LTD depends on the activation of NMDA receptors (3–5), we tested whether this dependence persisted after chronic blockade of NMDA receptors with CPP or whether alternative “reserve mechanisms” were involved (16). In the presence of bath-applied CPP (100 μM), SP and AP protocols did not result in potentiation (control, 98 ± 2% of baseline EPSP slope, n = 5; CPP, 99 ± 3% of baseline EPSP slope, n = 6) and depression, respectively (control, 97 ± 3% of baseline EPSP slope, n = 5; CPP, 97 ± 4% of baseline EPSP slope, n = 6). After washout of acutely applied CPP, significant potentiation (control, 137 ± 13% of baseline EPSP slope, n = 5; CPP, 167 ± 17% of baseline EPSP slope, n = 6) and depression (control, 53 ± 10% of baseline EPSP slope, n = 5; CPP, 70 ± 12% of baseline EPSP slope, n = 6) were induced, indicating that NMDA receptor activation is required for the induction of LTP and LTD in both control and CPP-treated slices.
Next, we tested whether the excitability of CA1 pyramidal cells was affected by chronic blockade of NMDA receptors, because an increase in cell excitability may account for the prevalence of LTP over LTD. The number of spikes that cells fired during injection of depolarizing current pulses (150 ms, 0.5 nA) was measured. No difference in spike number was observed between control and CPP-treated slices (control, 4.76 ± 0.17, n = 18; CPP, 4.83 ± 0.15, n = 21; P > 0.07). In addition, resting membrane potential (control, −63 ± 1.1 mV, n = 69; CPP, −64 ± 2.1 mV, n = 77; P > 0.06) and input resistance of CA1 pyramidal cells (control, 57.9 ± 2.8 MΩ, n = 69; CPP, 61.3 ± 3.4 MΩ, n = 77; P > 0.07) were equal in both conditions. These results indicate that the excitability of CA1 pyramidal cells was not changed after chronic NMDA receptor blockade.
Dynamic Range Is Not Affected by Chronic Blockade of NMDA Receptors.
To compare the dynamic range of CA3-CA1 synapses in control vs. CPP-treated slices, we normalized the maximal potentiation (saturated LTP) to the maximal depression (saturated LTD). Maximal potentiation in control and CPP-treated slices corresponded to a 2.49- and 2.40-fold increase of maximally depressed EPSPs, respectively, suggesting that the dynamic range was unaffected by CPP treatment (see graph in Fig. 1A).
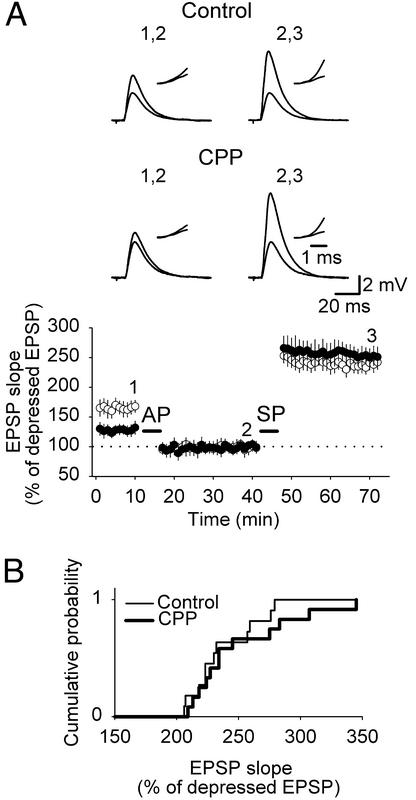
To determine the dynamic range directly, LTD and LTP were evoked at the same set of CA3-CA1 synapses. When LTP was induced in previously maximally depressed synapses, maximal potentiation was equal in both control and CPP-treated slices (control, 237.27 ± 9.2% of depressed EPSP slope, n = 11; CPP, 251.17 ± 12.24% of depressed EPSP slope, n = 12; P > 0.3, Fig. 3), again indicating no difference in the dynamic range between the two conditions. Together, these findings show that CA3-CA1 synapses in CPP-treated slices can be more potentiated and less depressed with respect to control slices, whereas the dynamic range stays equal. This finding suggests that synapses in CPP-treated slices are closer to their maximally depressed state than synapses in control slices, most likely because of the absence of spontaneous in vitro potentiation due to chronic blockade of NMDA receptors.
