Abstract
Biofilm formation in Staphylococcus epidermidis depends, in the majority of the strains, on the activity of the icaADBC locus. The expression of the operon that encodes the synthetic enzymes of the intercellular polysaccharide adhesin (PIA) depends on a variety of exogenic environmental conditions and is, at least in part, regulated by the alternative sigma factor σB. We investigated the transcriptional regulation of the ica operon and the respective phenotypes expressed under growth conditions differing in the content of glucose in the growth medium. In the presence of glucose, S. epidermidis exhibited a PIA- and biofilm-positive phenotype whereas ica transcription was down-regulated in the postexponential and stationary phases of growth. Surprisingly, maximum transcription of ica was detectable in the stationary phase of growth in the absence of glucose despite the expression of a PIA- and biofilm-negative phenotype. In vitro enzymatic assays and phenotypic characterization showed that the abundant amount of ica mRNA was functionally active because induction of stationary-phase cells with glucose led to immediate PIA synthesis. Induction of biofilm formation could be completely inhibited by chloramphenicol, which, given at a later stage of biofilm accumulation, also inhibited further development of preformed biofilm, indicating that continuous translation of an additional, icaADBC-independent factor is required for the expression of a biofilm-positive phenotype.
Coagulase-negative staphylococci, particularly Staphylococcus epidermidis, are the leading cause of foreign-body-associated infections and nosocomial bacteremia (3, 4, 51). Despite its low virulence as a commensal member of the human skin and mucous membrane flora, S. epidermidis equals or even outnumbers S. aureus as the causative organism of medical-device-related infections (3). This pathogenic association has been attributed to the ability of pathogenic strains to form multilayered biofilms on polymer surfaces. The process of biofilm formation has been divided into two distinct phases: initial attachment and biofilm accumulation (32). The genetic and biochemical mechanisms of each phase have been the subject of intense research in recent years. Multiple factors have been found to influence bacterial attachment, including bacterial surface proteins, polysaccharides, and physicochemical interactions (18, 26, 42, 46, 56, 57). The second phase is characterized by bacterial accumulation, which requires cell-to-cell interaction. The intercellular polysaccharide adhesin (PIA) has been found to mediate intercellular adhesion in the majority of clinical S. epidermidis isolates (13, 35, 41, 60). This linear glucosaminoglycan is composed of N-acetylglucosamine in β-1,6-glycosidic linkages containing deacetylated amino groups and succinate and phosphate substituents (34, 36). PIA is also functional in hemagglutination of erythrocytes (16, 37). The essential role of PIA expression in the pathogenesis of medical-device-associated infections has been shown in several animal models (52-54). An accumulation-associated protein may also be required for biofilm accumulation (22).
PIA synthesis depends on the expression of the icaADBC operon, which encodes three membrane proteins (IcaA, IcaD, and IcaC) with enzymatic activity and one extracellular protein (IcaB) (17, 19). The icaADBC gene locus has also been detected in S. aureus and a range of other coagulase-negative staphylococci (1, 9, 29, 43).
Phenotypically, the amount of biofilm produced by individual S. epidermidis strains is highly variable and is influenced by changing environmental conditions (2, 10, 12, 23, 28, 38, 40, 44, 47, 48, 50). Even though our knowledge of the regulation of the ica locus is limited, recent data seem to implicate a complex system involving the alternative sigma factor σB, icaR, and two additional, independent regulatory genetic loci (7, 27, 38, 48). PIA synthesis is also subject to an on-off mode of regulation in phase variants of S. epidermidis that, in part, is due to the alternate insertion of an IS element into the ica locus (61).
The expression of a biofilm-positive phenotype in S. epidermidis depends on the presence of glucose in the growth medium (5, 40, 48). Biofilm-positive strains exhibit a PIA- and biofilm-negative phenotype when cultivated in medium lacking glucose but can be induced to produce PIA by subsequent addition of glucose to the culture. In order to gain further insight into the regulatory mechanisms leading to the differential expression of PIA, we compared the transcriptional activity of icaADBC and the respective phenotype expressed under these two physiological growth conditions.
MATERIALS AND METHODS
Bacteria and growth media.
Biofilm-producing S. epidermidis 1457, 8400, RP62A, and 9142 and the isogenic biofilm-negative transductant 1457-M11 have been described previously (36, 37, 40, 45). Basic TA medium was assembled from the individual components and contained (wt/vol) 1.7% Tryptone (lot 208080; Oxoid, Basingstoke, England), 0.3% neutralized soya peptone (lot 14782150; Oxoid), 0.5% NaCl, and 0.25% dipotassium phosphate as recommended by the manufacturer. Glucose or fructose from filter-sterilized stock was added to a final concentration of 0.25% as indicated. Glucose-supplemented medium will be referred to as TA+glc.
RNA isolation and Northern blot analysis.
For RNA preparation from planktonic bacteria, an overnight culture (OC) of S. epidermidis 1457 was diluted 1:100 in fresh, prewarmed TA or TA+glc except when stated otherwise. The bacteria were incubated at 37°C under constant agitation, and aliquots were removed every hour to measure the optical density at 578 nm (OD578). Culture volumes of 20 to 40 ml were harvested at the early-, mid-, postexponential, and stationary growth phases, and total cellular RNA was isolated as described previously (15, 38, 39).
