Abstract
We show by using a regulated promoter that clpX of Streptococcus pneumoniae R6 is essential, whereas clpP, clpL, clpC, and clpE can be disrupted. The essentiality of clpX was initially missed because of duplication and rearrangement in the region of the chromosome containing clpX. Depletion of ClpX resulted in a rapid loss of viability without overt changes in cell morphology. Essentiality of clpX, but not clpP, has not been reported previously.
We reported previously that clpP-mediated proteolysis plays an important role in several pneumococcal stress responses, the development of competence, and virulence (22). ClpP proteases consist of an ATPase regulatory subunit from the Hsp100 family of chaperones and the ClpP protease subunit, which contains a serine protease active site (18; reviewed in references 8, 9, 11, 21, and 24). Streptococcus pneumoniae contains putative orthologs of four ATPase specificity factors (ClpC, ClpE, ClpL, and ClpX) (14, 28) and a single clpP gene that is in a monocistronic operon (22). clpP mutants showed temperature sensitivity for growth at 40°C on Trypticase soy agar blood (TSA-BA) plates but were not appreciably impaired for growth at 37°C (22). This observation was made independently by Chastanet and coworkers, who further showed that clpE mutants are temperature sensitive, suggesting a role for ClpE or ClpEP in temperature tolerance (5). Roles for ClpL and ClpX in S. pneumoniae have not yet been discovered, while the function of the ClpC specificity factor is at present in question (4, 5, 22).
We described elsewhere the construction of a ΔclpX::ermAM mutation (erythromycin resistance; Ermr) in S. pneumoniae R6 (strain EL873; Table 1) and found that this mutant did not show temperature-sensitive growth like the ΔclpP mutant (22). However, we also reported that, for reasons that we did not understand, we could not move this ΔclpX::ermAM amplicon into S. pneumoniae D39 (Table 1) by transformation (22) and that we could transfer the ΔclpX::ermAM mutation from R6 to D39 only by using chromosomal DNA isolated from strain EL873 (22). Further analyses of this problem have shown that clpX is actually essential in S. pneumoniae R6 and that the clpX region in the chromosome forms duplications and other rearrangements at low frequency. clpX was required at all temperatures tested, and depletion of ClpX resulted in rapid loss of cellular viability.
TABLE 1.
Bacterial strainsa
| Strain | Genotype or phenotype | Description | Source or reference |
|---|---|---|---|
| EL59 | S. pneumoniae R6, avirulent unen- capsulated parent strain | Derived from D39 isolate | A. Tomasz (14) |
| EL161 | S. pneumoniae D39, virulent encap- sulated type 2 parent strain | Subclone of original clinical isolate | J. Yother (25) |
| EL539 | EL59 ΔclpP::aad9 (Spcr) | EL59 transformed with linear ΔclpP::aad9 amplicon | 22 |
| EL854 | EL59 ΔclpC::ermAM (Ermr) | EL59 transformed with linear ΔclpC::ermAM amplicon | 22 |
| EL873 | EL59 ΔclpX::ermAM clpX+ (Ermr) | EL59 containing previously undetected merodiploid of ΔclpX::ermAM and clpX+ | 22 |
| EL1039 | EL161 ΔclpX::ermAM clpX+ (Ermr) | EL161 transformed with genomic DNA from EL873; contains a previously undetected merodiploid of ΔclpX::ermAM and clpX+ | 22 |
| EL1082 | EL59 ΔclpL::ermAM (Ermr) | EL59 transformed with linear ΔclpL::ermAM amplicon | This study |
| EL1259 | EL59 ΔclpE::aad9 (Spcr) | EL59 transformed with linear ΔclpE::aad9 amplicon | This study |
| EL1383 | EL59 ΔbgaA::(Pc::tetL::T1T2::PfcsK-clpX+) (Tetr) | EL59 transformed with linear ΔbgaA::(Pc::tetL::T1T2::PfcsK-clpX+) amplicon | This study |
| EL1387 | EL1383 ΔclpX<>ermAM (Ermr Tetr) | EL1383 transformed with linear ΔclpX<>ermAM amplicon | This study |
| EL1392 | ΔclpX<>ermAM clpX+ (Ermr) | EL59 transformed with ΔclpX<>ermAM, resulting in a partially characterized duplication of ΔclpX<>ermAM and clpX+ | This study |
Strains were constructed by transformation of indicated recipients with linear double-stranded synthetic PCR amplicon DNA. Following single colony isolation, all constructions were confirmed by PCR analysis of chromosomal DNA by using flanking and internal primers and additional restriction digestion of amplicons (details available at http://www.streppneumoniae.com).
