Abstract
Nitroxyl anion (HNO/NO−), the one-electron reduced form of nitric oxide (NO), induces positive cardiac inotropy and selective venodilation in the normal in vivo circulation. Here we tested whether HNO/NO− augments systolic and diastolic function of failing hearts, and whether contrary to NO/nitrates such modulation enhances rather than blunts β-adrenergic stimulation and is accompanied by increased plasma calcitonin gene-related peptide (CGRP). HNO/NO− generated by Angelis' salt (AS) was infused (10 μg/kg per min, i.v.) to conscious dogs with cardiac failure induced by chronic tachycardia pacing. AS nearly doubled contractility, enhanced relaxation, and lowered cardiac preload and afterload (all P < 0.001) without altering plasma cGMP. This contrasted to modest systolic depression induced by an NO donor diethylamine(DEA)/NO or nitroglycerin (NTG). Cardiotropic changes from AS were similar in failing hearts as in controls despite depressed β-adrenergic and calcium signaling in the former. Inotropic effects of AS were additive to dobutamine, whereas DEA/NO blunted β-stimulation and NTG was neutral. Administration of propranolol to nonfailing hearts fully blocked isoproterenol stimulation but had minimal effect on AS inotropy and enhanced lusitropy. Arterial plasma CGRP rose 3-fold with AS but was unaltered by DEA/NO or NTG, supporting a proposed role of this peptide to HNO/NO− cardiotropic action. Thus, HNO/NO− has positive inotropic and lusitropic action, which unlike NO/nitrates is independent and additive to β-adrenergic stimulation and stimulates CGRP release. This suggests potential of HNO/NO− donors for the treatment of heart failure.
Keywords: nitroxyl‖contractility‖heart failure‖nitric oxide‖CGRP
Organic nitrates such as nitroglycerin (NTG) are widely used for the treatment of congestive heart failure (CHF) because of their capacity to unload the heart by balanced venous and arterial vasodilation (1). However, these agents as well as direct nitric oxide (NO) donors also influence cardiac inotropy (2), particularly in the setting of enhanced β-sympathetic stimulation (3–6). NO-stimulated guanylyl cyclase increases cGMP thereby blunting adrenergic activation (7, 8). This modulation seems more pronounced in failing hearts where inhibition of NO synthase enhances β-stimulation more than in normal ventricles (5, 9). The interaction of NO/cGMP and adrenergic signaling is clinically important, because they are often concomitantly administered to treat cardiovascular decompensation.
The nitroxyl anion (HNO/NO−) is the one-electron reduced form of NO, and recent data suggest that this species may provide a very different hemodynamic profile and interaction with β-agonists. When administered i.v. to healthy conscious dogs, HNO/NO− [generated by Angelis' salt (AS)] has load- and reflex-independent, redox-sensitive positive inotropic and selective venodilator effects that are not mimicked by pure NO donors (10). Part of its mechanism involves release of calcitonin gene-related peptide (CGRP), a member of the nonadrenergic/noncholinergic fiber neuropeptides. It is unknown whether HNO/NO− has similar effects in the failing heart and vasculature and whether, unlike NO, its stimulation is independent of and/or positively interacts with β-adrenergic stimulation. The present study determined the in vivo pharmacology and physiology of HNO/NO− in a conscious canine model of CHF induced by rapid ventricular pacing. This widely used model displays many features common to human cardiac failure including β-adrenergic down-regulation, altered calcium homeostasis, repolarization delay, and neurohumoral activation (11–14). We provide evidence for unique favorable interactions between HNO/NO− and the failing heart under basal and β-stimulated conditions.
Methods
Chemicals.
HNO/NO− was generated from AS (Na2N2O3) and NO from diethylamine(DEA)/NO synthesized as described (15). Both were prepared in 0.9% NaCl immediately before use from a 100 mM stock solution in 10 mM NaOH. NTG was from American Reagent Laboratories (Shirley, NY), dobutamine hydrochloride and Dextran-40 were from Abbott, and propranolol was from Ayerst Laboratories.
Experimental Preparation and Protocols.
