Abstract
In autism, severe abnormalities in social behavior coexist with aberrant attention and deficient language. In the attentional domain, attention to people and socially relevant stimuli is impaired the most. Because socially meaningful stimulus events are physically complex, a deficiency in sensory processing of complex stimuli has been suggested to contribute to aberrant attention and language in autism. This study used event-related brain potentials (ERP) to examine the sensory and early attentional processing of sounds of different complexity in high-functioning children with autism. Acoustically matched simple tones, complex tones, and vowels were presented in separate oddball sequences, in which a repetitive “standard” sound was occasionally replaced by an infrequent “deviant” sound differing from the standard in frequency (by 10%). In addition to sensory responses, deviant sounds elicited an ERP index of automatic sound-change discrimination, the mismatch negativity, and an ERP index of attentional orienting, the P3a. The sensory sound processing was intact in the high-functioning children with autism and was not affected by sound complexity or “speechness.” In contrast, their involuntary orienting was affected by stimulus nature. It was normal to both simple- and complex-tone changes but was entirely abolished by vowel changes. These results demonstrate that, first, auditory orienting deficits in autism cannot be explained by sensory deficits and, second, that orienting deficit in autism might be speech–sound specific.
Autism (1) is a severely debilitating developmental disorder, characterized by aberrant social skills, deficient language, abnormal attention, stereotyped repetitive behaviors, and often also mental retardation.
One of the components of the social communication deficits in autism is peculiar attentional behavior. Individuals with autism show attentional preference to objects over people and a lack of a drive to communicate (2). Consistent with this, Swettenham et al. (3) found that 20-month-old infants with autistic features made significantly fewer attention shifts than did their controls from person to person and between a person and an object. They also spent more time looking at objects and less time looking at people. Further, 5-year-old children with autism oriented more poorly to social (both speech and nonspeech) than to nonsocial stimuli (4). Their impaired orienting to social stimuli correlated with the impairment of joint attention, one of the key features of autism.
The neurofunctional deficits underlying this pattern of behavior are not yet understood, although recent research revealed disorders in many aspects of attentional behavior in autism. They include abnormalities in spontaneous looking (3), focused attention (5–11), and voluntary shifting of attention (12–14). Courchesne et al. (12) proposed that the majority of the attentional deficits observed in autism might be caused by a cerebellar dysfunction that slows down the adaptation of the neural systems to the constantly changing neural processing demands. In particular, these authors suggested that the slowed shifting of voluntary attention in autism results in fragmented mental representation of the external world, lacking causative associations. The social realm ought to be highly susceptible to such an impairment due to its dynamic and highly interactive nature.
To further search for the causes of the attentional dysfunction in autism, one has to investigate the neural processing modules that provide input information to the attentional mechanisms. A major candidate would be sensory domain, which provides sensory information to perception and attentional processes.
It has been suggested that individuals with autism might have difficulty in encoding and representing sensory features of physically complex (but not simple) stimuli (15). Such a deficit would place autistic individuals at a disadvantage in processing signals of social communication (e.g., facial expressions or speech) because of their multifaceted and rapidly changing nature. If substantial, such an abnormality might severely compromise the extraction of the meaning from the ongoing stream of social information. Furthermore, if social stimuli are not particularly meaningful to individuals with autism, they might develop no motivation to attend to this type of information or, worse, they might even develop resentment to the stimuli and situations to which they feel they should but cannot relate. In support of this view, 5-year-old children with autism preferred listening to nonsense sounds to their mothers' talking (16).
On the other hand, individuals with autism not only perceive music well (17) but also outperform their peers in pitch discrimination (18) and in the perception of the detailed structure of segments of melodies (19). These findings suggest that individuals with autism are able to process well certain types of complex nonverbal auditory input.
The above-mentioned findings on social stimulus-specific deficits in autism (4, 16) did not differentiate between sensory and attentional processes nor did they evaluate the effects of the physical stimulus complexity on the autistic children's responses. Nonetheless, to advance our understanding of the pathophysiology of autism, one must determine whether the sensory processing of information is impaired in autism and, further, whether this possible impairment could be linked to the abnormalities of attention. To achieve this goal, one has to be able to separate the sensory and attentional processes involved in the processing of the same stimuli. Furthermore, the two processing modules should be studied as a quantitatively controlled function of physical stimulus complexity, with the speech sounds representing the most complex stimuli.
The goal of the present study was to determine whether children with autism have a disadvantage in the sensory representation of, and attentive orienting to, speech sounds (vowels) as compared with the sensory representation of, and attentive orienting to, acoustically matched complex and simple nonspeech sounds (tones). Recording cortical event-related brain potentials (ERP) allows one to examine several distinct and well-characterized stages of sensory and attentional processing. Therefore, the tone and vowel stimuli were presented in an oddball paradigm, permitting the elicitation of the auditory sensory ERPs, the mismatch negativity (MMN) component (20, 21) that taps the neural sound representation underlying conscious auditory perception (22), and the P3a component that indexes the involuntary switching of attention (attentional orienting) to salient environmental events (23, 24).
