Abstract
The β3-adrenoreceptor plays a major role in lipolysis but the role and distribution of β3-receptors in other specific sites have not been extensively studied. β3-adrenergic receptors are present not only in adipose tissue but also in human gall bladder, colon, prostate, and skeletal muscle. Recently, β3-adrenergic receptor stimulation was shown to elicit vasorelaxation of rat aorta through the NO–cGMP signal transduction pathway. Here we show that β3-receptors are present in human corpus cavernosum and are localized mainly in smooth muscle cells. After activation by a selective β3-adrenergic receptor agonist, BRL 37344, there was a cGMP-dependent but NO-independent vasorelaxation that was selectively blocked by a specific β3-receptor antagonist. In addition, we report that the human corpus cavernosum exhibits basal β3-receptor-mediated vasorelaxant tone and that β3-receptor activity is linked to inhibition of the RhoA/Rho-kinase pathway. These observations indicate that β3-receptors may play a physiological role in mediating penile erection and, therefore, could represent a therapeutic target for treatment of erectile dysfunction.
Keywords: erectile dysfunction‖Rho kinase‖endothelin
Erectile dysfunction (ED) is defined as the inability to achieve and maintain an erection sufficient to permit satisfactory sexual intercourse. ED is the result of psychological, neurologic, hormonal, arterial, or cavernosal impairment or a combination of two or more of these factors. After sexual stimulation, nerve impulses cause the release of neurotransmitters, including nitric oxide (NO), from the cavernous nerve terminal, resulting in the relaxation of arteries and arterioles with a consequent increase in penile blood flow. NO is also generated by vascular endothelial cells in the corpus cavernosum in response to increased penile blood flow. This process, together with relaxation of the trabecular smooth muscle leading to an increase in the compliance of sinusoids, results in the filling and expansion of the sinusoidal system (1).
NO seems to be the principal mediator released both by nerves and endothelial cells after sexual stimulation. The vascular smooth muscle relaxant effect of NO is mediated by cGMP, and the tissue levels of cGMP in the corpus cavernosum are regulated by phosphodiesterase-5 (2, 3). The pathophysiology of ED was not well understood until the discovery was made that NO is the principal neurotransmitter of the nonadrenergic/noncholinergic nerves that innervate the corpus cavernosum, and that the NO–cGMP signal transduction pathway is the main effector of penile erection (4–6). These findings were followed by the availability of sildenafil for the oral treatment of ED. Despite the clinical success of sildenafil, the physiology of the corpus cavernosum and the pathophysiology of ED are still incompletely understood (3).
The β3-adrenergic receptor subtype of sympathetic nervous system has been extensively characterized at the structural level (7, 8). Humans, other large mammalian species, and rodents possess β3-receptors that share most of the characteristic properties, although some species-specific differences have been identified. The β3-receptor plays a major role in lipolysis but the role and distribution of β3-receptors in other specific sites have not been extensively studied. Tissue distribution studies have demonstrated the presence of β3-receptors not only in adipose tissues but also in human gall bladder and more recently in colon, prostate, and skeletal muscle (9–11). In addition, β3-receptor stimulation has been recently demonstrated to elicit vasorelaxation of rat aorta through activation of NO synthase and the subsequent increase in tissue levels of cGMP (12). Here we show that β3-receptors are present in human corpus cavernosum (HCC) and that their activation causes a cGMP-dependent but NO-independent vasorelaxation that involves the Rho-kinase pathway.
Materials and Methods
Human Tissues.
In male-to-female transsexual surgical procedures, the penis and testicles are amputated and a neovagina is created to simulate female external genitalia by using techniques that have been described (13). Patients underwent appropriate hormonal pretreatment with antiandrogens and estrogens to adapt to female appearance, and the therapy was discontinued 2 months before surgery. The corpora cavernosa were carefully excised from the amputated penis and placed in ice-cold oxygenated Krebs solution and washed extensively with heparinized Krebs solution. After the lavage, the corpora cavernosa were placed in ice-cold Krebs solution and kept on ice until the experiments were conducted, within 2 h. All patients were informed of all procedures and gave their written consent. The protocol was approved by the Ethics Committee of the Medical School of the University of Naples.
HCC Strips.