Adult mongrel dogs (≈25 kg) were chronically instrumented for conscious hemodynamic studies as described (16). Briefly, after sodium thiamylal induction (3–5 mg/kg), dogs were anesthetized with 1–2% halothane, and the heart was exposed via lateral thoracotomy. A micromanometer (P22; Konigsberg Instruments, Pasadena, CA) was inserted into the left ventricle (LV) via an apical stab. Right atrial and descending aortic catheters were placed, the latter for LV manometer calibration. Endocardial sonomicrometers were positioned to measure anteroposterior short-axis dimension, and a pneumatic occluder was placed around the inferior vena cava to enable load reduction for LV pressure–dimension relation analysis. Epicardial pacing leads were sutured to the LV free wall and connected to a programmable stimulator (Spectrax, Medtronics, Minneapolis) inserted within a subcutaneous pocket. Additional leads were secured to the left atrium for pacing during hemodynamic recordings. The chest was closed, and catheters and leads were externalized. Morphine (10 mg s.c.) was administered postoperatively as required. Dogs were provided 10 days for recovery before the study. The surgical and experimental animal protocol was approved by The Johns Hopkins University Animal and Care Use Committee. Heart failure was induced by chronic rapid ventricular pacing at a rate of 210 beats per minute for 3 weeks followed by 240 beats per minute for 1 week (16). Tachypacing-induced failure was demonstrated by a decline in dP/dtmax, cardiac output of 43% and 51%, respectively (both P < 0.005), and doubling of end-diastolic pressure, similar to reported results with this model (16).
Cardiovascular effects of HNO/NO− (AS; 10 μg/kg per min for 5–20 min, i.v.) were assessed in 11 animals with cardiac failure and 9 normal controls. Studies were performed by using fixed-rate atrial pacing (130–160 min−1). In a subset of eight animals, reflex independence of HNO/NO− effects were assessed by offsetting load decline with intravascular volume expansion (10% Dextran 40 in 5% dextrose solution). The effects of HNO/NO− were contrasted further to those from an NO donor (DEA/NO) or from NTG, both titrated to yield the same systolic pressure decline observed with HNO/NO− (−15 ± 2.2%).
To test whether HNO/NO− modified β-adrenergic cardiostimulation, six studies were performed in which inotropic/lusitropic responses to dobutamine and HNO/NO− were tested both alone and in combination. Analogous studies were also conducted with dobutamine combined with either DEA/NO (n = 5) or NTG (n = 4). To explore further whether β-adrenergic stimulation was an important component of HNO/NO−-induced cardiac effects, healthy control animals were pretreated with the β-blocker propranolol (2 mg/kg, n = 6). The adequacy of β-receptor blockade was confirmed by its inhibition of maximal-response isoproterenol (0.4 μg/kg per min). Then HNO/NO− was infused, and its effect on the heart and vascular load was determined.
Hemodynamic Analysis.
Hemodynamic signals were sampled digitally at 250 Hz, and steady-state and pressure–dimension parameters were derived as described (16). In vivo contractility assessment required separation of primary cardiac effects from changes due to altered loading. Pressure–volume relations were used for this purpose, yielding load-insensitive indexes: end-systolic elastance (Ees) and slope of the dP/dtmax–end-diastolic dimension (DEDD) relation. The isovolumic relaxation time constant was determined from the pressure-decay waveform, assuming a nonzero asymptote. Other parameters for cardiac loading were derived as described (16).
Plasma CGRP and cGMP Analysis.
Blood was withdrawn from arterial, venous, and coronary sinus catheters and centrifuged at 1,600 × g for 20 min at 4°C, and plasma was separated and stored at −20°C. For analysis, 0.5 ml of plasma was mixed with 0.8 ml of ethanol and centrifuged at 1,600 × g for 20 min, and the supernatant was removed and air-dried at room temperature overnight. Samples were reconstituted with assay buffer according to manufacturer instructions (Peninsula Laboratories) and assayed for CGRP by RIA with CGRP antiserum (code RAS 6012). The dynamic assay range was 1–128 pg per 300 μl (17). Plasma cGMP levels (arterial, venous, and coronary sinus) were determined by enzyme immunoassay (Biotrak, Amersham Pharmacia) based on lyophilized samples. The assay has a detection sensitivity of 2 pmol/ml.
Statistical Analysis.
Data are presented as mean ± SEM. Analysis was performed by using paired t tests or one-way analysis of variance (for more than two comparisons) with a Tukey test for posthoc analysis.
Results
HNO/NO− Augments Inotropy and Lusitropy of Failing Heart.
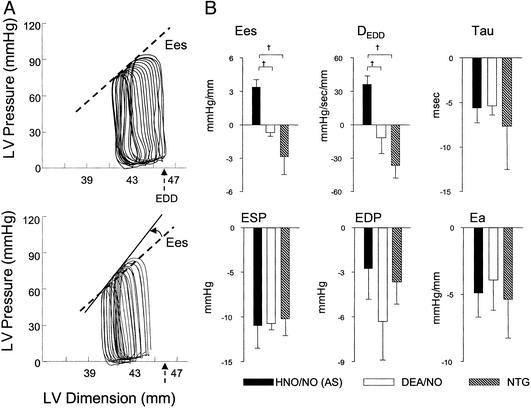
Fig. 1A displays example pressure–dimension loops and end-systolic relations in a failing heart before and after AS infusion. Chamber end-diastolic dimension (arrow shows baseline preload) and systolic pressure both declined, whereas the end-systolic pressure–dimension relation (contractile function) was enhanced, denoted by its left shift and higher slope. These AS responses were rapid (within 5 min) in failing hearts and remained stable for the duration of the infusion.