Auditory sensory ERPs reflect the neural processing of physical stimulus features (e.g., sound intensity or frequency). However, their relationship with the perception of those sound features is poor in adults (for reviews, see refs. 22 and 25) and has not yet been established in children. In contrast, the MMN correlates with the behavioral sound discrimination abilities in adults (26–28) and children (29, 30). The MMN is elicited by the “deviant” sounds occurring infrequently among the repetitive “standard” sounds, if the deviants are perceptually distinguishable from the standards. The MMN has been used to evaluate central auditory dysfunction in individuals with language and reading impairment (31–34), learning disability (29), and autism, associated with the structural brain lesions in tuberous sclerosis (35).
If a deviant stimulus is perceptually salient, then a call for attention toward it is generated, which may result in an actual attention switch. If attention is switched, then the P3a response is elicited (21, 24). The role of the P3a as an index of attention switch has been firmly established by studies demonstrating P3a elicitation by attention-catching novel or otherwise salient stimuli (36–39) and by correlations between stimulus salience and the P3a elicitation and between the P3a elicitation and the deterioration, due to distraction, of the behavioral task performance in adults (40–44) and children (45). In normally developing children, the P3a response correlated with their behavioral distractibility (46). Furthermore, the enhanced P3a indexed pathological distractibility in adults with chronic alcoholism (for a review, see ref. 24) and closed head injury (47) and in children with major depression (T.L., M. Soininen, R.Č., F. Almqvist, R.N., and E. Aronen, unpublished work). In turn, the P3a amplitude was diminished in adults with focal brain lesions, who manifested an impaired ability to orient to the stimuli (48, 49).
Summarizing, the present study aimed at determining whether high-functioning children with autism show abnormalities in the sensory module of information processing, attentive orienting, or both, and whether these possible abnormalities depend on stimulus complexity and speechness quality.
Materials and Methods
Subjects.
Brain responses were recorded in nine high-functioning autistic children (eight males, 6.3–12.4 yr old, mean 8.9 yr) and in 10 controls (nine males, 6.6–12.4 yr old, mean 8.4 yr) with no reported hearing or academic problems. The patients were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders–IV (DSM-IV) criteria for autism in Helsinki University Central Hospital (HUCH). As indexed by audiometry, their hearing sensitivity was normal. No abnormalities were revealed in their electroencephalograms or brain images (MRI in six and computer-assisted tomography in two patients; no quantitative volumetric analyses were performed). The Childhood Autism Rating Scale (50) was administered at the ages of 5–9 years by an experienced nurse in the HUCH and the Finnish version of Reynell Developmental Language Scale (51) was administered at the ages of 5–11 years (Table 1). At the time of the ERP testing, seven children were attending special education classes for autism, and two patients were integrated in regular classrooms with aides. Four children had very low language levels (needed pictures to communicate), whereas the rest spoke with sentences and were able to read. All were receiving speech therapy. No patient was on medication at the time of the ERP experiment. The study was approved by the Ethical Committee of the HUCH.
Table 1.
Behavioral and language characteristics of children with autism
| SS | Age at ERP test | Age at behavioral evaluation | PIQ | CARS scores
|
RDLS–verbal comprehension
|
|||
|---|---|---|---|---|---|---|---|---|
| Total* | Verbal† | Listen† | Raw score | Comprehension age | ||||
| S1 | 9.2 | 6.6 | 93 | 31 | 3 | 1.5 | 0 | <1.0 |
| S2 | 7.3 | 6.5 | 78 | 33 | 3 | 2 | 20 | 2.0 |
| S3 | 6.3 | 7.1 | 70 | 37 | 3 | 2 | 7 | 1.1 |
| S4 | 9.2 | 9.2 | 80 | 38.5 | 2.5 | 3 | 56 | 4.7 |
| S5 | 8.8 | 9.2 | 113 | 35 | 2 | 2.5 | 57 | 4.9 |
| S6 | 12.4 | 11.0 | 74 | 32.5 | 3 | 2 | — | 7.6§ |
| S7 | 8.7 | 7.1 | 88‡ | 31 | 3 | 2 | 47 | 3.8 |
| S8 | 10.3 | 4.4 | 73‡ | 38 | 2.5 | 2.5 | 38 | 3.0 |
| S9 | 6.11 | 6.10 | 102‡ | 36 | 3 | 2.5 | 32 | 2.8 |
CARS, Childhood Autism Rating Scale; RDLS, Reynell Developmental Language Scale.
Total score of >37, severe autism; 30–37, mild autism; and <30, no autism.
The seven-point score range is from 1 to 4; 1 is age-appropriate, and 4 is typical for autism.
Tested with WPPSI-R (52) or WISC-III (53), depending on age. The rest of the children were tested with the Leiter International Performance Scale (54).
Tested with the Finnish version of the Illinois Test of Psycholinguistic Abilities (ITPA, ref. 55) by using the Auditory Association subtest.
Stimuli and Experimental Design.