Longitudinal strips (2 cm) were dissected after the trabecular structure of the penis (5, 13). Isolated HCC strips were immediately placed in Krebs solution and kept at 4°C until use. Krebs solution had the following composition (mM): 115.3 NaCl/4.9 KCl/1.46 CaCl2/1.2 MgSO4/1.2 KH2PO4/25.0 NaHCO3/11.1 glucose. HCC strips were mounted in a 2-ml organ bath containing oxygenated (95% O2/5% CO2) Krebs solution at 37°C. HCC strips were connected to isometric force transducers (Model 7002, Ugo Basile, Comerio, Italy) and changes in tension were recorded continuously by using a polygraph linearcorder (WR3310, Graphtec, Yokohama, Japan). Tissues were preloaded with 2 g of tension and allowed to equilibrate for 90 min in Krebs solution that was changed at 15-min intervals. After equilibration, tissues were standardized by using repeated phenylephrine (PE; 1 μM)-elicited contractile responses until equal responses were obtained. After standardization, endothelial integrity was assessed by testing relaxant responses to 1 μM acetylcholine (ACh; Sigma). Strips that did not attain a relaxation of at least 70% were discarded. To evaluate tissue relaxant responsiveness, HCC strips were precontracted with 1 μM PE or human endothelin-1 (hET-1; 10 and 30 nM, respectively; Tocris Neuramin, Bristol, U.K.), and once a stable contraction was achieved (about 15 min), cumulative concentration responses to ACh (0.01–3 μM), the β3-receptor selective agonist BRL 37344 (10–300 μM; Tocris Neuramin), and sodium nitroprusside (SNP; 0.01–3 μM) were obtained. To assess the functional role of the vascular endothelium in relaxation of HCC strips, endothelium-denuded strips were tested. HCC strips were denuded of functional endothelium by incubating strips with distilled water for 15 sec. Strips that showed <5% relaxation to 1 μM ACh were used to obtain cumulative concentration responses to BRL 37344. The concentration responses to BRL 37344 were also performed in the presence of Krebs solution containing l-NG-nitroarginine-methylester (l-NAME; 100 μM; Sigma), methylene blue (10 μM; Sigma), atropine (1 μM; Sigma), guanethidine (5 μM; Sigma), tetraethylammonium (10 and 50 mM; Sigma), indomethacin (10 μM; Sigma), propranolol (30 μM; Sigma), butoxamine (30 μM; Sigma), 1-H-[1,2,4]oxadiazolo [4,3a]quinoxalin-1-one (ODQ; 10 μM; Tocris Neuramin), and charybdotoxin (100 nM; Sigma) plus apamin (1 μM; Sigma) or SR 59230A (Tocris Neuramin).
cGMP and cAMP Determinations.
HCC strips were placed in 24-well plates containing 1 ml of Krebs solution at 37°C and incubated for 30 min. 1-Methyl-3-isobutylxanthine (IBMX, 10 μM) was added to all wells 10 min before BRL 37344. All antagonists or inhibitors were added 5 min before the addition of BRL 37344. ACh (1 μM) and SNP (1 μM) were also tested as positive controls. After a 30-min incubation time, the strips were rapidly blotted and quick-frozen in liquid nitrogen. One milliliter of buffer (containing 5% trichloroacetic acid and 10 μM IBMX) per gram of tissue was added to each sample. Samples were homogenized and the precipitate was sedimented by centrifugation at 1,500 × g for 10 min. The supernatant was carefully removed and used in the next step (sediment was discarded). Residual trichloroacetic acid was removed by extraction into 5 vol of water-saturated diethyl ether (repeated twice for a total of 3 extractions). Any residual ether was removed by warming the samples at 50°C for 5 min. The samples were then processed according to the instructions provided with the kit (Cayman Chemical, Ann Arbor, MI) for determination of cAMP and cGMP levels. Data are expressed as pmol/g of wet weight tissue and each sample was run in duplicate.
Immunohistochemistry.