Duplications and rearrangements in the clpX region of the S. pneumoniae R6 chromosome.
The putative ClpX polypeptide of S. pneumoniae R6 shows high identity and similarity over its entire length to ClpX subunits defined for other bacteria (e.g., 58 and 76, 60 and 76, 65 and 81, and 75 and 87% identity and similarity, respectively, to ClpX of Escherichia coli K-12, Caulobacter crescentus, Bacillus subtilis, and Lactococcus lactis, respectively, in GenBank comparisons). The intercistronic regions that separate clpX from surrounding genes are minimal, and there are no obvious factor-independent transcription terminators in this region (Fig. 1A). Thus, clpX may be in a multifunctional operon that extends from dpr through the hypothetical gene spr1422 (Fig. 1A). The open reading frame immediately downstream from clpX designated spr1426 was recently shown to be essential and may encode a GTP binding protein (29). The dfr gene upstream from clpX (Fig. 1A) encodes dihydrofolate reductase, which is required for growth of a number of bacterial species (12). The other known gene upstream of clpX is dpr, which has been implicated in H2O2 resistance (33) and may be critical for growth of S. pneumoniae, which produces H2O2 during aerobic growth under laboratory conditions (26).
FIG. 1.
Organization of the clpX operon in S. pneumoniae R6 and construction of a nonpolar replacement of the clpX reading frame with ermAM (Ermr) (A) in a strain containing an ectopic copy of clpX+ under the control of the fucose-inducible PfcsK promoter located in the bgaA locus (B) (see text and Table 1 for details). Genes and predicted directions of transcription are depicted by arrows (drawn to scale). The lytC and trxB genes likely mark the boundaries of the clpX region based on the large intergenic region and the direction of transcription, respectively. The shaded regions depict the flanking genes surrounding clpX, and the hatched region corresponds to the ermAM open reading frame. Pc-tetL imparts constitutive resistance to 0.25 μg of tetracycline per ml and was used as a selection marker. T1T2 indicates transcription terminators to protect regulated expression from PfcsK.
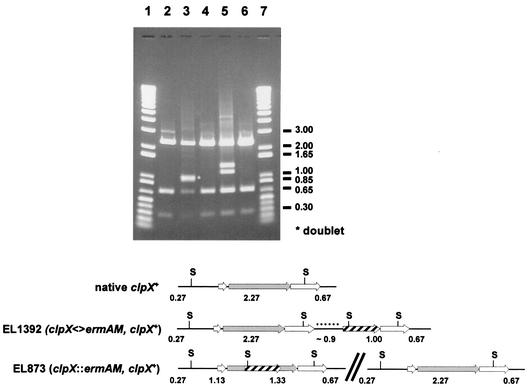
Previously, we confirmed the presence of the ΔclpX::ermAM allele in EL873 by PCR with primers located immediately outside the clpX reading frame (22). Although we thought that we had sufficient resolution on gels to resolve the ΔclpX::ermAM construct from the clpX+ gene, later analyses and further digestion of the PCR products with restriction enzymes revealed that copies of both the ΔclpX::ermAM and clpX+ genes were present in EL873 (Fig. 2, lane 5). Thus, EL873 is a merodiploid that most likely arose by recombination of the ΔclpX::ermAM allele into one copy of a duplication of the clpX region that occurred spontaneously in the chromosome of S. pneumoniae R6 (e.g., see reference 2). This conclusion was fully supported by the observation that EL873 reverted rapidly to sensitivity to erythromycin when antibiotic selection was removed from cultures (Table 2), and this reversion was accompanied by restoration of wild-type clpX+ in the chromosome (Fig. 2, lanes 5 and 6).