Figure 1.
Cardiovascular effects of HNO/NO− in failing myocardium and vasculature. (A) Example of pressure–dimension relationships at baseline (Upper) and after HNO/NO− (AS) (Lower). The basal cardiac cycle is the pressure–dimension loop to the far right of each set, with the subsequent data obtained during preload reduction by transient vena cava occlusion. AS lowered preload (end-diastolic dimension, EDD; arrow), shifting the set of loops leftward, and also increased contractility (denoted by upward-left shift of end-systolic pressure–dimension relation; arrow). (B) Mean data contrasting inotropic, lusitropic, and loading effects of HNO/NO− (AS), DEA/NO, and NTG. DEDD, dP/dtmax–end-diastolic dimension relation slope; Tau, relaxation time constant; ESP, end-systolic pressure; EDP, end-diastolic pressure; Ea, arterial elastance (afterload).
Summary data are provided in Table 1. On average, load-independent contractility indexed by Ees and DEDD rose 47 ± 7% and 51 ± 14%, respectively (both P < 0.01), and isovolumic relaxation decay time decreased −19 ± 3% (P < 0.005). As reported in normal hearts (10), AS induced marked declines in chamber preload and afterload, reflected by reduced end-diastolic pressure and dimension, and lower arterial elastance (all P < 0.001; Table 1). Importantly, both inotropic and lusitropic responses to AS persisted at a similar magnitude when preload and arterial pressures were returned toward baseline by dextran volume expansion. The combination of positive inotropy and load reduction from AS resulted in no net change in chamber shortening (i.e., output), and there was a modest (−20.8 ± 1.4%) decline in mean coronary flow (P < 0.001; Table 1). Both effects, however, were modified by preload restoration, with net ejection now increasing by 8.6 ± 1.2% (P < 0.001) and coronary flow returning to baseline levels.
Table 1.
Cardiovascular effects of HNO/NO− donated by AS in canine CHF
| Baseline (n = 9) | AS (HNO/NO−) (n = 9) | AS + volume expansion (n = 8) | |
|---|---|---|---|
| Heart rate, beats per minute | 138 ± 4.6 | 137 ± 5.4 | 134.7 ± 5.7 |
| Ees, mmHg/ml | 9.51 ± 0.9 | 13.8 ± 1.3* | 13.1 ± 1.2† |
| DEDD, mmHg/s/mm | 106.9 ± 11 | 155.6 ± 14‡ | 141.1 ± 15‡ |
| Ea, mmHg/mm | 26.1 ± 3.2 | 22.0 ± 1.9§ | 21.8 ± 2.3§ |
| Tau, dP/dt, ms | 39.8 ± 2.8 | 31.6 ± 1.1‡ | 33.0 ± 1.8‡ |
| LVESP, mmHg | 107.5 ± 4.8 | 92.2 ± 4.3* | 96.6 ± 4.5‡ |
| LVESD, mm | 35.9 ± 2.8 | 34.6 ± 2.9* | 34.8 ± 3.2* |
| LVEDP, mmHg | 16.6 ± 2.9 | 10.9 ± 2.8* | 16.1 ± 4.6 |
| LVEDD, mm | 40.7 ± 3.1 | 39.2 ± 3.2* | 39.8 ± 3.4‡ |
| CorF, ml/min | 31.4 ± 4.6 | 24.8 ± 2.7* | 29.2 ± 3.9 |
Data are mean ± SEM. Ees, slope of the end-systolic pressure–dimension relation; DEDD, preload-normalized maximal dP/dt; Ea, arterial elastance; LVESP, LV end-systolic pressure; LVESD, LV end-systolic dimension; LVEDP, LV end-diastolic pressure; LVEDV, LV end-diastolic dimension; CorF, coronary blood flow (circumflex territory).
P < 0.001 vs. baseline.
P < 0.01 vs. baseline.
P < 0.005 vs. baseline.
P < 0.05 vs. baseline.
In additional studies, we confirmed redox sensitivity of HNO/NO− by pretreatment (30 min) and co-infusion of N-acetyl-l-cysteine (6.7 μmol/kg per min), which inhibited both positive inotropic and lusitropic effects of AS (data not shown).
Cardiovascular effects of HNO/NO− next were contrasted to those induced by DEA/NO and NTG (Fig. 1B). To minimize potential co-influences of reflex activation, these studies were all performed with dextran co-infusion to minimize preload decline. All agents similarly lowered chamber preload and afterload and enhanced relaxation rate. However, inotropic responses were directionally opposite, with only HNO/NO− producing a positive response.
HNO/NO− Response Is Similar in Normal Vs. Failing Hearts.