Strips of HCC were placed in 4% formalin and embedded in paraffin. Sections were deparaffinized and permeabilized with 0.1% Triton X-100 in PBS for 10 min and rehydrated with PBS at room temperature. Endogenous peroxidase activity was quenched by addition of 0.3% H2O2 in 60% methanol. Nonspecific adsorption was minimized by incubating tissue sections with PBS containing 1% BSA. Tissue sections were incubated with a primary Ab, anti-human β3-adrenergic receptor (Alpha Diagnostic International, San Antonio, TX) in a dilution of 1:500. The secondary Ab was goat anti-rabbit biotinylated IgG in a dilution of 1:20,000 followed by treatment with streptavidin conjugated to alkaline phosphatase according to the directions provided by the manufacturer (DAKO). The development of the reaction was performed by using AS-MX naphthosulfate (0.02%) and new fucsin (4% in 2 M HCl) in 0.1 M Tris buffer, pH 8.2. Positively stained areas appeared in red.
Western Blot Analysis.
HCC strips were homogenized in lysis buffer (β-glycerophosphate, 0.5 mM/sodium orthovanadate, 0.1 mM/MgCl2, 2 mM/EGTA, 1 mM/DTT, 1 mM) and protease inhibitors by using an Ultra-Turrax homogenizer (IKA-LABORTECHNIK, Staufen, Germany). Protein concentrations were determined by using the Bradford assay (Bio-Rad). Protein samples (50 μg) were subjected to electrophoresis on an SDS/10% polyacrylamide gel and electrophoretically transferred onto a nitrocellulose transfer membrane (Protran, Schleicher & Schuell). The immunoblots were developed with 1:500 dilutions of the indicated Abs, and the signal was detected with the enhanced chemiluminescence system according to the manufacturer's instructions (Amersham Biosciences). Anti-β3 Ab was purchased from Santa Cruz Biotechnology and Alpha Diagnostic International. All salts used for Western blot analysis were purchased from ICN.
Results and Discussion
The principal findings of this study are the expression of β3-adrenergic receptors localized mainly in smooth muscle cells in HCC, and that activation by a selective β3-receptor agonist elicits relaxation of HCC smooth muscle via a cGMP-dependent but NO-independent mechanism.
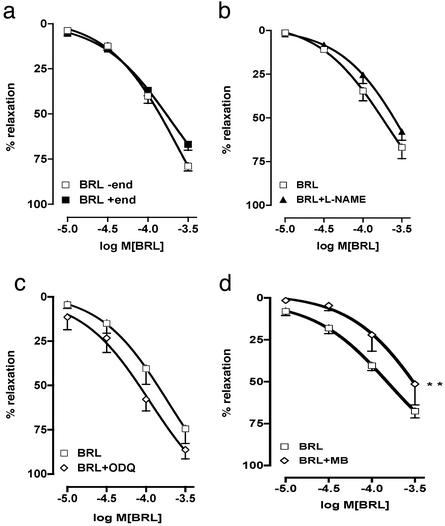
HCC strips that had been precontracted with PE relaxed in a concentration-dependent manner to the addition of cumulative concentrations of BRL 37344 both in the presence and absence of a functional endothelium, as shown in Fig. 1a. The EC50 for BRL 37344 in the presence of endothelium was 0.194 μM (0.100 μM, lower confidence limit, to 0.370 μM, upper confidence limit), whereas in the absence of endothelium the EC50 for BRL 37344 was 0.24 μM (0.130–0.370 μM). Therefore, the smooth muscle relaxant responses to β3-receptor stimulation were independent of the vascular endothelium. The complete lack of effect of 100 μM l-NAME (Fig. 1b), an inhibitor of NO synthase isoforms including endothelial NO synthase, is consistent with this view and indicates that endothelium-derived NO is not involved. To rule out any involvement of β1- and β2-adrenergic receptors in the relaxant responses to BRL 37344, propranolol (nonselective antagonist of β1- and β2-receptors) and butoxamine (selective antagonist of β2-receptors) were tested and found to have no effects (data not shown). The latter observations are in agreement with those from a report in which analysis of the effects of selective β1- and β2-adrenergic receptor antagonists on relaxant responses in HCC suggested the existence of a third receptor type (14). To determine any role for prostanoids or nonadrenergic/noncholinergic neurons in the relaxant responses to BRL 37344, indomethacin or atropine in the presence or absence of guanethidine, respectively, was tested and found to exert no appreciable influence (data not shown). Therefore, relaxation of HCC smooth muscle in response to BRL 37344 seemed to be mediated by endothelium- and NO-independent β3-adrenergic receptors.