FIG. 2.
ScaI restriction maps of the clpX regions of genomic DNA of S. pneumoniae strains EL59, EL873, and EL1392 amplified by PCR with the oligonucleotide primer pair clpX-a and clpX-c (22). Lanes 1 and 7, Gibco 1-kb Plus standard ladder; lane 2, wild-type clpX+ locus from parent strain EL59; lane 3, duplicated spr1426 locus from EL1392, which contains a copy of clpX+ and the clpX<>ermAM cassette; lane 4, Erms revertant of EL1392 containing the wild-type clpX+ region; lane 5, clpX::ermAM with duplicated clpX+ locus from EL873; lane 6, Erms revertant of EL873 containing the wild-type clpX+ region. Shaded arrows, clpX reading frame; hatched arrows, ermAM reading frame; open arrows, genes surrounding clpX (Fig. 1); black dots, ∼0.9 kb of unknown DNA present in EL1392 clpX+ region. ScaI restriction sites are indicated with the letter S and a horizontal line. The break denotes an unknown amount of chromosomal DNA in the EL873 merodiploid. Predicted restriction fragment sizes and indicated DNA standards are in kilobase pairs.
TABLE 2.
Reversion frequency of S. pneumoniae mutants containing duplications in the clpX region of the chromosome
| Strain | Selection during growtha | CFU/mlb | No. of colonies screened | No. (%) of Ermr and Erms isolates following spontaneous resolution of duplications
|
|
|---|---|---|---|---|---|
| Ermr | Erms | ||||
| EL873 | None | 4.7 × 106 | 31 | 4 (13) | 27 (87) |
| ERY | 4.9 × 105 | 31 | 31 (100) | 0 (0) | |
| EL1392 | None | 2.0 × 107 | 130 | 129 (99.2) | 1 (0.8) |
| ERY | 7.9 × 106 | 61 | 61 (100) | 0 (0) | |
Bacteria were grown statically in BHI containing or lacking 0.3 μg of erythromycin (ERY) per ml for ≈7 h at 37°C in 5% CO2, serially diluted in 0.9% (wt/vol) NaCl, and spread onto TSA-BA plates containing or lacking 0.3 μg of erythromycin per ml, respectively. Random colonies were screened for erythromycin resistance (Ermr) or sensitivity (Erms) by being patched onto TSA-BA plates containing or lacking 0.3 μg of erythromycin per ml.
Number of CFU per milliliter of culture from which cells were recovered at the time of plating.
Because of the potential for polarity of clpX insertions on expression of the essential downstream spr1426 gene (Fig. 1A), we attempted to cross the ΔclpX<>ermAM amplicon depicted in Fig. 1A, which has the clpX reading frame replaced exactly from its start to stop codon by the ermAM reading frame, into the chromosome of S. pneumoniae R6 by transformation induced by synthetic competence stimulatory peptide 1 (see reference 13). The frequency of transformation of this amplicon (<2.0 × 10−6 ΔclpX<>ermA transformants) was below the level of detection compared with that of control genomic DNA imparting resistance to novobiocin (7.1 × 10−4 Norr transformants) in R6 parent strain EL59. The frequency of transformation was calculated based on the number of recovered antibiotic-resistant colonies from 1 ml of transformation mixture divided by the total CFU per milliliter. However, one Ermr colony did appear (EL1392; Table 1), which contained another kind of rearrangement in the clpX region. Analysis of the clpX region from EL1392 by PCR showed the presence of an anomalous ∼5-kb amplicon that contained the ermAM cassette, clpX+, a duplication of the flanking downstream gene spr1426, and an uncharacterized region of ∼0.9 kb containing DNA of unknown origin (Fig. 2, lane 3). This arrangement probably arose by an aberrant recombination event in the clpX region. Unlike EL873, reversion of EL1392 to erythromycin sensitivity occurred at a very low frequency when antibiotic selection was removed from cultures (Table 2), and again, reversion was accompanied by restoration of wild-type clpX+ in the chromosome (Fig. 2, lanes 3 and 4).