One standard and one deviant stimulus were generated for each of the three stimulus classes: simple tones, complex tones, and vowels. The goal was to create a set of stimuli ranging from acoustically simple to complex and to ensure the best possible acoustic correspondence among the stimuli of the three classes. Therefore, the stimulus synthesis was started by generating the standard vowel. The vowel (Finnish/ö/) was synthesized by using the Semisynthetic Speech Generation (SSG) method (56), which enables one to create quantitatively controlled yet natural-sounding speech stimuli. The speech, synthesized with the SSG, sounds natural, because this method uses natural human utterances to extract a waveform of a glottal flow, representing the fluctuation of the vocal folds during utterance. The glottal waveform is then used to excite an artificial vocal tract, which shapes the waveform according to the phoneme to be produced. The formants‡‡ of a phoneme, created in such a way, can be adjusted as needed. The four lowest formants of the standard vowel/ö/(450, 1,420, 2,200, and 3,500 Hz) were neighbored by the lower-energy harmonics†† rendering the “speechness” quality to the stimulus. The deviant vowel/ö/was created by raising the frequencies of the formants of the standard vowel by 10% each. The complex tone was composed of four sinusoidal tones (458, 1,370, 2,054, and 3,537 Hz), with the frequency and intensity levels matching the strongest harmonics in the vicinity of the formants of the vowel/ö/. The simple tone corresponded to the strongest formant of the vowel/ö/spectrum, 458 Hz. Finally, the simple- and complex-tone deviants were created by raising all their frequencies by 10%.
Stimuli of each class were presented in separate blocks, 400 stimuli each, with a stimulus-onset asynchrony of 700 ms and an intensity of 55 dB sound-pressure level at the subject's head. Of all stimuli, 86% were standards (260 ms in duration), and the rest were randomly occurring frequency (P = 7%) or duration (P = 7%; responses not reported here) deviants of the same stimulus class. The frequency deviants were 10% higher in frequency, including all formants.
At least three blocks (1,200 stimuli) of each stimulus class were presented to each subject. Sounds were delivered via two loudspeakers, located at each side of a television monitor situated at a 1.8-m distance in front of the subject. Subjects were instructed to ignore auditory stimuli and concentrate on watching silent videos of their choice. Their attention was visually monitored throughout the recording.
Placing the sources of the unattended auditory stimuli in front of the subject and just outside the spatial focus of their visual attention facilitated the elicitation of the P3a (57, 58). Finally, moderate rather than salient stimulus changes were used, because both sensory discrimination of, and attentive orienting to, subtle changes in the environment are critical prerequisites to language and communication skills, both of which are aberrant in autism.
Electrophysiological Recordings.
The electroencephalogram (amplified by SynAmps (El Paso, TX) at DC-30 Hz and digitized at 250 Hz) was recorded at the left and right frontal (F3, F4) and central (C3, C4) electrode sites, according to the International Electrode Placement 10–20 system. Eye movements were monitored with two electrodes placed below and at the outer corner of the right eye. Off-line, the data were rereferenced to the algebraic average of the right and left mastoid recordings, digitally filtered (bandpass 1–15 Hz), and baseline corrected with respect to the mean amplitude of the 100-ms prestimulus period. Epochs with voltage exceeding 150 μV in any channel, the first three epochs of each block, and those immediately after the deviants were omitted from averaging. The deviant-stimulus averages included at least 90 trials per subject.
The MMN and P3a (Fig. 2) were measured from the difference waves obtained by subtracting the standard-stimulus ERP from the deviant-stimulus ERP of the same stimulus class. The peak latencies were measured from the time intervals that included the corresponding peak of each individual in that group. The amplitudes of the sensory ERP peaks (the P1 and N2, Fig. 1), the MMN, and the P3a (Fig. 2) were measured at the latencies of their maximal amplitudes in the grand-average waveforms, specific to each group (Table 2). The peak amplitudes were used in the sensory ERP peak analyses due to their high signal-to-noise ratio. The MMN and the P3a were measured as the means of the 50-ms intervals centered at their grand-average peak latencies.
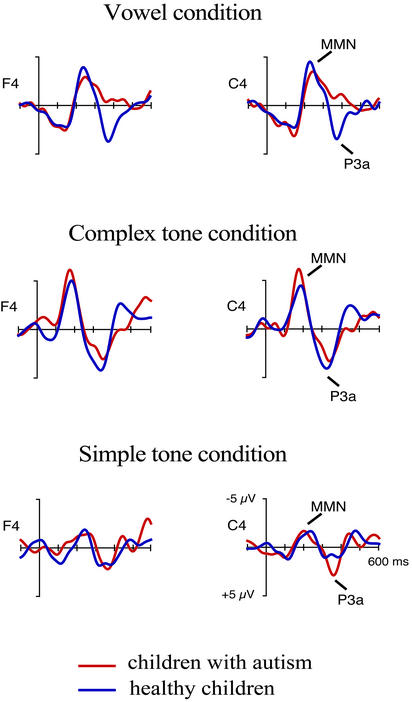
Figure 2.
The deviant-minus-standard difference waves in children with autism and in the controls, obtained in the vowel (Top), complex-tone (Middle), and simple-tone (Bottom) conditions. There were no significant group differences in the MMN amplitude. In contrast, the P3a response was absent in children with autism, but in the vowel condition only.
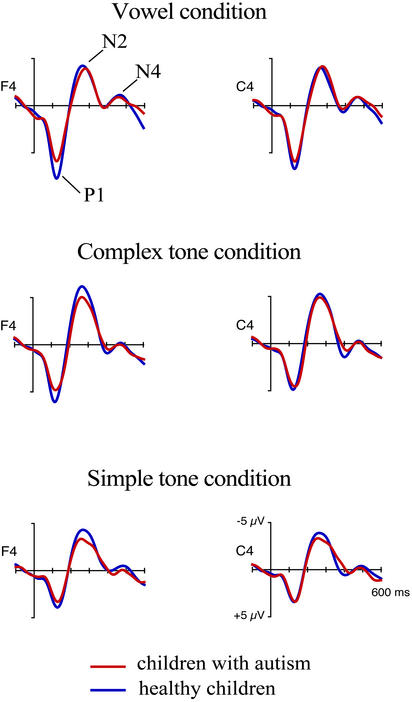
Figure 1.