Figure 1.
Relaxant effects of BRL 37344 on HCC strips. (a) BRL 37344 (BRL) relaxed HCC strips in the presence (+ end) and absence (− end) of endothelium. (b) l-NAME (100 μM) did not inhibit BRL 37344-induced vasorelaxation of HCC. (c) 1-H-[1,2,4]oxadiazolo [4,3a]quinoxalin-1-one (10 μM) did not inhibit BRL 37344-induced vasorelaxation. (d) Methylene blue (MB; 10 μM) inhibited BRL 37344-induced vasorelaxation. Data were analyzed by using ANOVA. **, P < 0.01, significantly different from corresponding control values. The data represent the mean ± SEM from eight separate experiments (eight strips per experiment).
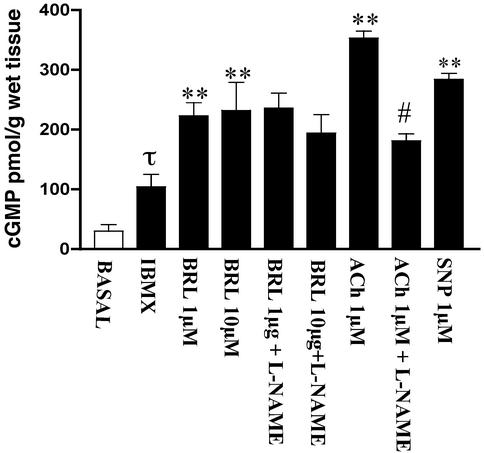
Activation of β3-adrenergic receptors in various tissues generally seems to be coupled to the generation of the second messenger, cAMP (7). It is well recognized that both cAMP and cGMP cause smooth muscle relaxation by lowering intracellular levels of free calcium. Therefore, the possible involvement of cyclic nucleotides in the smooth muscle relaxant responses to BRL 37344 was assessed by measuring tissue levels of cAMP and cGMP by specific ELISA. HCC strips possessing a functional endothelium were used in these experiments. BRL 37344 did not produce any significant effects on tissue cAMP levels. These observations are consistent with the findings that the relaxant response to BRL 37344 was not modified by antagonists of either β1- or β2-adrenergic receptors. Therefore, no further experiments involving cAMP were made in this study. IBMX was added to prevent any rapid hydrolysis of cyclic nucleotides by tissue phosphodiesterases. Addition of IBMX caused a 3-fold increase in cGMP levels (Fig. 2). In the presence of IBMX, BRL 37344 caused a >2-fold increase in tissue cGMP levels that was not affected by addition of 100 μM l-NAME, whereas the cGMP response to ACh was markedly inhibited by l-NAME (Fig. 2). These observations indicate that the cGMP response to BRL 37344 was not mediated by endothelium-derived NO. The increase in HCC levels of cGMP caused by BRL 37344 is not unique in that similar effects were observed in vascular tissue (12). Whether the increase in cGMP levels occurs in both vascular and nonvascular smooth muscle in HCC is unknown at present but it is likely that both are involved. These data suggest that cGMP rather than cAMP is involved in mediating the relaxant response to BRL 37344.
Figure 2.
Influence of BRL 37344 on cGMP levels in HCC. Refer to Materials and Methods for experimental details. Addition of IBMX caused a 3-fold increase in cGMP levels (τ, P < 0.05 vs. basal). Addition of BRL 37344 (BRL; 1 and 10 μM) caused a significant increase in cGMP (**, P < 0.01 vs. IBMX) that was not inhibited by l-NAME. In the same assay, ACh caused an increase in cGMP (P < 0.01 vs. IBMX) that was significantly inhibited by l-NAME (#, P < 0.01 vs. ACh). Experiments were from four different HCC strips and each sample was run in triplicate. The results are expressed as mean ± SEM, and data were compared by ANOVA followed by Bonferroni post hoc test.