We determined the frequency at which ΔclpX<>ermAM clpX+ merodiploids arose in transformations compared to that for the unlinked essential gyrB gene (29). Ten independent exponential cultures of EL59 (R6 parent strain) in brain heart infusion (BHI) medium were diluted 1:20 in 1 ml of BHI medium containing 10% heat-inactivated horse serum (Sigma), 10 mM glucose, and 100 ng of competence stimulatory peptide 1 to give a cell density of ∼7.0 × 105 CFU/ml (22). Competent cell suspensions were then mixed with the nonpolar ΔclpX<>ermAM amplicon, a ΔgyrB::ermAM deletion-insertion amplicon, or control genomic DNA bearing a point mutation which confers resistance to novobiocin (Novr) (16). Novr transformants were recovered from these transformations at an average frequency of ∼6 × 10−4, whereas ΔclpX<>ermAM or ΔgyrB::ermAM transformants appeared at a significantly lower frequency of ≤10−6. Thus, formation of ΔclpX<>ermAM clpX+ duplications occurred at a low detectable frequency comparable to that for other essential genes, such as ΔgyrB::ermAM gyrB+, elsewhere in the chromosome of S. pneumoniae R6.
clpX is essential in S. pneumoniae R6.
The fact that we could not obtain a simple transformant that replaced clpX with ΔclpX<>ermAM suggested that clpX was essential. To demonstrate essentiality, we needed to construct a merodiploid that put clpX+ transcription under the control of a regulatable promoter. Several catabolic promoters have been shown elsewhere to be regulated by sugars in S. pneumoniae and related species, including those for galactose, maltose, and fucose utilization (1, 3, 3a, 20; P. F. Chan, K. A. Ingraham, C. Y. So, M. Lonetto, M. Rosenberg, D. J. Holmes, and M. Zalacain, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. H-97, p. 370, 2000). We chose the promoter upstream from the fucose kinase gene (fcsK spr1973) (PfcsK) (14), because it had previously been reported to provide regulation in S. pneumoniae (3a; W. Bae, P. Chan, L. Palmer, V. Clausen, J. Throup, M. Noordewier, K. Koretke, D. Lunsford, A. Bryant, K. Ingraham, D. Holmes, M. Rosenberg, and M. Burnham, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. B-381, p. 126, 2001). We constructed the cassette shown in Fig. 1B in which the promoter region of clpX+ was replaced by PfcsK, which is protected by transcription terminators T1T2 and connected to a tetL (tetracycline resistance [Tetr]) gene driven from a constitutive promoter (Pc) (16). The Pc-tetL::T1T2::PfcsK-clpX+ cassette was crossed into the bgaA locus, which encodes a dispensable β-galactosidase (34) that was partly deleted in the construction, to yield strain EL1383 (Table 1). Tetr transformants were selected on TSA-BA plates containing 0.25% (wt/vol) l-fucose at 37°C in an atmosphere of 5% CO2. The location of the construction was confirmed by PCR (data not shown).