For analysis of static biofilm cultures, S. epidermidis was precultured in TA for 6 to 10 h with shaking at 160 rpm at 37°C. This culture was then diluted 1:100 into TA or TA+glc in several 9-cm-diameter tissue culture dishes (Nunc, Roskilde, Denmark) with an inoculum of 10 ml per dish. In parallel, aliquots (200 μl) of the prepared dilutions were inoculated in 4 wells of 96-well tissue culture plates (Nunc). After 16 h of incubation at 37°C, biofilm formation was induced by the addition of glucose (0.25% final concentration) at different time points so that the induced cultures could be harvested simultaneously (usually 8 h after induction). The cultures in the 96-well tissue culture plates were induced in parallel to allow monitoring of biofilm formation by the adherence assay (6, 40). Total cellular RNA was then recovered as described previously (15). A 10-μg portion of RNA from each sample was separated on a 1% formaldehyde gel and blotted onto nylon membranes. The amount and quality of the prepared RNA and the efficacy of blotting were controlled by visualization of the respective 16S and 23S rRNAs. Northern blot analysis was performed with a 32P-labeled icaC-specific (5′-GAA ATA GCC ATA CCA TTG TCC-3′) oligonucleotide that probed the approximately 3,600-kb length of the icaADBC operon (14, 15, 38).
Characterization of culture conditions.
One to three colonies of S. epidermidis 1457 were inoculated in 200 ml of prewarmed TA and incubated for 12 h at 37°C under constant shaking at 120 rpm. This culture was referred to as the OC. A subculture (SC) was generated by diluting these stationary-phase cells 1:100 in 200 ml of the same batch of fresh, prewarmed TA, which was again incubated for 12 h at 37°C to stationary phase. Usually, an OC and an SC of S. epidermidis 1457 were inoculated simultaneously so that they would reach the stationary phase of growth at the same time.
Analysis of cell aggregation.
Addition of 0.25% glucose to stationary-phase cells grown in TA led to the induction of PIA and biofilm production and the formation of cell aggregates (40). To monitor the respective cultures for the formation of cell aggregates, 3-ml culture samples were removed and transferred to 1 well of a 24-well plate (Nunc) before and every hour after the addition of glucose to the medium. The 24-well plate was stored at 4°C for approximately 8 h until termination of the experiment and then evaluated macroscopically.
Measurement of biofilm formation.
To determine the biofilm formation of S. epidermidis under different physiological conditions, a semiquantitative adherence assay with a 96-well tissue culture plate was performed as described earlier (6, 33). Adherent bacterial biofilms were stained with gentian violet. The amount of biofilm formation was quantified by measuring the OD570 with an automatic spectrophotometer (Behring, Marburg, Germany). An OD570 of >0.1 was defined as a biofilm-positive phenotype. The medium used for preculture was TA or TA+glc, except when stated otherwise.
Preparation of bacterial extracts and coagglutination assay for detection of PIA.
S. epidermidis 1457 was grown to stationary phase in TA or TA+glc. Twenty-milliliter volumes of the cultures were harvested at serial time points, and the cells were washed in phosphate-buffered saline, sedimented by centrifugation (3,000 × g for 10 min), and resuspended in 10 ml of phosphate-buffered saline. Bacterial extracts were prepared by sonication as described previously (36, 40).
To quantitate PIA in bacterial extracts, a specific coagglutination assay was performed as described earlier (33).
Hexosamine assay.
The presence of hexosamine in the culture supernatant or in bacterial extracts was determined by the 3-methyl-2-benzothiazolone hydrazone hydrochloride method with N-acetylglucosamine as the standard as described previously (30, 36).
In vitro synthesis of PIA.
An SC of S. epidermidis 1457 in TA was grown to stationary phase, and crude membranes were prepared as described previously (17). Cell disruption was performed by using the FastPrep shaking apparatus (FastPrep System; Bio 101, Inc., Vista, Calif.). For analysis of Ica enzymatic activity, the prepared membranes were incubated with 2 mM UDP-N-acetylglucosamine and 50 μg of d-cycloserine per ml for 15 h at 20°C as described previously (17). Membranes incubated without UDP-N-acetylglucosamine were used as negative controls, as well as crude membrane preparations of biofilm-negative transposon mutant 1457-M11. As a positive control, we used S. carnosus pTXicaADBC, which harbors the cloned icaADBC gene locus under the control of a xylose-inducible promoter (8). Crude membranes of S. carnosus pTXicaADBC were collected after induction with 0.5% xylose. In vitro synthesis of PIA was verified by coagglutination of the reaction supernatant. Aliquots of the reaction volume were also subjected to periodate oxidation (40).
RESULTS
Analysis of icaADBC transcription under different physiological conditions. (i) S. epidermidis shaking planktonic culture.
The transcriptional activity of icaADBC was analyzed with respect to the phenotypes expressed at different times during growth. To this end, S. epidermidis 1457 was grown in TA medium with and without supplementation with 0.25% glucose. In the adherence assay, S. epidermidis 1457 was biofilm negative in basic TA medium (OD570, <0.1) but established a strong biofilm in TA+glc (OD570, 2.5). An OC of S. epidermidis 1457 in TA+glc was diluted 1:100 either in fresh, prewarmed TA or in TA+glc. The cultures were grown with shaking at 37°C, and aliquots were removed to determine the OD. The cell density (OD578) of S. epidermidis grown in TA+glc was approximately three times higher than that grown in TA. However, the time course of the different growth phases seemed to be the same, independent of the medium used (data not shown). Thus, samples of glucose-supplemented medium and medium lacking glucose were taken at the same time points. Total bacterial RNA was extracted at the early-exponential (after 3 to 4 h), mid-exponential (5 to 6 h), postexponential (9 to 10 h), and stationary (16 h) phases of growth.
Northern blot analysis of S. epidermidis 1457 in TA+glc revealed a strong ica-specific transcript in the early (OD578, ∼1.3) and mid-exponential (OD578, ∼4.5) phases of growth, which then faded during the postexponential (OD578, ∼8.8) and stationary (OD578, ∼8.5) phases of growth (Fig. 1A). A biofilm assay performed in parallel confirmed strong biofilm formation in the presence of glucose (OD570, 2.5).
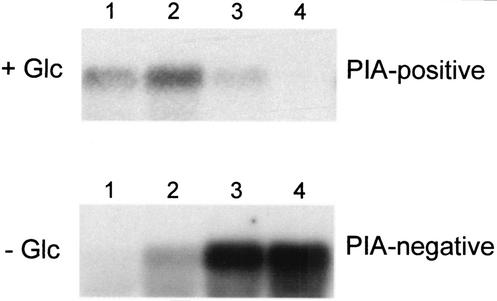
FIG. 1.