The increase in cGMP levels over basal levels in HCC after addition of BRL 37344 in the presence of IBMX was 6- to 7-fold, which is substantial. In these experiments, strips of HCC were incubated with BRL 37344 for 30 min before analysis of tissue cyclic nucleotide levels, which is the same time frame for the concomitant smooth muscle relaxation studies. Therefore, the cGMP data represented levels of cGMP that had accumulated over 30 min. This may explain why there is no clear-cut concentration–response relationship between 1 and 10 μM BRL 37344, which might have been more apparent had we used shorter incubation times. The lack of readily available HCC tissue precluded a more extensive analysis.
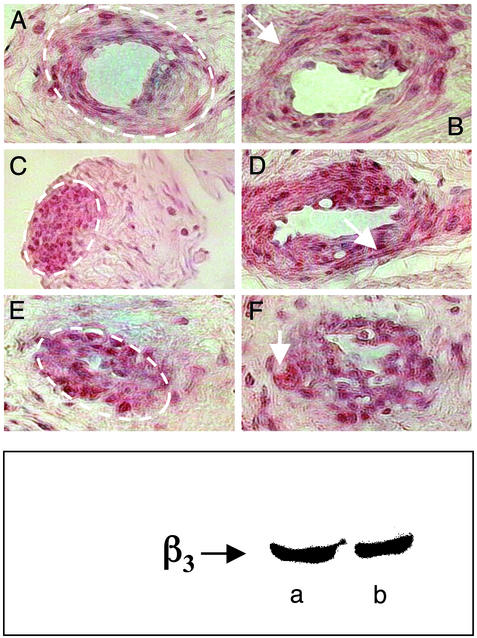
The presence of β3-adrenergic receptors in HCC was confirmed by Western blot experiments that were performed by using two different specific Abs to the β3-adrenergic receptor (Fig. 3 Lower). Immunohistochemical analysis of sections of HCC showed an intense staining for β3-adrenergic receptors that was localized to the smooth muscle tissue. The positive staining is present homogeneously in the vessel lumen in both transverse and longitudinal sections (Fig. 3 Upper).
Figure 3.
Immunohistochemical localization of β3-adrenergic receptors in HCC. (Upper) The red/brown color is indicative of the presence of β3-adrenergic receptors in the tissue. Positive areas are indicated by the dotted lines (A, C, and E) and by the arrows (B, D, and F). The positive staining is absent in the stromal component, e.g., the area outside the dotted line in A, C, and E. The positive reaction is homogeneously diffuse both in smooth muscle cells that transversally define the vascular lumen and longitudinally define the intimal cushions (A and B). The positive reaction is also evident in the sections where the smooth muscle cells are associated with the venous wall (F). There is also a positive reaction in the smooth muscle trabecular components that are interconnected with the more external layers of the venous wall (C). (Lower) Two examples of Western blots for β3-adrenergic receptors in HCC performed with 30 μg of protein and two different Abs (a, from Alpha Diagnostic International; b, from Santa Cruz Biotechnology).
Overall, the present data suggest that the smooth muscle relaxant action of BRL 37344 is linked to the activation of soluble guanylate cyclase in the smooth muscle cells in an NO-independent manner. This hypothesis is supported further by the finding that 1-H-[1,2,4]oxadiazolo [4,3a]quinoxalin-1-one, a selective inhibitor of the NO-activated guanylate cyclase, did not modify the relaxant response of HCC strips to BRL 37344 (Fig. 1c). To further test this hypothesis, methylene blue was used in certain experiments because methylene blue is known to inhibit soluble guanylate cyclase catalytic activity in the absence of enzyme activation by NO (15–17). Methylene blue significantly inhibited the smooth muscle relaxing effect of BRL 37344, as shown in Fig. 1d. Recently, an NO-independent regulatory site was found to be present on soluble guanylate cyclase (18), and a specific activator of this site, BAY 58-2667, caused enzyme activation even after the enzyme had been oxidized by addition of 1-H-[1,2,4]oxadiazolo [4,3a]quinoxalin-1-one or rendered hemedeficient (19). Thus, it is possible that β3-adrenergic receptor activation causes an increase in HCC cGMP levels through stimulation/activation of soluble guanylate cyclase at this NO-independent regulatory site.