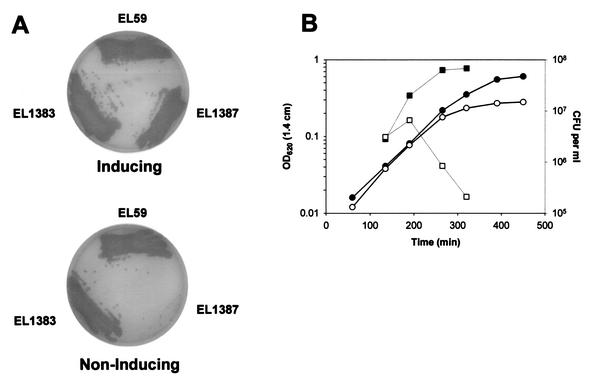
The ΔclpX<>ermAM amplicon was then crossed into the chromosome of EL1383, and Ermr mutants were selected on TSA-BA plates containing the inducer 0.25% (wt/vol) l-fucose. Now the frequency of recovery of Ermr transformants increased dramatically (2.1 × 10−3 ΔclpX<>ermA transformants compared to <2.0 × 10−6 in EL59 [see above]) and was comparable to that for the Novr control (4.5 × 10−4). The resulting regulated replacement EL1387 (Table 1) grew like EL59 (clpX+ parent) and EL1383 [ΔbgaA::(Pc-tetL::T1T2::PfcsK-clpX+) clpX+ merodiploid] on plates containing l-fucose but completely failed to grow when l-fucose was omitted from plates (Fig. 3A). The lack of growth of EL1387 in the absence of l-fucose is not consistent with a polar effect on expression of the essential downstream spr1426 gene, because only expression of the clpX+ gene was under the control of l-fucose in the ΔbgaA locus. Thus, expression of clpX+ is essential for the growth of S. pneumoniae R6.
FIG. 3.
Impaired growth of S. pneumoniae R6 strain EL1387 containing a regulated replacement of clpX (ΔclpX PfcsK::clpX+) on medium lacking fucose. (A) EL59 (clpX+ parent), EL1383 (regulated merodiploid clpX+ PfcsK::clpX+), and EL1387 (regulated replacement ΔclpX PfcsK::clpX+) were streaked onto TSA-BA plates containing 0.25% (wt/vol) fucose (inducing) or lacking additional fucose (noninducing). Plates were photographed after 24 h of incubation at 37°C in an atmosphere of 5% CO2. (B) EL1387 (regulated replacement ΔclpX PfcsK::clpX+) was grown statically overnight in BHI broth containing 0.1% (wt/vol) fucose at 37°C in 5% CO2. Cultures were then diluted 100-fold into fresh BHI containing or lacking 0.1% (wt/vol) fucose, and static incubation was continued at 37°C in 5% CO2. Filled circles and squares represent the optical densities and viable cell counts, respectively, of the culture containing 0.1% (wt/vol) fucose. Open circles and squares represent the optical densities and viable cell counts, respectively, of the culture lacking fucose (0.001% [wt/vol]) carryover fucose from starting culture). Results are representative of at least two independent experiments. OD620 (1.4 cm), optical density at 620 nm for a tube with a 1.4-cm diameter.
Although clpX is essential and clpP is not, it is formally possible that the essentiality of clpX could depend on a functional clpP+, clpC+, clpE+, or clpL+ gene. For example, ClpX could interact with the ClpP protease subunit or one of the other ATPase specificity subunits to limit ClpP proteolysis activity. To test this notion, we transformed EL1387 grown in fucose-containing medium with a clpP::kan::rpsL+, clpC::kan::rpsL+, clpE::kan::rpsL+, or clpL::kan::rpsL+ Janus cassette amplicon (27). For each transformation, we recovered hundreds of transformants on TSA-BA plates containing fucose and kanamycin but no transformants on TSA-BA plates containing kanamycin but lacking fucose (data not shown). Thus, clpX essentiality did not depend on a functional clpP+, clpC+, clpE+, or clpL+ gene.
Phenotypes of clpX underexpression.