Northern blot analysis of icaADBC in shaking cultures of S. epidermidis. An OC of S. epidermidis 1457 was diluted 1:100 in fresh, prewarmed TA (− Glc) and in the same medium supplemented with glucose (+ Glc). The bacteria were incubated at 37°C under constant agitation, and aliquots were removed for RNA extraction at different times during growth. Lanes: 1, early exponential phase of growth (3 to 4 h of incubation); 2, mid-exponential phase of growth (5 to 6 h of incubation); 3, postexponential phase of growth (9 to 10 h of incubation); 4, stationary phase of growth (16 h of incubation). In parallel, a biofilm assay was performed demonstrating a biofilm-negative phenotype in TA and a biofilm-positive phenotype in TA+glc. Hybridization was performed with an icaC-specific oligonucleotide. Data from representative experiments are shown.
Northern blot analysis of S. epidermidis 1457 grown in basic TA displayed a very different transcriptional pattern. Despite the expression of a biofilm-negative phenotype under these conditions, an ica-specific transcript was detectable in the mid-exponential phase of growth (OD578, 1.5) and the amount of ica-specific mRNA increased to a maximum level throughout the postexponential (OD578, 2.4) and stationary (OD578, 2.5) phases of growth (Fig. 1A). To exclude the possibility that the differential ica transcription in medium with and without glucose is specific to S. epidermidis 1457, we also examined three other biofilm-positive S. epidermidis strains (RP62A, 8400, and 9142), which displayed identical transcriptional patterns (data not shown).
To clearly demonstrate the lack of PIA production in medium without glucose supplementation, coagglutination assays with PIA-specific antiserum and hexosamine assays were performed with crude extracts of stationary-phase cells. In cultures of S. epidermidis 1457 grown in basic TA, no biofilm was produced and no PIA could be detected, whereas in cultures grown in the presence of glucose (TA+glc), a biofilm was generated and PIA was detected with a coagglutination titer of 1:64 (Table 1). Hexosamine assays also demonstrated the absence of hexosamine in extracts prepared from cells grown in the absence of glucose. Biofilm-negative mutant 1457-M11, an isogenic mutant of S. epidermidis 1457 with a Tn917 insertion in icaA, was used as a negative control (36) (Table 1). Thus, S. epidermidis also did not produce any precursor oligosaccharides in the absence of glucose that might have failed to be detected by the PIA-specific antiserum used.
TABLE 1.
Detection of PIA and hexosamine in crude extracts of S. epidermidis 1457 and biofilm-negative transposon mutant 1457-M11a
| Strain and medium | Biofilm formation (OD570) | Hexosamine concn (μg/ml)b | PIA titer |
|---|---|---|---|
| 1457 | |||
| TA + Glc | 2.50 | 9.88 | 1:64 |
| TA − Glc | 0.06 | 0 | Negative |
| 1457-M11 | |||
| TA + Glc | 0.02 | 0 | Negative |
| TA − Glc | 0.05 | 0 | Negative |
Both strains were grown in TA in the presence or absence of glucose.
Limit of detection, 1.3 μg/ml.
(ii) S. epidermidis static biofilm culture.
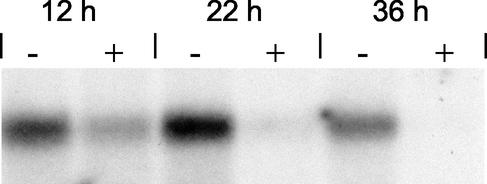
Similar patterns of ica transcription were observed when S. epidermidis 1457 was grown in 9-cm tissue culture dishes used for generation of S. epidermidis biofilms. Northern blot analysis showed a high level of ica-specific mRNA in cultures grown in TA lacking glucose after 12, 22, and 36 h of incubation (Fig. 2). The OD570 was <0.1 at each time point, indicating a biofilm-negative phenotype. In contrast, ica transcription in cultures grown in TA+glc was significantly less pronounced at each time point (Fig. 2), even though strong biofilm formation with OD570s of 1.6, 1.7, and 1.8 was detected at 12, 22, and 36 h, respectively. These findings indicate that the transcriptional patterns observed in shaking planktonic and static biofilm cultures were similar.
FIG. 2.
Northern blot analysis of icaADBC in static cultures of S. epidermidis in TA in the presence or absence of glucose. A 6-h preculture of S. epidermidis 1457 was diluted 1:100 in fresh TA (−) and in TA+glc (+) and cultivated in tissue culture plates at 37°C. After 12, 22, and 36 h of incubation, total bacterial RNA was extracted. Hybridization was performed with an icaC-specific oligonucleotide. In parallel, a biofilm assay was performed demonstrating a biofilm-positive phenotype in TA+glc. OD570s of 1.6, 1.7, and 1.8 were detected at 12, 22, and 36 h, respectively. In TA, S. epidermidis 1457 exhibited a biofilm-negative phenotype.
Analysis of Ica enzymatic activity in membranes of biofilm-negative cultures.