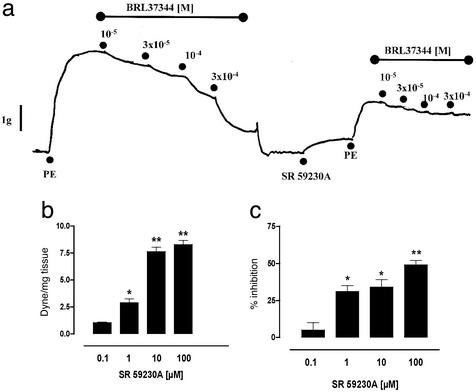
To verify the specificity of action of BRL 37344 on β3-adrenergic receptors, we used SR 59230A, a selective β3-adrenergic receptor antagonist. SR 59230A (0.1–100 μM) caused a concentration-dependent contraction of HCC strips (Fig. 4b), as well as a significant reduction in the magnitude of PE-elicited contraction (Fig. 4c). When strips were preincubated with SR 59230A, the relaxation response to BRL 37344 was nearly abolished, as shown in Fig. 4a. These data imply that the vascular smooth muscle in HCC possesses tonic or continuous β3-adrenergic receptor activity (mediating relaxation), as suggested by the vasoconstrictor effect that results from selectively antagonizing the β3-adrenergic receptors. The mechanism by which antagonism of β3-adrenergic receptors leads to diminished contractile responses to PE is unknown but suggests that β3-adrenergic receptors may play a dynamic role in modulating the state of vascular smooth muscle tone in HCC. Indeed, it is believed that the erectile response depends on the balance between vasoconstrictor and vasodilator tonic influences (20).
Figure 4.
Influence of the β3-adrenergic receptor antagonist SR 59230A on vasorelaxation and smooth muscle tone in HCC. (a) SR 59230A virtually abolished BRL 37344-induced vasorelaxation. (b) SR 59230A alone caused a concentration-dependent contraction. (c) SR 59230A inhibited the contractile effects of 1 μM PE. In these experiments, SR 59230A was added to organ baths 15 min before addition of PE. The data in a represent four separate experiments. The data in b and c represent the mean ± SEM of duplicate determinations from four to five separate experiments. Data are expressed as mean ± SEM. In b, * (P < 0.05) and ** (P < 0.01) signify that values were significantly different from smooth muscle tone in the absence of added SR 59230A. In c, * (P < 0.05) and ** (P < 0.01) signify that values were significantly different from PE-induced contractions in the absence of added SR 59230A. Data were analyzed by ANOVA followed by Bonferroni post hoc test.
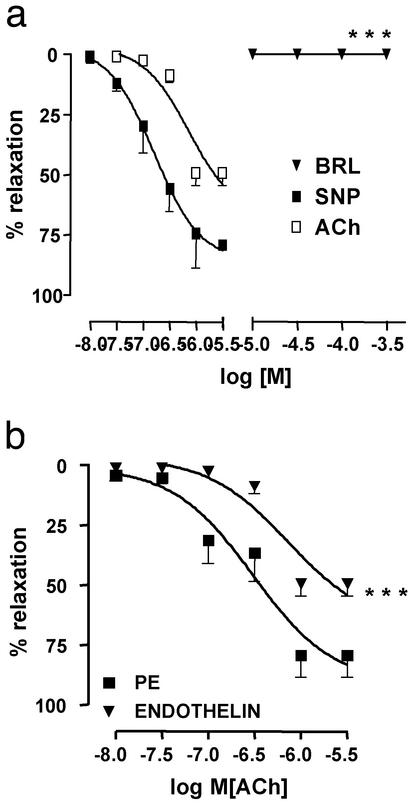
The RhoA/Rho-kinase pathway seems to be involved in the contractile pathway(s) of the HCC that lead to detumescence or the flaccid state (21, 22). RhoA is a monomeric GTPase that is inactive when GDP is bound but becomes active after binding GTP. The activated RhoA stimulates Rho kinase, a serine/threonine kinase. Rho kinase phosphorylates myosin light chain (MLC) phosphatase at the myosin-binding subunit, thereby inactivating MLC phosphatase. This results in an increase in phosphorylated MLC and consequent myosin binding to α-actin, thereby resulting in smooth muscle contraction. Selective inhibition of the RhoA/Rho-kinase pathway has been shown to promote erectile responses in the rat (23). High expression of RhoA occurs in HCC and is believed to mediate calcium sensitization that contributes to the flaccid state of HCC (22). Endothelin-1-elicited vasoconstriction and detumescence in the corpus cavernosum are also attributed to activation of the RhoA/Rho-kinase pathway (21, 22), and endothelin-1 stimulation causes a 17-fold increase in RhoA content in HCC (22). Accordingly, we studied the effect of BRL 37344 on