We examined phenotypes of bacteria depleted for ClpX to learn more about the basis for the essentiality of clpX. Repetition of the experiment shown in Fig. 3A at 30°C showed that clpX expression was essential at the lower temperature as well as at 37°C for bacteria spread onto TSA-BA plates (data not shown). We found that 0.05% (wt/vol) fucose was the minimum concentration that supported growth of regulated replacement mutant EL1387 in BHI broth at 37°C; however, this concentration of fucose led to lower growth yields of EL1387 than of the regulated merodiploid EL1383 (final culture optical density at 620 nm, 0.17 compared to 0.47, respectively). We next determined the viability of cells of the regulated replacement mutant EL1387 following downshift to a low fucose concentration (Fig. 3B). Addition of 0.1% (wt/vol) fucose supported full growth of viable cells in BHI broth at 37°C (Fig. 3B, closed symbols). In contrast, cultures of EL1387 downshifted to 0.001% (wt/vol) fucose prematurely stopped increasing in optical density and concomitantly rapidly lost cellular viability (Fig. 3B). The plateau in optical density suggested that EL1387 was not undergoing rapid autolysis. Consistent with this observation, examination of EL1387 by phase-contrast microscopy at various times after fucose downshift did not reveal significant changes in cellular morphology compared to that of EL59 and EL1383 controls (data not shown). At 360 min after fucose downshift, <1% of the EL1387 cells were irregularly shaped and large, especially at the end of short chains, compared with those of the EL59 or EL1383 controls (data not shown); however, at this point significant death was occurring in the EL1387 cultures.
The rapid cell death of EL1387 upon ClpX depletion (Fig. 3B) made analysis of global transcription patterns by microarrays (19, 22) problematic. Instead we tested whether potential overexpression of ClpX in the regulated merodiploid EL1383 changed the transcription pattern. We compared microarray patterns of RNA isolated from cultures of EL1383 grown to an optical density at 620 nm of ∼0.2 in BHI at 37°C lacking or induced for 60 min with 0.2% (wt/vol) fucose. On the basis of two independent experiments, we were unable to detect significant differences in transcript patterns of EL1383 cultured with and without fucose, with the exception of the clpX transcript (increased ∼1.7-fold) and those of genes spr1963 to spr1973, which comprise the fucose regulon (fcsK, fucA, fucU, PTS-EII [spr1970], PTS-EII [spr1969], PTS-EII [spr1968], PTS-EII [spr1967], spr1966, spr1965, fucI, and adh2 increased ∼23-, 16-, 15-, 11-, 39-, 25-, 10-, 10-, 36-, 28-, and 3-fold, respectively). It is possible that overexpression of clpX was not sufficient in these experiments to elicit a phenotype.
Implications.
Our finding that clpX is essential, but clpP is not, is unusual, because ClpX usually functions as regulatory subunit for a ClpXP protease (10, 32). We constructed and confirmed the presence of single-copy insertion-deletion mutations that inactivate clpP, clpC, clpL, or clpE (strain EL539, EL854, EL1082, or EL1259, respectively [Table 1]). None was essential for growth of S. pneumoniae R6 at 37°C (data not shown), and only clpP and clpE imparted temperature sensitivity in BHI liquid medium as reported previously (5, 22; data not shown). Curiously, the clpE mutant was not temperature sensitive on TSA-BA plates at 40°C (data not shown), whereas the clpP mutant was (22). The cyanobacterium Synechococcus sp. strain PCC 7942 has genes encoding three ClpP isoenzymes and a single copy of ClpX. Viable deletion-insertion mutants of Synechococcus were obtained only for clpPI and clpPII, suggesting that clpPIII and clpX are indispensable for growth (6, 23). ClpX and ClpP are both essential in C. crescentus (7, 15), and chaperone functions have been ascribed to ClpX besides its role in ClpXP proteolysis (17, 24, 30). A recent report shows that E. coli K-12 lacking functional ClpX or ClpP lost viability more rapidly than did wild-type parent strains during extended stationary phase (31).
The results reported here indicate that ClpX must play essential roles independent of ClpP in S. pneumoniae R6 (Fig. 3A; also see above). Furthermore, depletion of ClpX leads to rapid cell death without overtly affecting cell morphology (Fig. 3B; data not shown). Unlike clpP, clpC, clpE, and clpL, clpX appears not to be a member of the heat shock regulon in S. pneumoniae (22), and microarray analyses indicated that the clpX transcript amount was not significantly changed by phase of growth in chemically defined medium or by sublethal concentrations of the antibiotic triclosan or novobiocin or most common translation inhibitors, except for the macrolides erythromycin and roxithromycin, which caused marginal 1.7-fold induction of clpX transcript amounts (19; data not shown). Genetic and physiological experiments are in progress to learn the essential function(s) of ClpX and to understand the organization and possible regulation of the clpX operon (Fig. 1) in S. pneumoniae.