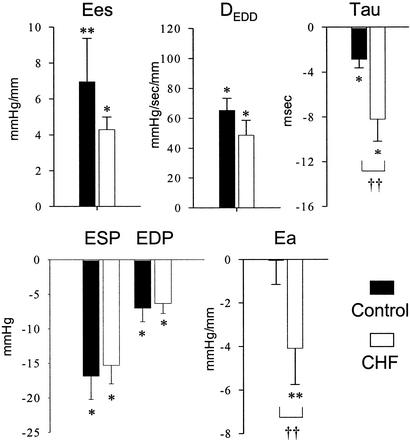
Many forms of cardiac inotropic stimulation are depressed in the failing heart, particularly those coupled to β-adrenergic signaling and sarcoplasmic reticular calcium release. Therefore, we contrasted the cardiovascular effects of HNO/NO− in failing hearts to those in healthy control animals (Fig. 2). The absolute increase in contractile function was nearly the same in both groups, as was the decline in systolic/diastolic pressures and volumes. Arterial vasodilation and improvement in ventricular relaxation (tau) due to HNO/NO− were even enhanced in animals with cardiac failure over healthy controls.
Figure 2.
HNO/NO−-induced changes in CHF vs. control animals. The data show HNO/NO− induced absolute change in each parameter from baseline in control normal (n = 9) and CHF (n = 9) dogs. Data are mean ± SEM. *, P < 0.01 vs. baseline; **, P < 0.05 vs. baseline; ††, P < 0.05 between groups. Abbreviations are as described for Fig. 1.
HNO/NO− Potentiates β-Adrenergic Response in Failing Hearts.
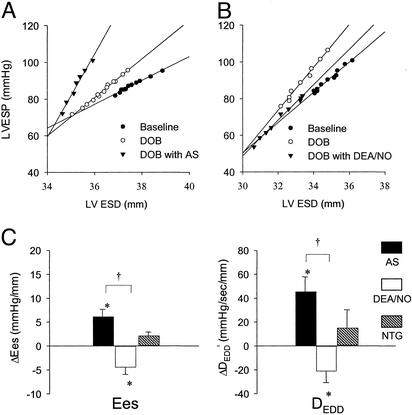
We next determined the interaction between HNO/NO−-mediated inotropy and β-adrenergic stimulation. Dobutamine alone elevated Ees (10.2 ± 1.0 to 13.3 ± 1.6, P < 0.005) and DEDD (112 ± 11.1 to 144.6 ± 13.4, P < 0.001). Co-infusion of dobutamine with HNO/NO− further enhanced myocardial contractility, reaching levels nearly twice that with dobutamine alone (Fig. 3A). This differed markedly from DEA/NO, which depressed β-stimulated function, and from NTG, which displayed a neutral impact on dobutamine co-stimulation (Fig. 3 B and C).
Figure 3.
Combined effects of HNO/NO−, DEA/NO, or NTG with dobutamine (DOB). (A) End-systolic pressure–volume relations shift upward and to the left with dobutamine alone compared with baseline and are shifted further (increased contractility) with the combination of dobutamine and HNO/NO−. (B) In contrast, the combination of DEA/NO with dobutamine reduced the effects of the latter on contractility toward baseline. ESD, end-systolic dimension. (C) Summary data for the change in contractility (the two indexes are as abbreviated in Fig. 1) due to each agent when combined with dobutamine. Only HNO/NO− significantly further enhanced contractility over dobutamine alone, whereas the other agents either reduced or were neutral to β-adrenergic stimulation.
HNO/NO−-Induced Inotropy: Independence from β-Adrenergic Stimulation.
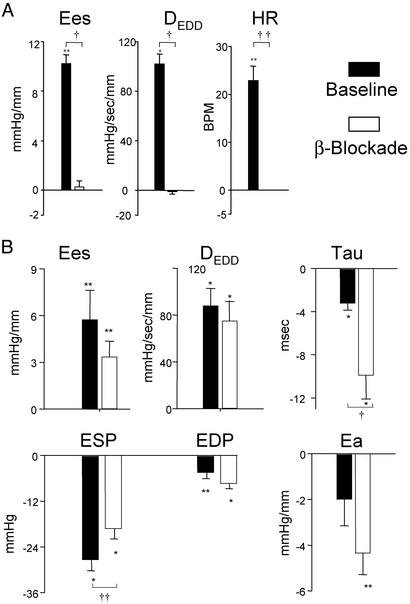
Previous results suggested that HNO/NO−-mediated inotropy did not depend on β-receptor stimulation or direct receptor-coupled signaling. To test this further, we studied six controls pretreated with the β-blocker propranolol. Propranolol fully inhibited isoproterenol-stimulated contractility (Fig. 4A); however, the inotropic response to HNO/NO− remained similar to non-β-blocked animals (Fig. 4B). Both systemic arterial vasodilation and improved relaxation induced by HNO/NO− were enhanced after β-blockade, similar to the augmented responses in failing over control hearts.