Figure 3.
The dynamic range of CA3-CA1 synapses is unaffected by chronic NMDA receptor blockade. (A Lower) LTD was induced with AP, and, subsequently, LTP was evoked by SP. All experiments performed in control (○) and CPP-treated (●) slices were pooled together and summarized in the graph, showing that maximal potentiation of previously maximally depressed EPSPs was equal in control and CPP-treated slices. (Upper) Representative traces of EPSPs evoked at labeled time points are illustrated. (Insets) A higher magnification of superimposed rising phases of evoked EPSPs. (B) Cumulative distributions of potentiations of previously depressed EPSPs in control and CPP-treated slices show no significant difference in the two conditions.
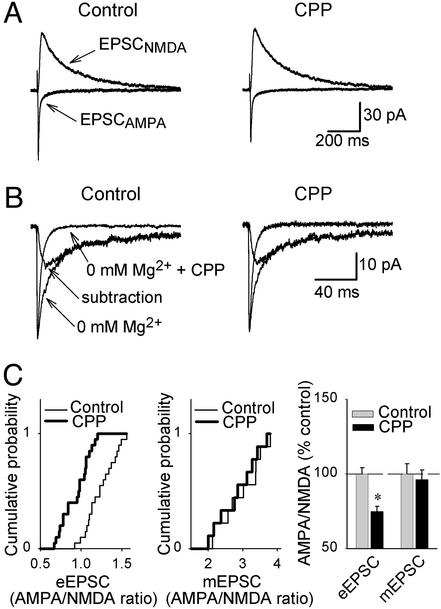
To test this hypothesis, we compared the state of synaptic potentiation in control vs. CPP-treated slices by measuring the relative contribution of AMPA and NMDA receptors to evoked synaptic transmission (the AMPA/NMDA current ratio), because LTP is known to increase this ratio (17). Therefore, AMPA and NMDA receptor-mediated EPSCs were elicited at holding potentials of −70 mV and +30 mV, respectively, and the amplitude ratio was determined. A significantly lower evoked AMPA/NMDA current ratio was found in CPP-treated slices compared with control slices (control, 1.24 ± 0.04; CPP, 0.93 ± 0.04, 75 ± 3.23% of control; n = 20 in each group, P < 0.001, Fig. 4 A and C). This alteration could reflect a higher proportion of silent synapses in CPP-treated slices, provided the AMPA vs. NMDA receptor content at functional synapses was unaffected by chronic blockade of NMDA receptors. In this context, we define silent synapses as synapses displaying only NMDA receptor-mediated EPSCs (18–21) and functional synapses as synapses displaying both AMPA and NMDA receptor-mediated postsynaptic responses. To assess whether chronic blockade of NMDA receptors leads to a change in the relative number of AMPA and NMDA receptors at functional synapses, we recorded dual-component mEPSCs in the absence of extracellular magnesium. We found that the amplitude ratio of current mediated by AMPA and NMDA receptors at functional synapses, respectively, was unchanged in CPP-treated slices compared with control slices (miniature AMPA/NMDA current ratio: control, 2.96 ± 0.2; CPP, 2.85 ± 0.19; n = 9 in each group, P > 0.6; Fig. 4 B and C). In addition, the amplitude of NMDA receptor-mediated mEPSCs, calculated by subtraction of pure AMPA receptor-mediated mEPSCs (recorded in the presence of CPP) from the dual-component mEPSCs, was equal in control and CPP-treated slices (control, 9.6 ± 0.3 pA; CPP, 9.8 ± 0.6 pA; n = 9 in each group, P > 0.2). The amplitude of AMPA receptor-mediated mEPSCs, recorded in a different set of experiments, also was unaffected by chronic blockade of NMDA receptors (control, 22.87 ± 1.16 pA; CPP, 20.53 ± 0.8 pA; n = 14 in each group, P > 0.1). These results suggest that the relative contribution of AMPA and NMDA receptors at functional synapses was not affected by chronic blockade of NMDA receptors.
Figure 4.