contraction of HCC elicited by hET-1. HCC strips were contracted with hET-1 and then challenged with BRL 37344, ACh, or SNP (Fig. 5). Strips did not relax to BRL 37344 and displayed a reduced relaxant response to ACh, and the relaxation response to SNP was unaffected. These results indicate that the relaxant activity of BRL 37344 in HCC is closely linked to the RhoA/Rho-kinase pathway. That is, activation of the RhoA/Rho-kinase pathway prevents the vasorelaxant effect of β3-adrenergic receptor stimulation. The maximal vasorelaxant response to β3-adrenergic receptor stimulation requires inactivity of the RhoA/Rho-kinase pathway. Therefore, the two pathways may function physiologically to regulate or modulate the state of tumescence of the HCC. Interestingly, the NO–cGMP signaling pathway mediated by cGMP-dependent protein kinase activation causes smooth muscle relaxation that is associated with phosphorylation and consequent inhibition of RhoA (23–27). This latter mechanism of smooth muscle relaxation is also likely to occur in HCC because cGMP-dependent protein kinase is expressed in HCC (24).
Figure 5.
Influence of hET-1 on vasorelaxant responses to BRL 37344 (BRL), ACh, and SNP. (a) Precontraction of HCC strips with 10 nM hET-1 resulted in the abolition of the smooth muscle relaxant action of BRL. The smooth muscle relaxant actions of ACh and SNP were maintained. The relaxant response to ACh was slightly reduced (see b) but that of SNP was not affected by hET-1 (data not shown). (b) HCC strips were precontracted with either PE (1 μM) or hET-1 (10 nM). The smooth muscle relaxant effect of ACh in HCC strips precontracted with hET-1 was significantly reduced when compared with HCC strips precontracted with PE. All strips were tested at the end of experiments for their capacity to relax in response to added SNP to be certain that the smooth muscle in HCC was functionally intact. Data are expressed as mean ± SEM of duplicate determinations from four to five separate experiments. In a, *** (P < 0.001) signifies that values for BRL in the presence of hET-1 were significantly different from values for BRL in the presence of PE (data not shown). In b, *** (P < 0.001) signifies that values for ACh in the presence of hET-1 (endothelin) were significantly different from values for ACh in the presence of PE. Data were analyzed by ANOVA followed by Bonferroni post hoc test.
In conclusion, the data presented here indicate that HCC possesses β3-adrenergic receptors that are localized primarily to smooth muscle cells. Selective activation of β3-adrenergic receptors causes an increase in tissue levels of cGMP, resulting in NO-independent vasorelaxation that seems to be mediated by cGMP-dependent phosphorylation and inhibition of the RhoA/Rho-kinase pathway. We also provide evidence for the existence of a close functional link between β3-adrenergic receptor-mediated vasorelaxation and RhoA/Rho-kinase-mediated vasoconstriction in HCC. The presence of β3-adrenergic receptors in HCC and their possible involvement in promoting erectile function may open new approaches to develop a better understanding not only of the physiology and pathophysiology of erectile function but also of potential novel therapeutic approaches to treat ED.