Figure 4.
Impact of β-blockade by propranolol on cardiovascular effects exerted by nitroxyl in normal hearts. (A) Pretreatment with i.v. propranolol fully inhibited both inotropic and chronotropic effects of high-dose isoproterenol. HR, heart rate; BPM, beats per minute. (B) However, β-blockade had negligible influence on the contractile or loading effects of HNO/NO−. Shortening of isovolumic relaxation time and arterial unloading was more prominent after β-blockade. *, P < 0.01 vs. baseline; **, P < 0.05 vs. baseline; †, P < 0.01 between groups; ††, P < 0.05 between groups. Abbreviations are as described for Fig. 1.
CGRP but Not cGMP Are Enhanced in Arterial and Venous Plasma by HNO/NO−.
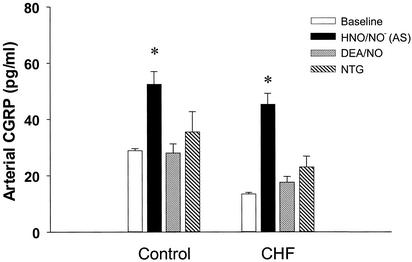
Fig. 5 displays arterial plasma CGRP concentration during HNO/NO−, DEA/NO, or NTG infusion in both healthy controls and CHF studies. HNO/NO− increased plasma CGRP by 100% in healthy controls and nearly 200% in failing dogs, whereas neither DEA/NO nor NTG altered CGRP plasma concentration. Basal CGRP was reduced in animals with heart failure; however levels stimulated by HNO/NO− rose to those in similarly treated stimulated controls, supporting the similar cardiotropic response in both groups. Data from venous and coronary sinus plasma were very similar to those shown for arterial blood.
Figure 5.
Arterial plasma CGRP stimulated by HNO/NO− vs. DEA/NO and NTG. Arterial levels increased only with HNO/NO− (AS) infusion. The NO donor DEA/NO and nitrate (NTG) did not alter levels significantly from initial baseline. Basal plasma CGRP was reduced in failing hearts (CHF) vs. control (P < 0.01), whereas after HNO/NO− infusion, the elevated levels were similar between both groups.
In contrast, we found no effect of HNO/NO− on arterial, venous, or coronary sinus plasma cGMP levels. For example, baseline arterial cGMP was 57.5 ± 6.3 pmol/ml in failing dogs vs. 54.5 ± 7.6 pmol/ml after AS infusion. This lack of change in cGMP with AS infusion is very similar to prior data in healthy controls (10). Basal cGMP levels themselves, however, were higher in CHF compared with controls (22.3 ± 6.6 pmol/ml, P < 0.005 vs. CHF).
Discussion
This study reveals features of HNO/NO− derived from AS with marked venous and arterial unloading accompanied by positive inotropic and lusitropic effects in dogs with cardiac failure. Importantly, we provide evidence that these effects are essentially independent of β-receptor activation and indeed additive to concomitant β-stimulation. This is strikingly different to traditional NO donors such as NTG or DEA/NO, which either blunt the dobutamine response or provide no additive effect. The uniqueness of AS activity was supported by demonstration of enhanced plasma CGRP levels that was not observed with NO donors or nitrates and seemed enhanced in heart failure. Basal plasma cGMP levels were elevated in failure over control dogs but unchanged by AS as reported in normal controls (10).
The combination of vasodilation derived from NO/nitrates and inotropic stimulation from β-agonists is among the most common pharmacologic treatment for acute cardiac decompensation. However, NO/nitrates can themselves blunt adrenergic signaling. This has been revealed indirectly by showing enhanced dobutamine or isoproterenol-stimulated function in mice lacking the endothelial NO synthase gene (18, 19) or in control animals and humans after NO synthase inhibition (5, 9, 20). Importantly, this negative interaction appears enhanced in failing myocardium, which has been attributed to altered inducible NO synthase activity (21), down-regulated cGMP catabolism (22), enhanced β3-receptor signaling (23, 24), and enhanced oxidant stress (25–27). β-Adrenergic stimulation further increases NO release (28) and can amplify its depressant modulation. Importantly, this negative interaction seems enhanced in failing myocardium. This has been proposed to be due to altered inducible NO synthase.
Given these findings, the disparity between NO donors or nitrates and HNO/NO− is particularly striking. Although each agent induced similar levels of veno- and arterial dilation in heart failure, only AS conveyed positive inotropic effects. The addition of HNO/NO− and dobutamine was particularly potent yet occurred without arrhythmia, further tachycardia, or unacceptable load reduction. Although β-stimulation is widely used for acute hemodynamic support, more sustained use can be cardiotoxic (29, 30). The present results suggest an alternative approach whereby the dobutamine dose might be lowered substantially or even eliminated by the addition of HNO/NO− rather than nitrates.