The auditory sensory ERPs elicited by the repetitive “standard” sounds in the children with autism and in the controls in the vowel (Top), complex-tone (Middle), and simple-tone (Bottom) conditions. There was a tendency for the P1 peak to be smaller in children with autism as compared with that of the controls.
Table 2.
The latencies (in milliseconds) used for ERP amplitude measurements
| Standard class | Group | P1 | N2 | MMN | P3a |
|---|---|---|---|---|---|
| Vowels | A | 120 | 272 | 240–248 | 360–368* |
| C | 120 | 264 | 220–232 | 360–368 | |
| Complex tones | A | 120–128 | 260 | 176 | 336–360 |
| C | 120 | 264–272 | 184–192 | 328–340 | |
| Simple tones | A | 128 | 256 | 200–268 | 348–360 |
| C | 124 | 264 | 208–224 | 304–324 |
The noted latency intervals were used in those cases in which there were latency differences among the four electrodes (A, autism group; C, control group).
Because in the autism group no distinctive P3a could be distinguished in the vowel condition, the P3a amplitudes in this group were measured at the latencies of the grand average P3a of the control group.
Two-tailed t tests were used to determine the statistical presence (difference from 0 μV) of the ERP peaks across the groups and conditions. To test the between-group differences and the effects of the stimulus class, three-way mixed ANOVAs (group × stimulus class × electrode) were performed on the component amplitudes and latencies. The least-significant difference post hoc test was used to calculate the sources of the significant main effects and interactions. Greenhouse–Geisser adjustments were performed when appropriate.
Results
Sensory ERPs.
In both groups, the stimuli of each type elicited the P1-N2-N4 complex, typically obtained in children with fast stimulus presentation (refs. 59–61; Fig. 1). These peaks were highly significant in both healthy children and those with autism. Statistical analyses revealed only a trend for the P1 to be smaller in amplitude in the autistic children than in the healthy controls (P < 0.07). No group differences were found in the latencies of the sensory ERP peaks.
The MMN.
A significant MMN was elicited by all stimulus classes in both groups of children (Fig. 2 and Table 3), and there were no significant ANOVA effects involving group. The stimulus class effect was significant at F(2, 32) = 8.73, P < 0.001 and originated from the complex-tone MMN being larger in amplitude than that elicited by simple tones (P < 0.0003) in both groups of children. These results indicated that, in the sensory domain, children with autism were able to discriminate frequency changes in the simple and complex tones and in the vowel at the level of accuracy comparable to that of their healthy peers.
Table 3.
The mean amplitudes (over all four electrodes, in microvolts) and latencies (at the F3 electrode, in milliseconds) of the MMN and P3a in children with autism (A) and their controls (C)
| Stimulus class | MMN
|
P3a
|
MMN
|
P3a
|
||||
|---|---|---|---|---|---|---|---|---|
| Amplitude
|
Latency (SD)
|
|||||||
| A | C | A | C | A | C | A | C | |
| Vowels | −2.92* | −3.26† | −0.52 (ns) | 3.52† | 252 (34) | 245 (30) | 394 (43) | 395 (33) |
| Complex tones | −5.15† | −5.05‡ | 2.66* | 3.94† | 184 (36) | 187 (22) | 342 (43) | 323 (29) |
| Simple tones | −1.61 (ns) | −1.70* | 2.08† | 2.23‡ | 231 (45) | 242 (37) | 356 (34) | 340 (41) |
At the F3 electrode:
, P < 0.05;
, P < 0.01;
, P < 0.005; ns, nonsignificant.
The P3a.
In healthy children, the P3a was significant in all stimulus conditions and was smaller in response to changes in the simple tone than to changes in either the complex tone or the vowel (P < 0.01 and P < 0.05, respectively). In contrast, in children with autism, no P3a was elicited by changes in the vowel (Fig. 2 and Table 2), whereas changes in both types of tones elicited significant P3a responses. Accordingly, the group × stimulus class interaction in the ANOVA analysis was significant at F(2, 34) = 3.82, P < 0.03. This interaction resulted from a significant between-group difference in the vowel–P3a amplitude (P < 0.0004), whereas there was only a slight trend toward a group difference in the complex-tone P3a amplitude (P = 0.14) and no significant group difference in the simple-tone P3a amplitude (P = 0.96). There was also a slight trend toward P3a latency being longer in children with autism than in control children (P = 0.19; Table 2), regardless of stimulus type.
Discussion
The present study was aimed at determining whether high-functioning children with autism have difficulty in sensory processing of complex sounds and in particular vowels, and whether such a possible difficulty could be linked with attentional preferences. Our results indicated that sensory sound processing, including pitch discrimination, was largely intact in high-functioning children with autism, regardless of the acoustic sound complexity or “speechness” quality. In contrast, their attentional orienting to sound changes was impaired, albeit exclusively for the speech sound (vowel).