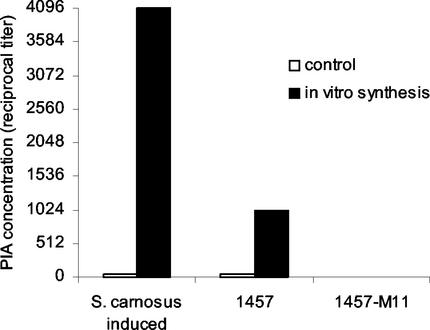
In order to show that the large amount of ica-specific transcripts in stationary-phase cells grown in medium lacking glucose were translated to functionally active proteins, we conducted in vitro enzymatic assays with crude membranes of S. epidermidis 1457. In membrane preparations incubated with UDP-N-acetylglucosamine, coagglutination titers of 1:1,024 were detected, compared to the 1:32 titer of the control membranes (Fig. 3). This result demonstrated in vitro synthesis of PIA and confirmed the presence of functionally active Ica proteins in biofilm-negative cells. In contrast, in vitro assays conducted with membrane preparations of transposon mutant 1457-M11, which is biofilm negative because of a Tn917 insertion in icaA, yielded no PIA because no coagglutination activity could be detected in the reaction supernatant. Membranes prepared from S. carnosus/pTXicaADBC in which icaADBC expression was induced by xylose were used as a positive control. Incubation with UDP-N-acetylglucosamine yielded a coagglutination titer of 1:4,096, compared to the 1:32 titer of the control membranes (Fig. 3). The coagglutination activities of both in vitro reactions of strain 1457 and the control, S. carnosus/pTXicaADBC, were completely abolished by periodate oxidation, whereas the coagglutination titers remained unchanged after incubation with water, thus confirming specific detection of PIA (40).
FIG. 3.
In vitro synthesis of PIA. Crude membranes of S. epidermidis 1457 and biofilm-negative transposon mutant 1457-M11 were prepared from cells grown in TA SC to the stationary phase of growth. The in vitro assay was performed by incubating the collected membranes with UDP-N-acetylglucosamine as described previously (17). The control reaction mixtures did not contain UDP-N-acetylglucosamine. Xylose-inducible S. carnosus pTXicaADBC was used as a positive control.
Correlation of ica transcription with phenotypic features of PIA production.
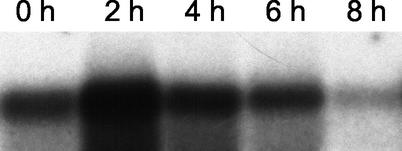
In order to address the question of whether the amount of ica-specific mRNA was relevant with respect to the phenotype expressed after the induction of PIA synthesis by glucose, we compared two simultaneously grown S. epidermidis cultures in TA expressing different basic steady-state levels of ica mRNA. To this end, an SC and an OC of S. epidermidis 1457 were grown with shaking to stationary phase in TA as described in Materials and Methods. Northern blot analysis and densitometric measurements revealed that the level of icaADBC transcription in the S. epidermidis SC was approximately fivefold higher than that of the OC (Fig. 4A). In a kinetic study, both cultures (OC and SC) were analyzed in parallel after induction of PIA synthesis by addition of 0.25% glucose to the medium. Transcriptional analysis revealed an increased level of ica-specific mRNA 2 h after glucose induction in both cultures. The level of mRNA was higher in the SC than in the OC, corresponding to its fivefold increase in basal ica transcription. Four hours after glucose induction, the level of ica-specific mRNA decreased and ica-specific mRNA was then undetectable after 6 to 8 h (Fig. 4A). We subsequently compared the phenotypic features of PIA production in these two cultures (OC and SC) to determine if the larger amount of ica-specific mRNA in the SC consequently led to a greater amount of PIA produced.
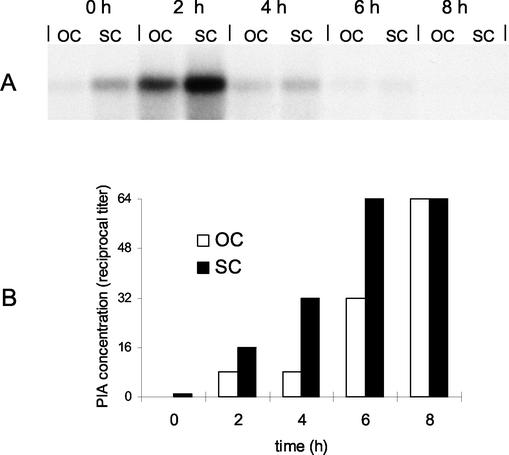
FIG. 4.
Correlation of different levels of ica transcription in an OC and a repeated SC and phenotypical features of PIA production. (A) Northern blot analysis of icaADBC after glucose induction of an OC and a repeated SC of S. epidermidis. An OC and an SC of S. epidermidis 1457 in TA were grown to the stationary phase as described in Materials and Methods. Total bacterial RNA was extracted from the cultures before the addition of glucose (0 h) and at 2-h intervals after the addition of glucose. Hybridization was performed with an icaC-specific oligonucleotide. (B) PIA production after glucose induction of an OC and a repeated SC of S. epidermidis. Twenty-milliliter aliquots were removed from each culture before (0 h) and every 2 h after glucose induction and adjusted to the same OD. Bacterial extracts were prepared by sonication, and the presence of PIA was detected by coagglutination of serial dilutions with PIA-specific antibodies.
(i) PIA production.
In TA, stationary-phase cells of both cultures (OC and SC) did not produce detectable amounts of PIA, as determined by the coagglutination assay (Fig. 4B). Following the addition of glucose, aliquots of the cultures were taken at the same time points as for RNA extraction. Increasing amounts of PIA were detectable after 2, 4, 6, and 8 h of glucose induction in both cultures. In the SC, the coagglutination titer was two- to fourfold higher than that in the OC within the first 6 h after glucose induction. The two cultures reached similar concentrations of PIA after 8 h (Fig. 4B).
(ii) Cell aggregation.
The addition of glucose to the S. epidermidis SC and OC in TA led to macroscopic aggregation of the cells because of the production of PIA. In the SC, aggregation occurred approximately 1 h earlier and to a higher degree than in the OC (data not shown). Apparently, the higher degree of ica expression in the SC enabled the cells to produce PIA more rapidly after induction, indicating that a larger amount of Ica proteins was present before glucose was added to the culture.
(iii) Biofilm formation.