Chronic blockade of NMDA receptors decreases the AMPA/NMDA ratio of evoked EPSCs (eEPSCs) but not of mEPSCs. (A) Representative traces of evoked EPSCAMPA and EPSCNMDA recorded in the same cell at −70 mV and +30 mV, respectively, from control and CPP-treated slices. (B) Superimposed averaged traces of mEPSCs from control and CPP-treated slices recorded in the absence of extracellular magnesium and during application of CPP (100 μM), together with traces of mEPSCNMDA obtained by their subtraction. (C) Cumulative distributions of the AMPA/NMDA ratios of evoked EPSCs (Left), miniature EPSCs (Center), and their normalized values (Right) in control and CPP-treated slices. A significantly lower evoked AMPA/NMDA current ratio was found in CPP-treated slices. * indicates statistical significance (P < 0.05).
In summary, chronic blockade of NMDA receptors leads to a lower AMPA/NMDA ratio of evoked EPSCs but not of mEPSCs, suggesting reduced spontaneous conversion of silent synapses to functional synapses in CPP-treated slices.
An alternative explanation for a lower AMPA/NMDA ratio of evoked EPSCs could be that CPP-treated slices exhibit a decreased efficiency of glutamate uptake compared with control slices. As a consequence of decreased uptake, spillover of glutamate might lead to the activation of NMDA receptors at neighboring synapses, thereby resulting in a lower overall AMPA/NMDA ratio. Because it has been demonstrated that interfering with glutamate uptake prolongs the time course of evoked NMDA receptor-mediated synaptic currents (22), we compared the decay time of evoked NMDA receptor-mediated EPSCs in control and CPP-treated slices. No change in the decay time was observed between the two conditions (control, 171 ± 14 ms, n = 18; CPP, 167 ± 11 ms, n = 21; P > 0.2), suggesting that chronic blockade of NMDA receptors does not affect glutamate uptake.
Lower LTP Threshold upon Chronic Blockade of NMDA Receptors.
It has been shown that synaptic plasticity thresholds are modifiable, thereby affecting the capacity of synapses for plastic changes (8–11). We therefore investigated, using two different approaches, whether the LTP threshold is affected by chronic blockade of NMDA receptors during development. First, we modified the temporal relationship between presynaptic and postsynaptic activity, which is known to determine the magnitude and direction of synaptic plasticity (15, 23), by introducing a delay of 50 ms between postsynaptic bursting and synaptic stimulation (AP* protocol). This stimulation protocol, repeated 100 times, induced moderate LTP in CPP-treated slices but no potentiation in control slices (control, 102.33 ± 2.96% of baseline EPSP slope, n = 9, P > 0.1; CPP, 129.2 ± 4.02% of baseline EPSP slope, n = 10, P < 0.01; Fig. 5A). To confirm that the stimulated CA3-CA1 synapses in control slices could be potentiated, LTP was induced with the SP protocol repeated 100 times (146.66 ± 9.7% of baseline EPSP slope, n = 9, Fig. 5A). As predicted, SP induced further potentiation in CPP-treated slices (176.4 ± 12.72% of baseline EPSP slope, n = 10, Fig. 5A), reaching a level of potentiation similar to that already shown to be saturating (see Fig. 1A).
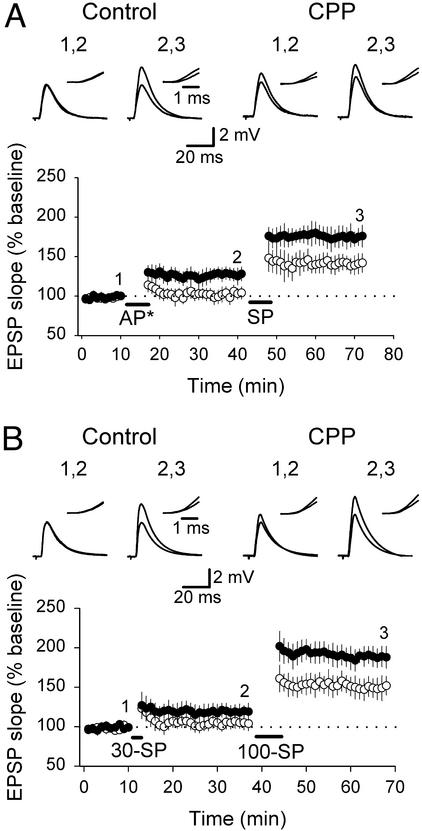
Figure 5.