One of the aims of the present study was to determine how children with autism centrally process sounds of different acoustic complexities. Sound detection by the sensory systems and the transient encoding of acoustic sound features are reflected by sensory ERPs (22, 62). A typical sensory childhood response, consisting of the P1, N2, and N4 peaks (60–62), was elicited in children of both groups by all three types of standard stimuli (Fig. 1). Among these sensory peaks, only the P1 showed a tendency to be smaller in children with autism (Fig. 1), albeit regardless of stimulus type. The possibly diminished P1, echoing the diminished P1 in adults with autism (ref. 63, but see ref. 64), and an abnormal intensity modulation of the auditory N1 wave in children with autism (65, 66) suggest that certain early sensory processes might be mildly dysfunctional in autism. These dysfunctions, however, probably concern the feature nonspecific aspects of stimulus processing (e.g., stimulus detection, facilitation/inhibition, and sensorimotor integration), because both the P1 and N1 are sensitive to arousal (67, 68) but show poor sound-feature specificity (22, 25). Further, the N2 and N4 peaks of auditory sensory ERP were normal in the autism group (see also ref. 69). Therefore, the present study found no evidence for abnormalities in the processing of the sensory sound features (refs. 63, 70 and 71; for reviews, see refs. 72 and 73) in high-functioning children with autism.
As reflected by the MMN, an ERP index of sound discrimination, the integrated sound representation, underlying auditory perception, was also normal in these children. This is in agreement with Gomot et al. (74), who found no significant differences in the tone-frequency MMN amplitude between children with autism and their controls, and with Kemner et al. (75), who found no abnormalities in the speech-sound-elicited MMN in children with autism.
As indexed by the present MMN data, children with autism were able to form short-term neural representations of repetitive sounds with an accuracy permitting the detection of moderate pitch deviations in these stimuli. Further, neither the acoustic complexity nor the “speechness” quality of these stimuli affected the sensory discrimination of pitch in the autism group, as shown by their normal MMN amplitudes. This finding is in good agreement with the generally good behavioral performance on auditory target (acoustically simple and complex) detection tasks by adults and adolescents with autism (6, 7, 11) and with the normal behavioral pitch discrimination abilities in adults with autism (18).
In contrast to the largely intact auditory sensory processing, the P3a response, indexing involuntary attention switch (21, 23, 24, 39, 41), was abnormal in high-functioning children with autism. Importantly, this abnormality varied as a function of stimulus type: in these children, changes in both simple and complex tones elicited normal P3a responses, whereas changes in the vowel elicited no P3a (Fig. 2).
In both groups of children, the simple tones evoked the smallest P3a responses (Table 2, Fig. 2 Bottom), and there was no difference in P3a amplitude between the groups (see also ref. 11). Thus, simple auditory tones appear to effectively catch attention in high-functioning children with autism.
The complex-tone deviants, rich in acoustic content, elicited the largest P3a responses in both groups of children (Table 3, Fig. 2). In children with autism, the complex-tone P3a tended to be somewhat smaller in amplitude than that in their healthy peers, albeit insignificantly. This finding indicated that, to a certain extent, acoustic complexity might affect autistic children's orienting. However, according to existing behavioral evidence (4, 76), it is unlikely that physical stimulus complexity could entirely account for social attention deficits in autism, because children with autism oriented to physically complex nonsocial stimuli similarly as their healthy peers.
In contrast, the present children with autism oriented very differently to vowel deviants than to complex tone deviants, regardless of the fact that both of these stimuli were acoustically complex. Strikingly, children with autism showed no P3a, an ERP index of orienting, to vowel changes.
One might suggest that orienting to vowel changes was abolished in children with autism, because acoustically, vowels were even more complex than complex tones. However, the present MMN data show that frequency changes were discriminated equally well in vowels as in the complex tones by children with autism. Therefore, accurate sensory information on the vowel and its changes was available in children with autism. Furthermore, the complexity of the complex tones of the present study was substantial; it covered a broad range of sound frequency and intensity, typical for speech. Also, as a function of tone complexity, the autistic children's P3a changed in the same manner as did that in the controls; it was larger in response to the complex-tone than simple-tone changes (Table 2). Therefore, it is feasible that the “speechness” quality of the vowel stimulus was the main factor compromising the autistic children's orienting. Consistent with the present ERP results, behavioral evidence indicates that children with autism orient and attend to physically complex nonsocial stimuli similarly as do their healthy peers but show deficient orienting to complex social stimuli (4, 76).
Therefore, the present findings demonstrate that, despite the availability of the sensory information on the vowel and changes in its pitch, autistic children's orienting could not be triggered by those changes. Therefore, there appears to be a striking dissociation between the sensory (normal) and attentional (impaired) processing of speech sounds in high-functioning children with autism.
In summary, high-functioning verbal children with autism showed no significant abnormalities in sensory processing of the spectral characteristics of a simple tone, a complex tone, or a vowel. In contrast, their involuntary orienting was selectively impaired to changes in the vowel but not to changes in simple or even complex tones. Therefore, it appears that the impairment of auditory processing of vowels in these children occurred beyond the stage of sensory processing, and that the vowel-exclusive attentional orienting deficit might be contingent on the “speechness” quality of sound stimuli. If such an orientation deficit is present as early as in infancy, it profoundly compromises the development of verbal and nonverbal communication skills in the affected children.