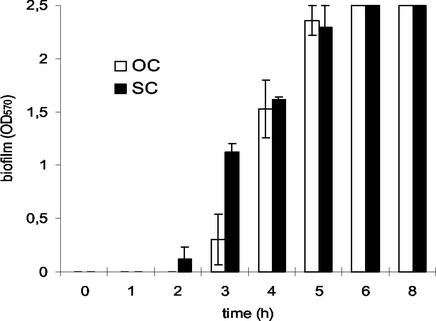
To compare the kinetics of biofilm formation, SC and OC cultures of S. epidermidis were inoculated as described for shaking culture but were instead dispensed into 96-well tissue culture plates and grown to stationary phase as a static culture. Biofilm formation was induced by addition of glucose to both stationary-phase cultures (40). Corresponding to the increased level of ica transcription in the SC, biofilm formation could already be observed 2 h after glucose induction, which was 1 h earlier than in the OC (Fig. 5). Three hours after glucose induction, the OD570 of the SC was twice as high as that of the OC but both cultures subsequently reached the same values and both had a maximum OD570 of 2.5 6 h after induction.
FIG. 5.
Biofilm formation after glucose induction of an OC and an SC of S. epidermidis. A 12-h OC and an SC of S. epidermidis 1457 in TA were diluted 1:100 in the same medium, and each culture was dispensed to one-half of a 96-well tissue culture plate. After 15 h of incubation at 37°C, both cultures were induced by the addition of glucose (0.25% final concentration) at 1-h intervals until the experiment was finished after 8 h of incubation. Biofilm formation was determined as described in Materials and Methods. Mean values of three independent experiments are shown.
The cell density, as determined by measuring the OD578, 8 h after glucose induction increased approximately twofold, indicating that the cells did not re-enter exponential growth as a response to glucose induction.
As a negative control, all assays were also performed with transposon mutant 1457-M11 (37) and no biofilm formation, PIA production, or cell aggregation could be detected in this mutant (data not shown).
Inhibition of PIA production and biofilm formation by blocking protein synthesis.
The lack of PIA production despite the presence of large amounts of ica-specific mRNA in cells grown in the absence of glucose could be due to the lack of a substrate for PIA synthesis or to the absence of an additional, glucose-dependent factor. In order to address this question, we inhibited protein synthesis by adding chloramphenicol to cells grown in TA before inducing biofilm formation with glucose. To this end, S. epidermidis 1457 was grown in TA for 15 h in 96-well tissue culture plates for the induction experiments and in 9-cm tissue culture dishes for RNA extraction. Northern blot analysis demonstrated a high level of ica-specific mRNA before glucose induction, corresponding to the results obtained with a shaking culture (Fig. 6). Therefore, the icaADBC-encoded proteins were expected to be present in the cells before glucose induction, as shown by the in vitro experiments described above.
FIG. 6.
Northern blot analysis of icaADBC after glucose induction of a static S. epidermidis culture. A 6-h preculture of S. epidermidis 1457 was diluted 1:100 in TA and grown in tissue culture plates for 15 h at 37°C. The culture was induced by the addition of glucose at 2-h intervals and so that the induced cultures could be harvested simultaneously for RNA extraction after 8 h of incubation. 0 h, time point without glucose induction; 2, 4, 6, and 8 h, respective time after glucose induction. Six hours after induction, a fully established biofilm was detectable.
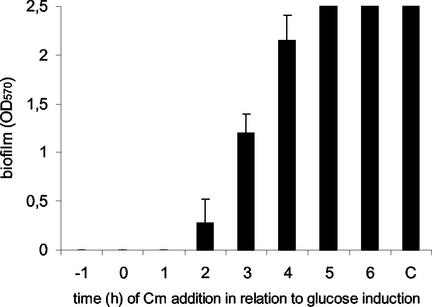
Chloramphenicol was added to the 96-well tissue culture plates in a kinetic study 1 h before induction, at the time of induction, and at different intervals after the addition of glucose. The adherent biofilms were subsequently stained 12 h after induction. Complete inhibition of biofilm formation was observed when chloramphenicol was added before and up to 2 h after glucose induction (Fig. 7). Blocking of protein synthesis 3 to 4 h after glucose induction led to a reduced OD570, but no significant effect was detectable when chloramphenicol was added to an established biofilm at later stages. Apparently, the presence of Ica proteins in S. epidermidis was not sufficient for PIA synthesis after the addition of glucose but translation of an additional factor was required at each stage of biofilm maturation. The number of CFU, as determined by plating, was independent of chloramphenicol treatment, indicating that bacterial cells were still viable in the chloramphenicol concentration used.
FIG. 7.
Inhibition of biofilm formation by blocking of protein synthesis prior to glucose induction. A 6-h preculture of S. epidermidis was diluted 1:100 in TA and incubated in a 96-well tissue culture plate for 15 h at 37°C. Chloramphenicol (Cm) was added (2-mg/ml final concentration) 1 h before (−1), simultaneously with (0), and every hour (1 to 6) after addition of glucose to the culture. The OD570s of four wells were determined after 12 h of incubation. The control (C) did not contain chloramphenicol.
Similar results were obtained in experiments performed with fructose instead of glucose, a sugar that has also been shown to stimulate biofilm formation in S. epidermidis 1457 (data not shown) (40). We therefore concluded that the additional factor required for biofilm formation was induced not specifically by the addition of glucose but also by other monosaccharides supporting biofilm formation.