Induction of LTP in CPP-treated slices occurs at a lower threshold. (A Lower) Summary plot showing that AP* (synaptic stimulation 50 ms after the postsynaptic burst) failed to induce LTP in control slices (○) whereas it produced LTP in CPP-treated slices (●). Subsequent SP resulted in additional potentiation in CPP-treated slices and induced LTP in control slices. (B) SP repeated 30 times induced LTP in CPP-treated slices (●) but no potentiation in control slices (○). Subsequent SP repeated 100 times resulted in LTP in control slices and induced even higher potentiation in CPP-treated slices. (A and B Upper) Example traces of EPSPs evoked at labeled time points are illustrated. (Insets) Superimposed rising phases of evoked EPSCs at a higher magnification.
In the second approach, we maintained the synchrony between presynaptic and postsynaptic activity but reduced the number of pairings from 100 to 30. Repeating SP 30 times failed to induce LTP in control slices but resulted in a significant potentiation in CPP-treated slices (control, 105.43 ± 3.12% of baseline EPSP slope, n = 5, P > 0.1; CPP, 118.4 ± 5.02% of baseline EPSP slope, n = 7, P < 0.03; Fig. 5B). When SP was repeated 100 times, LTP was induced in control slices (151 ± 10.34% of baseline EPSP slope, n = 5, Fig. 5B), indicating that these CA3-CA1 synapses had the ability to undergo potentiation but that 30 pairings were not sufficient to induce LTP. Additional potentiation was induced in CPP-treated slices with 100 SPs (188 ± 11.32% of baseline EPSP slope, n = 7, Fig. 5B).
In summary, these results indicate that the LTP threshold is lower in CPP-treated slices compared with control slices, thereby facilitating induction of LTP.
It has been reported that an increase in the 2B subunit of NMDA receptors after binocular deprivation can account for a decrease in LTP threshold in the visual cortex (24). Therefore, we tested the sensitivity of evoked NMDA receptor-mediated EPSCs to ifenprodil (3 μM), a selective antagonist of 2B subunit-containing NMDA receptors. The ifenprodil-induced current decrease was not significantly different between control and CPP-treated slices (control, 60.26 ± 3.62% of decrease; CPP, 66.07 ± 4.48% of decrease; n = 13 in each group, P > 0.3). This result indicates that the lower LTP threshold in CPP-treated slices is not caused by changes in the proportion of the 2B subunit-containing NMDA receptors.
Discussion
The principal finding of this study is that chronic blockade of NMDA receptors during early development fails to affect the dynamic range within which the strength of CA3-CA1 synapses can be modulated but results in a lower threshold for synaptic potentiation.
Our data show that the dynamic range of CA3-CA1 synapses, defined as the range between the minimal (saturated LTD) and maximal (saturated LTP) levels of synaptic transmission, was equal in control and CPP-treated slices. CA3-CA1 synapses were in the middle of the available dynamic range in control slices but closer to LTD saturation in CPP-treated slices (see Figs. 1A and 3A). This finding can be explained either by different levels of potentiation of functional synapses in control vs. CPP-treated slices or by a difference in the ratio of silent to functional synapses in the two conditions. We did not find changes in the amplitude of AMPA receptor-mediated mEPSCs and the AMPA/NMDA ratio of mEPSCs, but a significantly higher AMPA/NMDA ratio of evoked synaptic currents was observed in control slices with respect to CPP-treated slices. In addition, our results rule out the possibility that a lower AMPA/NMDA ratio of evoked EPSPs arises from a decreased efficiency of glutamate uptake in CPP-treated slices. Taken together, these results suggest the presence of a smaller proportion of silent synapses in control slices than in CPP-treated slices. Given the role of LTP in the conversion of silent synapses into functional ones (18–21), it is likely that spontaneous synaptic potentiation reduced the proportion of silent synapses in control slices. In contrast, NMDA receptor-dependent synaptic plasticity was absent in CPP-treated slices, and, therefore, the functional conversion of silent synapses was prevented. In accordance with our findings, a developmentally regulated gradual increase in the AMPA/NMDA ratio of evoked synaptic currents (25) without concomitant change in the amplitude of mEPSCs (25, 26) was described in the hippocampus. In our previous report (27), we showed that 2 weeks of CPP treatment did not change the evoked AMPA/NMDA current ratio, suggesting that, in our preparation, spontaneous potentiation occurred mainly during the third week of in vitro development (see also ref. 28).