In the normal cardiovascular system, AS is a preferential venodilator, yielding little change in systemic arterial resistance (10), whereas in cardiac failure, the net result was more balanced dilation. Analogous data have been reported with nitrates in human normal vs. failing hearts (31), which may relate to the higher basal systemic resistance in the latter condition, thus enhancing the arterial effects of nitrates or HNO/NO−. However, other factors may contribute. For example, baroreflex blockade in normal dogs converts the AS response to more balanced venous and arterial dilation (10), and in the present study similar changes in response were achieved with β-adrenergic blockade. Cardiac failure is associated not only with β-down-regulation but also with blunting of arterial baroreflex activation (25, 32), and this could play a role in the more balanced dilator response.
The present results provide in vivo evidence for direct stimulation of CGRP by HNO/NO−. This finding corroborates prior studies (10) and further clarifies differential effects between HNO/NO− and NO donors (33–35). CGRP has positive inotropic activity involving enhanced calcium release (36) and is active in failing human hearts (37). The similar elevation in arterial, venous, and coronary sinus blood from AS in the present study precludes identification of the primary source (e.g., perivascular and organ-specific neurons). Our observation of reduced basal plasma CGRP levels in failing hearts supports prior findings in humans (38). However, CGRP receptors are reportedly up-regulated in experimental CHF, suggesting enhanced expression of both CGRP-specific receptor activity-modifying protein 1 (39) and possibly calcitonin receptor-like receptor (40). This is intriguing, because it might explain the preserved physiologic response to AS in failing hearts. In addition to enhancing systolic function, CGRP also may be a myocardial protectant during ischemia (41). AS also provides cardiac preconditioning, reducing the impact of subsequent myocardial ischemia (42), and this may share a CGRP-mediated mechanism (43).
Although CGRP signaling likely plays a role in HNO/NO− signaling, the present findings suggest that additional factors are also involved. AS was equally effective in the presence or absence of β-receptor blockade, yet CGRP-stimulated positive inotropy is thought to be coupled to protein kinase A stimulation via increased cAMP (36) and thus would be blunted by heart failure. One possibility is that alternative nonadrenergic/noncholinergic peptides are released by AS. Alternatively, there may be other direct effects of HNO/NO− on cardiomyocytes to influence contractility or sensitize them to CGRP signaling.
The present investigation has limitations inherent to any integrative in vivo study in that the precise fate of AS and its cellular interactions cannot be readily defined. Nevertheless, this preparation is important given the potent interaction of HNO/NO− with a neurotransmission system. Whether HNO/NO− is generated in vivo remains unknown, but this was not the focus of the present investigation, which rather sought to define its physiologic/pharmacologic effects in the setting of heart failure.
In conclusion, we have shown that HNO/NO− enhances cardiac inotropy and lusitropy while unloading the failing heart both at baseline and notably during β-stimulation. These latter features and an involvement with nonadrenergic/noncholinergic–CGRP fiber activation are strikingly different from the pharmacologic profile of pure NO donors and nitrates, which suggests that nitroxyl-generating compounds may be an attractive alternative for the treatment of cardiac failure.
Acknowledgments
We thank Drs. W. F. Saavedra and J. M. Hare for assistance and facilitation of the studies; Dr. T. J. Bivalacqua for helping with the CGRP measurements; and Richard S. Tunin and Amy McDonald for excellent surgical support. This research was supported by American Heart Association Beginning Grant-in-Aid 0265435U (to N.P.), a fellowship grant from the University of Tokyo (to T.K.), and National Institutes of Health Grants HL-47511 and P50-HL52307 (to D.A.K.).
Abbreviations
- NTG
nitroglycerin
- CHF
congestive heart failure
- HNO/NO−
nitroxyl anion
- AS
Angelis' salt
- CGRP
calcitonin gene-related peptide
- DEA
diethylamine
- LV
left ventricle
- Ees
end-systolic elastance
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 4978.