Acknowledgments
We thank colleagues listed in Table 1 for bacterial strains; D. A. Morrison for the Pc-Tetr cassette used in these studies; and K. Kazmierczak, D. Mytelka T. Nicas, and G. Zhao for comments on the manuscript.
This work was supported by resources provided by Lilly Research Laboratories and Indiana University Bloomington. Gregory T. Robertson was supported by a Lilly Postdoctoral Fellowship.
REFERENCES
- 1.Ajdic, D., and J. J. Ferretti. 1998. Transcriptional regulation of the Streptococcus mutans gal operon by the GalR repressor. J. Bacteriol. 180:5727-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, R. P., and J. R. Roth. 1977. Tandem genetic duplications in phage and bacteria. Annu. Rev. Microbiol. 31:473-505. [DOI] [PubMed] [Google Scholar]
- 3.Bettenbrock, K., and C. A. Alpert. 1998. The gal genes for the Leloir pathway of Lactobacillus casei 64H. Appl. Environ. Microbiol. 64:2013-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Chan, P. F., K. M. O’Dwyer, L. M. Palmer, J. D. Ambrad, K. A. Ingraham, C. So, M. A. Lonetto, S. Biswas, M. Rosenberg, D. J. Holmes, and M. Zalacain. 2003. Characterization of a novel fucose-regulated promoter (PfcsK) suitable for gene essentiality and antibacterial mode-of-action studies in Streptococcus pneumoniae. J. Bacteriol. 185:2051-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charpentier, E., R. Novak, and E. Tuomanen. 2000. Regulation of growth inhibition at high temperature, autolysis, transformation and adherence in Streptococcus pneumoniae by clpC. Mol. Microbiol. 37:717-726. [DOI] [PubMed] [Google Scholar]
- 5.Chastanet, A., M. Prudhomme, J. P. Claverys, and T. Msadek. 2001. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J. Bacteriol. 183:7295-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke, A. K., J. Schelin, and J. Porankiewicz. 1998. Inactivation of the clpP1 gene for the proteolytic subunit of the ATP-dependent Clp protease in the cyanobacterium Synechococcus limits growth and light acclimation. Plant Mol. Biol. 37:791-801. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs, T., P. Wiget, M. Osteras, and U. Jenal. 2001. Precise amounts of a novel member of a phosphotransferase superfamily are essential for growth and normal morphology in Caulobacter crescentus. Mol. Microbiol. 39:679-692. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30:465-506. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman, S. 1999. Regulation by proteolysis: developmental switches. Curr. Opin. Microbiol. 2:142-147. [DOI] [PubMed] [Google Scholar]
- 10.Gottesman, S., W. P. Clark, V. de Crecy-Lagard, and M. R. Maurizi. 1993. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. Sequence and in vivo activities. J. Biol. Chem. 268:22618-22626. [PubMed] [Google Scholar]
- 11.Gottesman, S., and M. R. Maurizi. 1992. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol. Rev. 56:592-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartman, P. G. 1993. Molecular aspects and mechanism of action of dihydrofolate reductase inhibitors. J. Chemother. 5:369-376. [PubMed] [Google Scholar]
- 13.Havarstein, L. S., P. Gaustad, I. F. Nes, and D. A. Morrison. 1996. Identification of the streptococcal competence-pheromone receptor. Mol. Microbiol. 21:863-869. [DOI] [PubMed] [Google Scholar]
- 14.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenal, U., and T. Fuchs. 1998. An essential protease involved in bacterial cell-cycle control. EMBO J. 17:5658-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, M. S., and D. A. Morrison. 1999. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J. Bacteriol. 181:5004-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levchenko, I., L. Luo, and T. A. Baker. 1995. Disassembly of the Mu transposase tetramer by the ClpX chaperone. Genes Dev. 9:2399-2408. [DOI] [PubMed] [Google Scholar]