In principle, the prevalence of LTP over LTD in CPP-treated cultures could be explained by an increased excitability of CA1 pyramidal cells or by up-regulation of NMDA receptors in functional synapses. Our results are not consistent with these possibilities but, rather, suggest an NMDA receptor-dependent shift in baseline synaptic efficacy within a stable dynamic range during hippocampal development. It has been shown recently in the cortex that motor skill learning increases baseline synaptic efficacy without affecting the “synaptic modification range” (29). This opens the possibility that stability of the dynamic range may be a general synaptic property during development and learning.
We propose that after 3 weeks of in vitro development, the baseline synaptic efficacy settles in the middle of the available dynamic range, thereby maximizing the capacity of CA3-CA1 synapses for bidirectional changes in efficacy. Similar results have been reported in acute hippocampal slices from 2-week-old rats (30) and hippocampal slice cultures prepared from 6-day-old rats and maintained for at least 2 weeks in vitro (31).
An intriguing question concerns the limits confining the dynamic range of CA3-CA1 synapses. Although the concept of LTP saturation has been challenged (13, 32), it may represent the state in which all silent synapses are converted into functional synapses. Similarly, saturated LTD may reflect the silencing of all plastic functional synapses, whereas nonplastic synapses (33) maintain the minimal level of synaptic transmission between connected neurons. Consistent with this possibility are the findings that upon LTD induction only a proportion of CA3-CA1 pairs becomes silent (34) and that interruption of the N-ethyl-maleimide-sensitive fusion protein–GluR2 interaction, an intracellular pathway involved in the expression of LTD, only partially blocks evoked EPSCs (35). It could be argued that the limits in the dynamic range are imposed by the stimulation protocols used to induce synaptic plasticity. This possibility is unlikely because 100-Hz tetanic stimulation failed to further potentiate synapses in which saturated LTP had been induced by using the SP protocol. Similarly, the magnitude of LTD induced with AP could not be increased by 1-Hz tetanic stimulation.
In addition, our data show that the LTP threshold is lower in CPP-treated slices than in control slices, thereby accounting for the facilitated induction of LTP. Because CPP treatment did not affect the sensitivity of NMDA receptor-mediated evoked synaptic currents to ifenprodil, it is unlikely that the lower LTP threshold in CPP-treated slices resulted from changes in the subunit composition of NMDA receptors. Our results show that a higher AMPA/NMDA ratio of evoked EPSCs and a higher LTP threshold were found in control slices with respect to CPP-treated slices. This correlation between the AMPA/NMDA ratio and the LTP threshold suggests the following scenario: Individual CA3-CA1 synapses have different LTP thresholds (36). During development, silent synapses with a lower threshold are potentiated first, and, as development proceeds, the remaining synapses have a higher threshold for potentiation. Therefore, after 3 weeks of in vitro development, the average LTP threshold in control slices is higher than in CPP-treated slices, where potentiation of synapses with lower threshold is prevented.
Taken together, our data show that NMDA receptor-dependent functional maturation of CA3-CA1 synapses does not affect the dynamic range over which their efficacy can be modulated but results in a higher threshold for the induction of LTP, thereby decreasing the probability that further synaptic potentiation will occur. Thus, CA3-CA1 synapses formed in the absence of NMDA receptor-mediated synaptic activity and those that experienced NMDA receptor-mediated synaptic plasticity during development operate within the same dynamic range. Stability of the dynamic range during hippocampal development may be important in preventing excessive changes in synaptic gain, thus ensuring that neurons will remain functional within a meaningful physiological range.
Acknowledgments
We thank L. Rietschin for excellent technical assistance and Dr. M. Abegg and Profs. M. Scanziani and U. Gerber for stimulating discussions and reading of the manuscript. This work was supported by Swiss National Science Foundation Grant 31-61518.00.