Acknowledgments
We acknowledge Mr. J. Arkkila and Ms. J. Meskanen for data collection and Ms. S. Wolkenhauer for language editing. Our sincere thanks to the children and parents who participated in the study. This study was supported by the Academy of Finland (project no. 70252).
Abbreviations
- ERP
event-related potential
- MMN
mismatch negativity
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
A formant is the resonance of a vocal tract (expressed in frequency, Hz), specific to a speaker and a phoneme; the formants are defined by the characteristics of the vocal tract.
The harmonics are lower-frequency acoustic components neighboring the formants; they are defined by the characteristics of the vocal folds of the speaker.
References
- 1.Kanner L. Nervous Child. 1943;2:217–250. [Google Scholar]
- 2.Rapin I. N Engl J Med. 1997;337:97–104. doi: 10.1056/NEJM199707103370206. [DOI] [PubMed] [Google Scholar]
- 3.Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, Rees L, Wheelwright S. J Child Psychol Psychiatr. 1998;39:747–753. [PubMed] [Google Scholar]
- 4.Dawson G, Meltzoff A N, Osterling J, Rinaldi J, Brown E. J Autism Dev Disord. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- 5.Novick B, Vaughan H G, Jr, Kurtzberg D, Simson R. Psychiatry Res. 1980;3:107–114. doi: 10.1016/0165-1781(80)90052-9. [DOI] [PubMed] [Google Scholar]
- 6.Courchesne E, Kilman B A, Galambos R, Lincoln A J. Electroencephalogr Clin Neurophysiol. 1984;59:238–248. doi: 10.1016/0168-5597(84)90063-7. [DOI] [PubMed] [Google Scholar]
- 7.Courchesne E, Lincoln A J, Kilman B A, Galambos R. J Autism Dev Disord. 1985;15:55–76. doi: 10.1007/BF01837899. [DOI] [PubMed] [Google Scholar]
- 8.Oades R D, Walker M K, Geffen L B, Stern L M. Int J Psychophysiol. 1988;6:25–37. doi: 10.1016/0167-8760(88)90032-3. [DOI] [PubMed] [Google Scholar]
- 9.Courchesne E, Lincoln A J, Yeung-Courchesne R, Elmasian R, Grillon C. J Autism Dev Disord. 1989;19:1–17. doi: 10.1007/BF02212714. [DOI] [PubMed] [Google Scholar]
- 10.Ciecielski K T, Courchesne E, Elmasian R. Electroencephalogr Clin Neurophysiol. 1990;75:207–220. doi: 10.1016/0013-4694(90)90174-i. [DOI] [PubMed] [Google Scholar]
- 11.Lincoln A J, Courchensne E, Harms L, Allen M. J Autism Dev Disord. 1993;23:37–58. doi: 10.1007/BF01066417. [DOI] [PubMed] [Google Scholar]
- 12.Courchesne E, Townsend J, Akshoomoff N A, Saitoh O, Yeung-Courchesne R, Linkoln A, James H E, Haas R H, Schreibman L, Lau L. Behav Neurosci. 1994b;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- 13.Townsend J, Courchesne E, Egaas B. Dev Pshychopathol. 1996;8:563–584. [Google Scholar]
- 14.Townsend J, Westerfield M, Leaver E, Makeig S, Jung T-P, Pieerce K, Courchesne E. Cognit Brain Res. 2001;11:127–145. doi: 10.1016/s0926-6410(00)00072-0. [DOI] [PubMed] [Google Scholar]
- 15.Dawson G, Lewy A. In: Autism: Nature, Diagnosis, and Treatment. Dawson G, editor. New York: Guilford; 1989. pp. 49–74. [Google Scholar]
- 16.Klin A. J Autism Dev Disord. 1991;21:29–42. doi: 10.1007/BF02206995. [DOI] [PubMed] [Google Scholar]
- 17.Heaton P, Hermelin B, Pring L. Music Percept. 1998;15:291–305. [Google Scholar]
- 18.Mottron L, Burack J. In: The Development of Autism: Prospects from Theory and Research. Burack J A, Charman T, Yirmiya N, Zelazo P R, editors. Mahwah, N.J.: Erlbaum; 2001. pp. 131–148. [Google Scholar]
- 19.Mottron L, Peretz I, Menard E. J Child Psychol Psychiatr. 2000;41:1057–1065. [PubMed] [Google Scholar]
- 20.Näätänen R, Gaillard A W K, Mäntysalo S. Acta Psychol. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- 21.Näätänen R. Behav Brain Sci. 1990;13:201–288. [Google Scholar]
- 22.Näätänen R, Winkler I. Psychol Bull. 1999;125:826–859. doi: 10.1037/0033-2909.125.6.826. [DOI] [PubMed] [Google Scholar]
- 23.Squires K C, Squires N K, Hillyard S A. J Exp Psychol Hum Percept Perform. 1975;1:268–279. doi: 10.1037//0096-1523.1.3.268. [DOI] [PubMed] [Google Scholar]
- 24.Escera C, Alho K, Schröger E, Winkler I. Audiol Neurootol. 2000;5:151–166. doi: 10.1159/000013877. [DOI] [PubMed] [Google Scholar]