Abbreviations
- HCC
human corpus cavernosum
- ED
erectile dysfunction
- PE
phenylephrine
- ACh
acetylcholine
- hET-1
human endothelin-1
- l-NAME
l-NG-nitroarginine-methylester
- IBMX
1-methyl-3-isobutylxanthine
- SNP
sodium nitroprusside
References
- 1.Lue T. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 2.Andersson K-E. Pharmacol Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- 3.Wallis R M, Corbin J D, Francis S H. Am J Cardiol. 1999;83:3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 4.Ignarro L J, Bush P A, Buga G M, Wood K S, Fukuto J M, Rajfer J. Biochem Biophys Res Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- 5.Rajfer J, Aronson W J, Bush P A, Dorey F J, Ignarro L J. N Engl J Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 6.Bivalacqua T J, Champion H C, Hellstrom W J G, Kadowitz P J. Trends Pharmacol Sci. 2000;21:484–489. doi: 10.1016/s0165-6147(00)01587-x. [DOI] [PubMed] [Google Scholar]
- 7.Strosberg A D. Annu Rev Pharmacol Toxicol. 1997;37:421–450. doi: 10.1146/annurev.pharmtox.37.1.421. [DOI] [PubMed] [Google Scholar]
- 8.Guimaraes S, Moura D. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- 9.Guillaume J L, Petitjean F, Haasemann M, Bianchi C, Eshdat Y, Strosberg A D. Eur J Biochem. 1994;224:761–770. doi: 10.1111/j.1432-1033.1994.00761.x. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain P D, Jennings K H, Paul F, Cordell J, Berry A, Holmes S D, Park J, Chambers J, Sennitt M V, Stock M J, et al. Int J Obes Relat Metab Disord. 1999;23:1057–1065. doi: 10.1038/sj.ijo.0801039. [DOI] [PubMed] [Google Scholar]
- 11.Krief S, Lonqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P, Strosberg A D, Ricquier D, Emorine L J. J Clin Invest. 1993;91:344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trochu J, Leblais V, Rautureau Y, Beverelli F, Le Marec H, Berdeaux A, Gauthier C. Br J Pharmacol. 1999;128:69–76. doi: 10.1038/sj.bjp.0702797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirone V, Sorrentino R, d'Emmanuele di Villabianca R, Imbimbo C, Palmieri A, Fusco F, Tajana G, Cirino G. J Pharmacol Methods. 2000;44:477–482. doi: 10.1016/s1056-8719(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 14.Adaikan P G, Karim S M. J Auton Pharmacol. 1981;1:199–203. doi: 10.1111/j.1474-8673.1981.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 15.Martin W, Villani G M, Jothianadan D, Furchgott R F. J Pharmacol Exp Ther. 1985;232:708–714. [PubMed] [Google Scholar]
- 16.Kontos H A, Wei E P. Stroke (Dallas) 1993;24:427–431. doi: 10.1161/01.str.24.3.427. [DOI] [PubMed] [Google Scholar]
- 17.Beasley D, McGuiggin M. J Exp Med. 1994;179:71–80. doi: 10.1084/jem.179.1.71. [DOI] [PubMed] [Google Scholar]
- 18.Stasch J, Becker E M, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleib U, et al. Nature. 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- 19.Stasch J-P, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, et al. Br J Pharmacol. 2002;136:773–783. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills T M, Chitaley K, Lewis R W. Int J Impot Res. 2001;13:S29–S34. doi: 10.1038/sj.ijir.3900774. [DOI] [PubMed] [Google Scholar]
- 21.Chitaley K, Wingard C J, Webb R C, Branam H, Stopper V S, Lewis R W, Mills T M. Nat Med. 2001;7:119–122. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Eto M, Steers W D, Somlyo A P, Somlyo A V. J Biol Chem. 2002;277:30614–30621. doi: 10.1074/jbc.M204262200. [DOI] [PubMed] [Google Scholar]
- 23.Mills T M, Chitaley K, Wingard C J, Lewis R W, Webb R C. J Appl Physiol. 2001;91:1269–1273. doi: 10.1152/jappl.2001.91.3.1269. [DOI] [PubMed] [Google Scholar]
- 24.Sawada N, Itoh H, Yamashita J, Doi K, Inoue M, Masatsugu K, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, et al. Biochem Biophys Res Commun. 2001;280:798–805. doi: 10.1006/bbrc.2000.4194. [DOI] [PubMed] [Google Scholar]
- 25.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann S M, Bertoglio J, Chardin P, Pacaud P, Loirand G. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 26.Klotz T, Bloch W, Zimmermann J, Ruth P, Engelmann U, Addicks K. Int J Impotence Res. 2000;12:157–164. doi: 10.1038/sj.ijir.3900524. [DOI] [PubMed] [Google Scholar]
- 27.Chitaley K, Webb R C. Hypertension. 2002;39:438–442. doi: 10.1161/hy02t2.102960. [DOI] [PubMed] [Google Scholar]