Abbreviations
- LTP
long-term potentiation
- LTD
long-term depression
- NMDA
N-methyl-d-aspartate
- AMPA
(RS)-α-amino-3-hydroxy-5-methyl-4-isoxadepropionate
- EPSP
excitatory postsynaptic potential
- EPSC
excitatory postsynaptic current
- mEPSC
miniature EPSC
- SP
synchronous pairing
- AP
asynchronous pairing
- CPP
(R,S)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid
References
- 1.Bliss T V, Collingridge G L. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Malenka R C, Nicoll R A. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 3.Dudek S M, Bear M F. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulkey R M, Malenka R C. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 5.Collingridge G L, Kehl S J, McLennan H. J Physiol (London) 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings J A, Mulkey R M, Nicoll R A, Malenka R C. Neuron. 1996;16:825–833. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- 7.Zucker R S. Curr Opin Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]
- 8.Kirkwood A, Rioult M C, Bear M F. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 9.Abraham W C, Mason-Parker S E, Bear M F, Webb S, Tate W P. Proc Natl Acad Sci USA. 2001;98:10924–10929. doi: 10.1073/pnas.181342098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wexler E M, Stanton P K. NeuroReport. 1993;4:591–594. doi: 10.1097/00001756-199305000-00034. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y Y, Colino A, Selig D K, Malenka R C. Science. 1992;255:730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- 12.Bienenstock E L, Cooper L N, Munro P W. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham W C, Bear M F. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 14.Gähwiler B H, Thompson S M, McKinney R A, Debanne D, Robertson R T. In: Culturing Nerve Cells. Banker G, Goslin K, editors. Cambridge, MA: MIT Press; 1998. pp. 461–498. [Google Scholar]
- 15.Debanne D, Gähwiler B H, Thompson S M. Proc Natl Acad Sci USA. 1994;91:1148–1152. doi: 10.1073/pnas.91.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J J, Malinow R. Nat Neurosci. 2002;5:513–514. doi: 10.1038/nn0602-850. [DOI] [PubMed] [Google Scholar]
- 17.Kauer J A, Malenka R C, Nicoll R A. Neuron. 1988;1:911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- 18.Durand G M, Kovalchuk Y, Konnerth A. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 19.Isaac J T, Nicoll R A, Malenka R C. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Liao D, Hessler N A, Malinow R. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery J M, Pavlidis P, Madison D V. Neuron. 2001;29:691–701. doi: 10.1016/s0896-6273(01)00244-6. [DOI] [PubMed] [Google Scholar]
- 22.Arnth-Jensen N, Jabaudon D, Scanziani M. Nat Neurosci. 2002;5:325–331. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- 23.Markram H, Lubke J, Frotscher M, Sakmann B. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 24.Philpot B D, Sekhar A K, Shouval H Z, Bear M F. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 25.Hsia A Y, Malenka R C, Nicoll R A. J Neurophysiol. 1998;79:2013–2024. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- 26.Petralia R S, Esteban J A, Wang Y X, Partridge J G, Zhao H M, Wenthold R J, Malinow R. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- 27.Lüthi A, Schwyzer L, Mateos J M, Gähwiler B H, McKinney R A. Nat Neurosci. 2001;4:1102–1107. doi: 10.1038/nn744. [DOI] [PubMed] [Google Scholar]
- 28.Muller D, Buchs P-A, Stoppini L. Dev Brain Res. 1993;71:93–100. doi: 10.1016/0165-3806(93)90109-n. [DOI] [PubMed] [Google Scholar]
- 29.Rioult-Pedotti M-S, Friedman D, Donoghue J P. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 30.Dudek S M, Bear M F. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debanne D, Gähwiler B H, Thompson S M. J Neurophysiol. 1997;77:2851–2855. doi: 10.1152/jn.1997.77.5.2851. [DOI] [PubMed] [Google Scholar]
- 32.Frey U, Schollmeier K, Reymann K G, Seidenbecher T. Neuroscience. 1995;67:799–807. doi: 10.1016/0306-4522(95)00117-2. [DOI] [PubMed] [Google Scholar]
- 33.Debanne D, Gähwiler B H, Thompson S M. J Neurosci. 1999;19:10664–10671. doi: 10.1523/JNEUROSCI.19-24-10664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montgomery J M, Madison D V. Neuron. 2002;33:765–777. doi: 10.1016/s0896-6273(02)00606-2. [DOI] [PubMed] [Google Scholar]
- 35.Lüthi A, Chittajallu R, Duprat F, Palmer M J, Benke T A, Kidd F L, Henley J M, Isaac J T, Collingridge G L. Neuron. 1999;24:389–399. doi: 10.1016/s0896-6273(00)80852-1. [DOI] [PubMed] [Google Scholar]
- 36.Petersen C C, Malenka R C, Nicoll R A, Hopfield J J. Proc Natl Acad Sci USA. 1998;95:4732–4737. doi: 10.1073/pnas.95.8.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]