DISCUSSION
Expression of the icaADBC operon is essential for synthesis of PIA, which mediates cell-to-cell adhesion and promotes a biofilm-positive phenotype of S. epidermidis (19, 34). In the present study, we analyzed the transcriptional activity of ica under growth conditions supporting a biofilm-positive phenotype of S. epidermidis 1457 compared to conditions leading to a biofilm-negative phenotype. By using Northern blot analysis, we demonstrated two different ica transcriptional patterns, depending on the presence or absence of glucose, that were in contrast to the respective phenotypes expressed. In biofilm-negative cultures grown in the absence of glucose, transcription of icaADBC was highly upregulated in the stationary phase of growth whereas biofilm-positive cultures generated significantly less ica mRNA at the same time point. The level of ica mRNA could even be increased by subculturing stationary-phase cells a second time in medium lacking glucose, which might be due to the increased basic transcriptional level expressed at the start of the culture. Growth in the absence of glucose apparently promoted an unusual condition in which high-level steady-state ica mRNA accumulated even though PIA synthesis was inhibited at some stage of its biosynthetic pathway. The detected mRNA was functionally active because in vitro synthesis of PIA was demonstrated with membrane preparations of cells grown in the absence of glucose. The steady-state level of ica mRNA also correlated with the activity of the encoded Ica proteins. A fivefold higher level of mRNA (SC) in stationary-phase cells led to earlier cell aggregation, PIA production, and biofilm formation following induction with glucose.
Glucose first enhances and subsequently inhibits ica transcription. In Bacillus subtilis and some other gram-positive bacteria, glucose-mediated regulation is conferred by catabolite control protein A (CcpA) (20). However, there is no evidence of a CcpA binding site termed cre (catabolite-responsive element) in the sequence upstream of icaADBC (accession no. SEU43366) (19, 21). In S. aureus, glucose-mediated repression of gene expression independent of CcpA has been reported, which mainly involves the synthesis of several virulence factors, including enterotoxins A, B, and C (25, 49). However, up- and downregulation has not been described in these cases.
Interestingly, the downregulation of icaADBC by glucose did not influence the subsequent production of PIA. Four hours after glucose induction, the level of steady-state ica mRNA approximately equaled the level of mRNA before induction; still, a similar increase in the PIA concentration continued to be detected within the next hours. Apparently, the continuous transcription of ica was not required for the accumulation of PIA. The activity of the synthetic apparatus remained intact for several hours even though the transcript was no longer detectable in the cells. This finding is in contrast to observations made on polysaccharide expression in a variety of other bacteria. In S. aureus strain Becker grown in complex Trypticase soy medium, capsular polysaccharide 8 transcription remained at a constant level during the early and late phases of growth, which was compatible with the detected synthesis of CP8 (31). The presence of the has operon mRNA of group A streptococci was also closely correlated to the phenotypic expression of the hyaluronate capsule. Encapsulated strains expressed the has operon mRNA in the exponential phase of growth, as well as the hyaluronate capsule. As the cells entered the stationary phase of growth, they lost the has operon mRNA, which was shown to be concomitant with the loss of their capsule (11).
In staphylococci, knowledge of higher regulatory systems influencing biofilm formation is still limited. The global regulator agr, which is activated in the postexponential growth phase, was shown to influence biofilm formation in S. aureus even though the expression of PIA is not under the control of agr (58). Different environmental conditions were shown to induce biofilm formation by the activation of the alternative sigma factor σB (27, 47). It is conceivable that growth in the absence of glucose is a further stimulus for the activation of σB, which might explain the strong upregulation of ica transcription in the postexponential and stationary phases of growth. Further experimental data are required to evaluate this hypothesis because upregulation of ica transcription in the absence of glucose did not result in the stimulation of a biofilm-positive phenotype in S. epidermidis. Recently, icaR has been identified as a transcriptional repressor of ica expression and its presence was required for ica activation by ethanol (7). However, induction of ica transcription by NaCl-glucose did not require the icaR gene, indicating that different regulatory pathways are operative in ica expression. Our results provide evidence of the existence of additional factors essential for icaADBC activity because even though icaR influences ica transcription, it is apparently also not the factor required for PIA synthesis (because there is no PIA synthesis) in medium lacking glucose. In Escherichia coli, the global regulator CsrA has been shown to affect biofilm formation by negative regulation of intracellular glycogen biosynthesis and catabolism. Biofilm formation was stimulated by disruption of csrA in knockout mutants, demonstrating the importance of carbon flux for biofilm formation (24, 59).
The lack of PIA synthesis despite high-level mRNA and its encoded proteins in the absence of glucose suggested the presence of an additional, icaADBC-independent factor active in the expression of a PIA- and biofilm-positive phenotype. To gain further insight into the process of PIA production and biofilm accumulation after glucose induction, we inhibited protein synthesis by chloramphenicol before the addition of glucose. Northern blot analysis confirmed a high level of preformed ica mRNA before the cells were induced with glucose, so that these cells were already expressing IcaADBC proteins at that time point. Blocking protein synthesis 1 h before and up to 2 h after induction with glucose led to complete inhibition of biofilm formation. These results suggested that translation of an additional, icaADBC-independent factor is required for PIA synthesis and biofilm accumulation. Interestingly, preformed biofilm 3 to 5 h after glucose induction failed to develop further after inhibition of protein synthesis, which implies the need for continuous translation of the additional factor. Since this effect could also be observed with fructose as the inducing monosaccharide, the additional factor does not seem to be glucose specific. Potential candidates include enzymes providing energy or UDP precursors for PIA synthesis. Currently, besides icaADBC, virtually nothing is known about the biosynthetic pathway of PIA. However, since PIA is composed exclusively of N-acetylglucosamine, which is partly de-N-acetylated, the synthesis of their respective sugar nucleotide UDP-N-acetylglucosamine is required as an immediate precursor (17, 55). UDP-N-acetylglucosamine is also used in the formation of cell wall components, so that these two pathways seem to compete for the supply of the same sugar nucleotide. Growth in the absence of glucose might lead to a relative lack of UDP-N-acetylglucosamine available for cell wall synthesis, as can be deduced from the reduced cell density in the stationary phase of growth. It is conceivable that factors involved in the distribution of precursors or energy for PIA synthesis might play a role since the addition of the immediate precursor UDP-N-acetylglucosamine to crude membranes of cells grown without glucose abolished the blockage of PIA synthesis. Apparently, the enzymatic apparatus for assembly and modification of PIA was already present in the cell membranes under conditions lacking glucose.