References
- 1.Opie L H, White H D. In: Drugs for the Heart. Opie L H, Gersh B J, editors. Philadelphia: Saunders; 2002. pp. 33–52. [Google Scholar]
- 2.Paulus W J, Bronzwaer J G. Heart Fail Rev. 2002;7:371–383. doi: 10.1023/a:1020754232359. [DOI] [PubMed] [Google Scholar]
- 3.Balligand J L. Cardiovasc Res. 1999;43:607–620. doi: 10.1016/s0008-6363(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 4.Hare J M, Stamler J S. Nat Med. 1999;5:273–274. doi: 10.1038/6486. [DOI] [PubMed] [Google Scholar]
- 5.Hare J M, Givertz M M, Creager M A, Colucci W S. Circulation. 1998;97:161–166. doi: 10.1161/01.cir.97.2.161. [DOI] [PubMed] [Google Scholar]
- 6.Shinke T, Takaoka H, Takeuchi M, Hata K, Kawai H, Okubo H, Kijima Y, Murata T, Yokoyama M. Circulation. 2000;101:1925–1930. doi: 10.1161/01.cir.101.16.1925. [DOI] [PubMed] [Google Scholar]
- 7.Vila-Petroff M G, Younes A, Egan J, Lakatta E G, Sollott S J. Circ Res. 1999;84:1020–1031. doi: 10.1161/01.res.84.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Layland J, Li J M, Shah A M. J Physiol (London) 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hare J M, Lofthouse R A, Juang G J, Colman L, Ricker K M, Kim B, Senzaki H, Cao S, Tunin R S, Kass D A. Circ Res. 2000;86:1085–1092. doi: 10.1161/01.res.86.10.1085. [DOI] [PubMed] [Google Scholar]
- 10.Paolocci N, Saavedra W F, Miranda K M, Martignani C, Isoda T, Hare J M, Espey M G, Fukuto J M, Feelisch M, Wink D A, et al. Proc Natl Acad Sci USA. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vatner D E, Sato N, Ishikawa Y, Kiuchi K, Shannon R P, Vatner S F. Clin Exp Pharmacol Physiol. 1996;23:688–692. doi: 10.1111/j.1440-1681.1996.tb01760.x. [DOI] [PubMed] [Google Scholar]
- 12.O'Rourke B, Kass D A, Tomaselli G F, Kääb S, Tunin R, Marban E. Circ Res. 1999;84:562–570. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 13.Luchner A, Stevens T L, Borgeson D D, Redfield M M, Bailey J E, Sandberg S M, Heublein D M, Burnett J C., Jr Hypertension. 1996;28:472–477. doi: 10.1161/01.hyp.28.3.472. [DOI] [PubMed] [Google Scholar]
- 14.Pak P H, Nuss H B, Tunin R, Kääb S, Tomaselli G F, Marban E, Kass D A. J Am Coll Cardiol. 1997;30:576–584. doi: 10.1016/s0735-1097(97)00193-9. [DOI] [PubMed] [Google Scholar]
- 15.Wink D A, Feelisch M, Fukuto J, Chistodoulou D, Jourd'heuil D, Grisham M B, Vodovotz Y, Cook J A, Krishna M, DeGraff W G, et al. Arch Biochem Biophys. 1998;351:66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- 16.Senzaki H, Isoda T, Paolocci N, Ekelund U, Hare J M, Kass D A. Circulation. 2000;101:1040–1048. doi: 10.1161/01.cir.101.9.1040. [DOI] [PubMed] [Google Scholar]
- 17.Onuoha G N, Nugent A M, Hunter S J, Alpar E K, McEneaney D J, Campbell N P, Shaw C, Buchanan K D, Nicholls D P. Eur J Clin Invest. 2000;30:570–577. doi: 10.1046/j.1365-2362.2000.00676.x. [DOI] [PubMed] [Google Scholar]
- 18.Gyurko R, Kuhlencordt P, Fishman M C, Huang P L. Am J Physiol. 2000;278:H971–H981. doi: 10.1152/ajpheart.2000.278.3.H971. [DOI] [PubMed] [Google Scholar]
- 19.Barouch L A, Harrison R W, Skaf M W, Rosas G O, Cappola T P, Kobeissi Z A, Hobai I A, Lemmon C A, Burnett A L, O'Rourke B, et al. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 20.Hart C Y, Hahn E L, Meyer D M, Burnett J C, Jr, Redfield M M. Am J Physiol. 2001;281:H146–H154. doi: 10.1152/ajpheart.2001.281.1.H146. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Traverse J H, Du R, Hou M, Bache R J. Circulation. 2002;106:273–279. doi: 10.1161/01.cir.0000021120.90970.b9. [DOI] [PubMed] [Google Scholar]