- 18.Maurizi, M. R., W. P. Clark, S. H. Kim, and S. Gottesman. 1990. ClpP represents a unique family of serine proteases. J. Biol. Chem. 265:12546-12552. [PubMed] [Google Scholar]
- 19.Ng, W. L., K. M. Kazmierczak, G. T. Robertson, R. Gilmour, and M. E. Winkler. 2003. Transcriptional regulation and signature patterns revealed by microarray analyses of Streptococcus pneumoniae R6 challenged with sublethal concentrations of translation inhibitors. J. Bacteriol. 185:359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieto, C., P. Fernandez de Palencia, P. Lopez, and M. Espinosa. 2000. Construction of a tightly regulated plasmid vector for Streptococcus pneumoniae: controlled expression of the green fluorescent protein. Plasmid 43:205-213. [DOI] [PubMed] [Google Scholar]
- 21.Porankiewicz, J., J. Wang, and A. K. Clarke. 1999. New insights into the ATP-dependent Clp protease: Escherichia coli and beyond. Mol. Microbiol. 32:449-458. [DOI] [PubMed] [Google Scholar]
- 22.Robertson, G. T., W. L. Ng, J. Foley, R. Gilmour, and M. E. Winkler. 2002. Global transcriptional analysis of clpP mutations of type 2 Streptococcus pneumoniae and their effects on physiology and virulence. J. Bacteriol. 184:3508-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schelin, J., F. Lindmark, and A. K. Clarke. 2002. The clpP multigene family for the ATP-dependent Clp protease in the cyanobacterium Synechococcus. Microbiology 148:2255-2265. [DOI] [PubMed] [Google Scholar]
- 24.Schirmer, E. C., J. R. Glover, M. A. Singer, and S. Lindquist. 1996. HSP100/Clp proteins: a common mechanism explains diverse functions. Trends Biochem. Sci. 21:289-296. [PubMed] [Google Scholar]
- 25.Smith, M. D., and W. R. Guild. 1979. A plasmid in Streptococcus pneumoniae. J. Bacteriol. 137:735-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. Idanpaan-Heikkila, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803-813. [DOI] [PubMed] [Google Scholar]
- 27.Sung, C. K., H. Li, J. P. Claverys, and D. A. Morrison. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 29.Thanassi, J. A., S. L. Hartman-Neumann, T. J. Dougherty, B. A. Dougherty, and M. J. Pucci. 2002. Identification of 113 conserved essential genes using a high-throughput gene disruption system in Streptococcus pneumoniae. Nucleic Acids Res. 30:3152-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wawrzynow, A., D. Wojtkowiak, J. Marszalek, B. Banecki, M. Jonsen, B. Graves, C. Georgopoulos, and M. Zylicz. 1995. The ClpX heat-shock protein of Escherichia coli, the ATP-dependent substrate specificity component of the ClpP-ClpX protease, is a novel molecular chaperone. EMBO J. 14:1867-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weichart, D., N. Querfurth, M. Dreger, and R. Hengge-Aronis. 2003. Global role for ClpP-containing proteases in stationary-phase adaptation of Escherichia coli. J. Bacteriol. 185:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wojtkowiak, D., C. Georgopoulos, and M. Zylicz. 1993. Isolation and characterization of ClpX, a new ATP-dependent specificity component of the Clp protease of Escherichia coli. J. Biol. Chem. 268:22609-22617. [PubMed] [Google Scholar]
- 33.Yamamoto, Y., M. Higuchi, L. B. Poole, and Y. Kamio. 2000. Role of the dpr product in oxygen tolerance in Streptococcus mutans. J. Bacteriol. 182:3740-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zahner, D., and R. Hakenbeck. 2000. The Streptococcus pneumoniae beta-galactosidase is a surface protein. J. Bacteriol. 182:5919-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]