Further studies are necessary to elucidate the regulation and biosynthetic pathway of PIA synthesis, which will help to explain the ability of S. epidermidis to colonize human niches and foreign medical devices and may open new ways to interfere with this important virulence-associated factor.
Acknowledgments
We thank Rainer Laufs for continuous support. The kind gift of S. carnosus/pTXicaADBC from Friedrich Götz, Molekulare Genetik, University of Tübingen, Tübingen, Germany, is gratefully acknowledged. The photographic work of C. Schlüter is acknowledged.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to D.M. and the Forschungsförderungsfonds Fachbereich Medizin (FFM) of the Universitätsklinikum Hamburg-Eppendorf.
REFERENCES
- 1.Allignet, J., S. Aubert, K. G. Dyke, and N. El Solh. 2001. Staphylococcus caprae strains carry determinants known to be involved in pathogenicity: a gene encoding an autolysin-binding fibronectin and the ica operon involved in biofilm formation. Infect. Immun. 69:712-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker, L. P., W. A. Simpson, and G. D. Christensen. 1990. Differential production of slime under aerobic and anaerobic conditions. J. Clin. Microbiol. 28:2578-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce, J. M. 1997. Epidemiology and prevention of nosocomial infections, p. 309-329. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingston, New York, N.Y.
- 4.Christensen, G. D., L. Baldassarri, and W. A. Simpson. 1994. Colonization of medical devices by coagulase-negative staphylococci, p. 45-78. In A. L. Bisno and F. A. Waldvogel (ed.), Infections associated with indwelling medical devices. American Society for Microbiology, Washington, D.C.
- 5.Christensen, G. D., W. A. Simpson, A. L. Bisno, and E. H. Beachey. 1982. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37:318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlon, K. M., H. Humphreys, and J. P. O'Gara. 2002. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J. Bacteriol. 184:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramton, S. E., C. Gerke, and F. Götz. 2001. In vitro methods to study staphylococcal biofilm formation. Methods Enzymol. 336:239-255. [DOI] [PubMed] [Google Scholar]
- 9.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cramton, S. E., M. Ulrich, F. Götz, and G. Döring. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69:4079-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crater, D. L., and I. van de Rijn. 1995. Hyaluronic acid synthesis operon (has) expression in group A streptococci. J. Biol. Chem. 270:18452-18458. [DOI] [PubMed] [Google Scholar]
- 12.Deighton, M., and R. Borland. 1993. Regulation of slime production in Staphylococcus epidermidis by iron limitation. Infect. Immun. 61:4473-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Silva, G. D., M. Kantzanou, A. Justice, R. C. Massey, A. R. Wilkinson, N. P. Day, and S. J. Peacock. 2002. The ica operon and biofilm production in coagulase-negative staphylococci associated with carriage and disease in a neonatal intensive care unit. J. Clin. Microbiol. 40:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobinsky, S., K. Bartscht, and D. Mack. 2002. Influence of Tn917 insertion on transcription of the icaADBC operon in six biofilm-negative transposon mutants of Staphylococcus epidermidis. Plasmid 47:10-17. [DOI] [PubMed] [Google Scholar]
- 15.Dobinsky, S., and D. Mack. 2001. Efficient RNA isolation method for analysis of transcription in sessile Staphylococcus epidermidis biofilm cultures. Methods Enzymol. 336:255-262. [DOI] [PubMed] [Google Scholar]
- 16.Fey, P. D., J. S. Ulphani, F. Götz, C. Heilmann, D. Mack, and M. E. Rupp. 1999. Characterization of the relationship between polysaccharide intercellular adhesin and hemagglutination in Staphylococcus epidermidis. J. Infect. Dis. 179:1561-1564. [DOI] [PubMed] [Google Scholar]
- 17.Gerke, C., A. Kraft, R. Süssmuth, O. Schweitzer, and F. Götz. 1998. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J. Biol. Chem. 273:18586-18593. [DOI] [PubMed] [Google Scholar]
- 18.Heilmann, C., M. Hussain, G. Peters, and F. Götz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 19.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 20.Henkin, T. M. 1996. The role of CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol. Lett. 135:9-15. [DOI] [PubMed] [Google Scholar]
- 21.Hueck, C. J., W. Hillen, and M. H. Saier, Jr. 1994. Analysis of a cis-active sequence mediating catabolite repression in gram-positive bacteria. Res. Microbiol. 145:503-518. [DOI] [PubMed] [Google Scholar]
- 22.Hussain, M., M. Herrmann, C. von Eiff, F. Perdreau-Remington, and G. Peters. 1997. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect. Immun. 65:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain, M., M. H. Wilcox, P. J. White, M. K. Faulkner, and R. C. Spencer. 1992. Importance of medium and atmosphere type to both slime production and adherence by coagulase-negative staphylococci. J. Hosp. Infect. 20:173-184. [DOI] [PubMed] [Google Scholar]
- 24.Jackson, D. W., K. Suzuki, L. Oakford, J. W. Simecka, M. E. Hart, and T. Romeo. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis, A. W., R. C. Lawrence, and G. G. Pritchard. 1975. Glucose repression of enterotoxins A, B and C and other extracellular proteins in staphylococci in batch and continuous culture. J. Gen. Microbiol. 86:75-87. [DOI] [PubMed] [Google Scholar]
- 26.Kloos, W. E. 1997. Taxonomy and systematics of staphylococci indigenous to humans, p. 113-137. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingston, New York, N.Y.