- 22.Senzaki H, Smith C J, Juang G J, Isoda T, Mayer S P, Ohler A, Paolocci N, Tomaselli G F, Hare J M, Kass D A. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 23.Moniotte S, Kobzik L, Feron O, Trochu J N, Gauthier C, Balligand J L. Circulation. 2001;103:1649–1655. doi: 10.1161/01.cir.103.12.1649. [DOI] [PubMed] [Google Scholar]
- 24.Cheng H J, Zhang Z S, Onishi K, Ukai T, Sane D C, Cheng C P. Circ Res. 2001;89:599–606. doi: 10.1161/hh1901.098042. [DOI] [PubMed] [Google Scholar]
- 25.Liu J L, Zucker I H. Circ Res. 1999;84:417–423. doi: 10.1161/01.res.84.4.417. [DOI] [PubMed] [Google Scholar]
- 26.Singh N, Dhalla A K, Seneviratne C, Singal P K. Mol Cell Biochem. 1995;147:77–81. doi: 10.1007/BF00944786. [DOI] [PubMed] [Google Scholar]
- 27.Saavedra W F, Paolocci N, St. John M E, Skaf M W, Stewart G C, Xie J S, Harrison R W, Zeichner J, Mudrick D, Marban E, et al. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 28.Kanai A J, Mesaros S, Finkel M S, Oddis C V, Birder L A, Malinski T. Am J Physiol. 1997;273:C1371–C1377. doi: 10.1152/ajpcell.1997.273.4.C1371. [DOI] [PubMed] [Google Scholar]
- 29.Capomolla S, Febo O, Opasich C, Guazzotti G, Caporotondi A, La Rovere M T, Gnemmi M, Mortara A, Vona M, Pinna G D, et al. Eur J Heart Fail. 2001;3:601–610. doi: 10.1016/s1388-9842(01)00165-9. [DOI] [PubMed] [Google Scholar]
- 30.Thackray S, Easthaugh J, Freemantle N, Cleland J G. Eur J Heart Fail. 2002;4:515–529. doi: 10.1016/s1388-9842(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 31.Haber H L, Simek C L, Bergin J D, Sadun A, Gimple L W, Powers E R, Feldman M D. J Am Coll Cardiol. 1993;22:251–257. doi: 10.1016/0735-1097(93)90841-n. [DOI] [PubMed] [Google Scholar]
- 32.Creager M A, Creager S J. J Am Coll Cardiol. 1994;23:401–405. doi: 10.1016/0735-1097(94)90427-8. [DOI] [PubMed] [Google Scholar]
- 33.Ma X L, Gao F, Liu G L, Lopez B L, Christopher T A, Fukuto J M, Wink D A, Feelisch M. Proc Natl Acad Sci USA. 1999;96:14617–14622. doi: 10.1073/pnas.96.25.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartberger M D, Liu W, Ford E, Miranda K M, Switzer C, Fukuto J M, Farmer P J, Wink D A, Houk K N. Proc Natl Acad Sci USA. 2002;99:10958–10963. doi: 10.1073/pnas.162095599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miranda K M, Espey M G, Yamada K, Krishna M, Ludwick N, Kim S, Jourd'heuil D, Grisham M B, Feelisch M, Fukuto J M, et al. J Biol Chem. 2001;276:1720–1727. doi: 10.1074/jbc.M006174200. [DOI] [PubMed] [Google Scholar]
- 36.Huang M H, Knight P R, III, Izzo J L., Jr Am J Physiol. 1999;276:R259–R264. doi: 10.1152/ajpregu.1999.276.1.R259. [DOI] [PubMed] [Google Scholar]
- 37.Gennari C, Nami R, Agnusdei D, Fischer J A. Cardiovasc Res. 1990;24:239–241. doi: 10.1093/cvr/24.3.239. [DOI] [PubMed] [Google Scholar]
- 38.Taquet H, Komajda M, Grenier O, Belas F, Landault C, Carayon A, Lechat P, Grosgogeat Y, Legrand J C. Eur Heart J. 1992;13:1473–1476. doi: 10.1093/oxfordjournals.eurheartj.a060088. [DOI] [PubMed] [Google Scholar]
- 39.Cueille C, Pidoux E, de Vernejoul M C, Ventura-Clapier R, Garel J M. Biochem Biophys Res Commun. 2002;294:340–346. doi: 10.1016/S0006-291X(02)00487-4. [DOI] [PubMed] [Google Scholar]
- 40.Totsune K, Takahashi K, Mackenzie H S, Murakami O, Arihara Z, Sone M, Mouri T, Brenner B M, Ito S. Clin Sci. 2000;99:541–546. [PubMed] [Google Scholar]
- 41.Kallner G, Gonon A, Franco-Cereceda A. Cardiovasc Res. 1998;38:493–499. doi: 10.1016/s0008-6363(98)00016-9. [DOI] [PubMed] [Google Scholar]
- 42.Pagliaro P, Mancardi D, Rastaldo R, Penna C, Gattullo D, Miranda K M F M, Wink D A, Kass D A, Paolocci N. Free Radical Biol Med. 2003;34:33–43. doi: 10.1016/s0891-5849(02)01179-6. [DOI] [PubMed] [Google Scholar]
- 43.Li Y J, Peng J. Eur J Pharmacol. 2002;442:173–177. doi: 10.1016/s0014-2999(02)01538-8. [DOI] [PubMed] [Google Scholar]