- 25.Näätänen R, Picton T. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 26.Lang H, Nyrke T, Ek M, Aaltonen O, Raimo I, Näätänen R. In: Psychophysiological Brain Research. Brunia C H M, Gaillard A W K, Kok A, Mulder G, Verbaten M N, editors. Vol. 1. Tilburg, The Netherlands: Tilburg Univ. Press; 1990. pp. 294–298. [Google Scholar]
- 27.Winkler I, Tervaniemi M, Huotilainen M, Ilmoniemi R, Ahonen A, Salonen O, Standertskjöld-Nordenstam C-G, Näätänen R. NeuroReport. 1995;6:2317–2320. [PubMed] [Google Scholar]
- 28.Näätänen R, Lehtokoski A, Lennes M, Cheour M, Huotilainen M, Iivonen A, Vainio M, Alku P, Ilmoniemi R, Luuk A, et al. Nature. 1997;385:432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- 29.Kraus N, McGee T J, Carrell T D, Zecker S G, Nicol T G, Koch D B. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- 30.Čeponienė R, Service E, Kurjenluoma S, Cheour M, Näätänen R. Dev Psychol. 1999;35:709–720. doi: 10.1037//0012-1649.35.3.709. [DOI] [PubMed] [Google Scholar]
- 31.Schulte-Körne G, Deimel W, Bartling J, Remschmidt H. NeuroReport. 1998;9:337–340. doi: 10.1097/00001756-199801260-00029. [DOI] [PubMed] [Google Scholar]
- 32.Baldeweg T, Richardson A, Watkins S, Foale C, Gruzelier J. Ann Neurol. 1999;45:495–503. doi: 10.1002/1531-8249(199904)45:4<495::aid-ana11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 33.Kujala T, Karma K, Čeponienė R, Belitz S, Turkkila P, Tervaniemi M, Näätänen R. Proc Natl Acad Sci USA. 2001;98:10509–10514. doi: 10.1073/pnas.181589198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulte-Körne G, Deimel W, Bartling J, Remschmidt H. Int J Psychophysiol. 2001;40:77–87. doi: 10.1016/s0167-8760(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 35.Seri S, Cerquiglini A, Pisani F, Curatolo P. Clin Neurophysiol. 1999;110:1825–1830. doi: 10.1016/s1388-2457(99)00137-6. [DOI] [PubMed] [Google Scholar]
- 36.Courchesne E, Hillyard S A, Galambos R. Electroencephalogr Clin Neurophysiol. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- 37.Donchin E, Coles M G. Behav Brain Sci. 1988;11:357–427. [Google Scholar]
- 38.Knight R T, Scabini D. J Clin Neurophysiol. 1989;15:3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 39. Jeon, Y. W. & Polich, J. (2001) Clin. Neurophysiol.112. [DOI] [PubMed]
- 40.Schröger E. J Cognit Neurosci. 1996;8:527–539. doi: 10.1162/jocn.1996.8.6.527. [DOI] [PubMed] [Google Scholar]
- 41.Escera C, Alho K, Winkler I, Näätänen R. J Cognit Neurosci. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- 42.Berti S, Schröger E. Cognit Brain Res. 2001;10:265–273. doi: 10.1016/s0926-6410(00)00044-6. [DOI] [PubMed] [Google Scholar]
- 43.Escera C, Yago E, Alho K. Eur J Neurosci. 2001;14:877–883. doi: 10.1046/j.0953-816x.2001.01707.x. [DOI] [PubMed] [Google Scholar]
- 44.Gaeta H, Friedman D, Ritter W, Cheng J E. Psychol Aging. 2001;16:55–68. doi: 10.1037/0882-7974.16.1.55. [DOI] [PubMed] [Google Scholar]
- 45.Gumenyuk V, Korzyukov O, Alho K, Escera C, Schröger E, Ilmoniemi R, Näätänen R. Neurosci Lett. 2001;314:147–150. doi: 10.1016/s0304-3940(01)02308-4. [DOI] [PubMed] [Google Scholar]
- 46.Kilpeläinen R, Luoma L, Herrgard E, Sipila P, Ypparila H, Partanen J, Karhu J. NeuroReport. 1999;10:3405–3410. doi: 10.1097/00001756-199911080-00027. [DOI] [PubMed] [Google Scholar]
- 47.Kaipio M-L, Cheour M, Čeponienė R, Öhman J, Alku P, Näätänen R. NeuroReport. 2000;11:1463–1468. doi: 10.1097/00001756-200005150-00021. [DOI] [PubMed] [Google Scholar]
- 48.Knight R T. J Electroencephalogr Clin Neurophysiol. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- 49.Knight R T, Scabini D, Woods D L, Clayworth C. Brain Res. 1989;502:109–116. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- 50.Schopler E, Reichler R, Rochen Renner B. Childhood Autism Rating Scale. Los Angeles: Western Psychological Services; 1993. [Google Scholar]
- 51.Reynell J K, Huntley M. Reynell Developmental Language Scales. Second Revision. Windsor, U.K.: NFER-Nelson; 1985. [Google Scholar]
- 52.Wechsler D. WPPSI-R Manual: Wechsler Preschool and Primary Intelligence Scales, Revised. Helsinki: Psykologien Kustannus Oy; 1990. ; (1995) WPPSI-R Käsikirja. Wechslerin älykkyystestistö esikouluikäisille (Psykologien Kustannus Oy, Helsinki). [Google Scholar]
- 53.Wechsler D. Wechsler Intelligence Scale for Children (WISC-IIII Manual) Helsinki: Psykologien Kustannus Oy; 1991. ; (1999) WISC-III Käsikirja. Wechslerin Lasten Alykkyysasteikko (Psykologien Kustannus Oy, Helsinki). [Google Scholar]
- 54.Leiter R G. Leiter International Performance Scale. Chicago: Stoelting; 1948. [Google Scholar]
- 55.Kirk S A, McCarthy J J, Kirk W D. Illinois Test of Psycholinguistic Abilities (ITPA), Revised Ed. Chicago: Univ. of Illinois Press; 1968. [Google Scholar]
- 56.Alku P, Tiitinen H, Näätänen R. Clin Neurophysiol. 1999;110:1329–1333. doi: 10.1016/s1388-2457(99)00088-7. [DOI] [PubMed] [Google Scholar]
- 57.Teder-Sälejarvi W A, Hillyard S A, Roder B, Neville H J. Cognit Brain Res. 1999;8:213–227. doi: 10.1016/s0926-6410(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 58.Shestakova A, Čeponienė R, Huotilainen M, Yaguchi K. Clin Neurophysiol. 2002;113:162–168. doi: 10.1016/s1388-2457(01)00706-4. [DOI] [PubMed] [Google Scholar]
- 59.Neville H, Coffey S, Holcomb P, Tallal P. J Cognit Neurosci. 1993;5:235–253. doi: 10.1162/jocn.1993.5.2.235. [DOI] [PubMed] [Google Scholar]
- 60.Ponton C W, Eggermont J J, Kwong B, Don M. Clin Neurophysiol. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- 61.Čeponienė R, Rinne T, Näätänen R. Clin Neurophysiol. 2002;113:870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- 62.Čeponienė R, Shestakova A, Balan P, Alku P, Yaguchi K, Näätänen R. Int J Neurosci. 2001;109:245–260. doi: 10.3109/00207450108986536. [DOI] [PubMed] [Google Scholar]
- 63.Buchwald J S, Erwin R, Van Lancker D, Guthrie D, Schwafel J, Tanguay P. J Electroencephalogr Clin Neurophysiol. 1992;84:164–171. doi: 10.1016/0168-5597(92)90021-3. [DOI] [PubMed] [Google Scholar]
- 64.Bruneau N, Roux S, Guérin C, Barthélémy C, Lelord G. Psychophysiology. 1997;34:32–38. doi: 10.1111/j.1469-8986.1997.tb02413.x. [DOI] [PubMed] [Google Scholar]
- 65.Bruneau N, Garreau B, Roux S, Lelord G. In: Current Trends in Evoked Potential Research (EEG Suppl. 40) Johnson R, Parasuraman R, Rohrbaugh J W, editors. New York: Elsevier; 1987. pp. 584–589. [Google Scholar]
- 66.Bruneau N, Roux S, Adrien J L, Barthélémy C. Clin Neurophysiol. 1999;110:1927–1934. doi: 10.1016/s1388-2457(99)00149-2. [DOI] [PubMed] [Google Scholar]
- 67.Erwin R, Buchwald J S. Electroencephalogr Clin Neurophysiol. 1986;65:383–392. doi: 10.1016/0168-5597(86)90017-1. [DOI] [PubMed] [Google Scholar]
- 68.Paavilainen P, Camman R, Alho K, Reinikainen K, Sams M, Näätänen R. In: Current Trends in Event-Related Potential Research (EEG Suppl. 40) Johnson R J, Rohrbaugh J W, Parasuraman R, editors. Amsterdam: Elsevier; 1987. pp. 246–255. [Google Scholar]
- 69.Dawson G, Finley C, Philips S, Galpert L. J Autism Dev Disord. 1988;18:493–504. doi: 10.1007/BF02211869. [DOI] [PubMed] [Google Scholar]
- 70.Grillon C, Courchesne E, Akshoomoff N. J Autism Dev Disord. 1989;19:255–269. doi: 10.1007/BF02211845. [DOI] [PubMed] [Google Scholar]
- 71.Erwin R, Van Lancker D, Guthrie D, Schwafel J, Tanguay P, Buchwald J, S. J Electroencephalogr Clin Neurophysiol. 1991;80:561–571. doi: 10.1016/0168-5597(91)90139-o. [DOI] [PubMed] [Google Scholar]
- 72.Courchesne E. In: Neurobiological Tissues in Autism (Current Issues in Autism) Schopler E, Mesibov G B, editors. New York: Plenum; 1987. [Google Scholar]
- 73.Klin A. J Autism Dev Disord. 1993;23:15–35. doi: 10.1007/BF01066416. [DOI] [PubMed] [Google Scholar]
- 74.Gomot M, Giard M H, Adrien J L, Barthelemy C, Bruneau N. Psychophysiology. 2002;39:577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- 75.Kemner C, Verbaten M, Cuperus J, Camfferman G, van Engeland H. Biol Psychiatry. 1995;38:150–165. doi: 10.1016/0006-3223(94)00247-Z. [DOI] [PubMed] [Google Scholar]
- 76.Maestro S, Muratori F, Cavallaro M C, Pei F, Stern D, Golse B, Palacio-Espasa F. J Am Acad Child Adolesc Psychiatry. 2002;41:1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]