- 27.Knobloch, J. K. M., K. Bartscht, A. Sabottke, H. Rohde, H. H. Feucht, and D. Mack. 2001. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183:2624-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knobloch, J. K. M., M. A. Horstkotte, H. Rohde, P.-M. Kaulfers, and D. Mack. 2002. Alcoholic ingredients in skin disinfectants increase biofilm expression of Staphylococcus epidermidis. J. Antimicrob. Chemother. 49:683-687. [DOI] [PubMed] [Google Scholar]
- 29.Knobloch, J. K. M., M. A. Horstkotte, H. Rohde, and D. Mack. 2002. Evaluation of different detection methods for biofilm formation in Staphylococcus aureus. Med. Microbiol. Immunol. 191:101-106. [DOI] [PubMed] [Google Scholar]
- 30.Lane-Smith, R., and E. Gilkerson. 1979. Quantitation of glycosaminoglycan hexosamine using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Anal. Biochem. 98:478-480. [DOI] [PubMed] [Google Scholar]
- 31.Luong, T., S. Sau, M. Gomez, J. C. Lee, and C. Y. Lee. 2002. Regulation of Staphylococcus aureus capsular polysaccharide expression by agr and sarA. Infect. Immun. 70:444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mack, D. 1999. Molecular mechanisms of Staphylococcus epidermidis biofilm formation. J. Hosp. Infect. 43(Suppl.):S113-S125. [DOI] [PubMed] [Google Scholar]
- 33.Mack, D., K. Bartscht, C. Fischer, H. Rohde, C. de Grahl, S. Dobinsky, M. A. Horstkotte, K. Kiel, and J. K. M. Knobloch. 2001. Genetic and biochemical analysis of Staphylococcus epidermidis biofilm accumulation. Methods Enzymol. 336:215-239. [DOI] [PubMed] [Google Scholar]
- 34.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mack, D., M. Haeder, N. Siemssen, and R. Laufs. 1996. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J. Infect. Dis. 174:881-884. [DOI] [PubMed] [Google Scholar]
- 36.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 62:3244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mack, D., J. Riedewald, H. Rohde, T. Magnus, H. H. Feucht, H. A. Elsner, R. Laufs, and M. E. Rupp. 1999. Essential functional role of the polysaccharide intercellular adhesin of Staphylococcus epidermidis in hemagglutination. Infect. Immun. 67:1004-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mack, D., H. Rohde, S. Dobinsky, J. Riedewald, M. Nedelmann, J. K. M. Knobloch, H.-A. Elsner, and H. H. Feucht. 2000. Identification of three essential regulatory gene loci governing expression of the Staphylococcus epidermidis polysaccharide intercellular adhesin and biofilm formation. Infect. Immun. 68:3799-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mack, D., A. Sabottke, S. Dobinsky, H. Rohde, M. A. Horstkotte, and J. K. M. Knobloch. 2002. Differential expression of methicillin resistance by different biofilm-negative Staphylococcus epidermidis transposon mutant classes. Antimicrob. Agents Chemother. 46:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mack, D., N. Siemssen, and R. Laufs. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mack, D., N. Siemssen, and R. Laufs. 1994. Identification of a cell cluster associated antigen specific for plastic-adherent Staphylococcus epidermidis which is functional related to intercellular adhesion. Zentbl. Bakteriol. Suppl. 26:411-413. [Google Scholar]
- 42.McKenney, D., J. Hübner, E. Muller, Y. Wang, D. A. Goldmann, and G. B. Pier. 1998. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect. Immun. 66:4711-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 44.Muller, E., J. Hübner, N. Gutierrez, S. Takeda, D. A. Goldmann, and G. B. Pier. 1993. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect. Immun. 61:551-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nedelmann, M., A. Sabottke, R. Laufs, and D. Mack. 1998. Generalized transduction for genetic linkage analysis and transfer of transposon insertions in different Staphylococcus epidermidis strains. Zentbl. Bakteriol. 287:85-92. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson, M., L. Frykberg, J. I. Flock, L. Pei, M. Lindberg, and B. Guss. 1998. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect. Immun. 66:2666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rachid, S., K. Ohlsen, W. Witte, J. Hacker, and W. Ziebuhr. 2000. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44:3357-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Regassa, L. B., J. L. Couch, and M. J. Betley. 1991. Steady-state staphylococcal enterotoxin type C mRNA is affected by a product of the accessory gene regulator (agr) and by glucose. Infect. Immun. 59:955-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rohde, H., J. K. M. Knobloch, M. A. Horstkotte, and D. Mack. 2001. Correlation of biofilm expression types of Staphylococcus epidermidis with polysaccharide intercellular adhesin synthesis: evidence for involvement of icaADBC genotype-independent factors. Med. Microbiol. Immunol. 190:105-112. [DOI] [PubMed] [Google Scholar]
- 51.Rupp, M. E., and G. L. Archer. 1994. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin. Infect. Dis. 19:231-243. [DOI] [PubMed] [Google Scholar]
- 52.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Götz. 2001. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed] [Google Scholar]
- 53.Rupp, M. E., J. S. Ulphani, P. D. Fey, K. Bartscht, and D. Mack. 1999. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect. Immun. 67:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutherland, I. W. 1982. Biosynthesis of microbial exopolysaccharides. Adv. Microb. Physiol 23:79-150. [DOI] [PubMed] [Google Scholar]
- 56.Tojo, M., N. Yamashita, D. A. Goldmann, and G. B. Pier. 1988. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J. Infect. Dis. 157:713-722. [Erratum, 158:268, 1988.] [DOI] [PubMed]
- 57.Veenstra, G. J., F. F. Cremers, H. van Dijk, and A. Fleer. 1996. Ultrastructural organization and regulation of a biomaterial adhesin of Staphylococcus epidermidis. J. Bacteriol. 178:537-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vuong, C., H. L. Saenz, F. Götz, and M. Otto. 2000. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 182:1688-1693. [DOI] [PubMed] [Google Scholar]
- 59.Yang, H., M. Y. Liu, and T. Romeo. 1996. Coordinate genetic regulation of glycogen catabolism and biosynthesis in Escherichia coli via the CsrA gene product. J. Bacteriol. 178:1012-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ziebuhr, W., C. Heilmann, F. Götz, P. Meyer, K. Wilms, E. Straube, and J. Hacker. 1997. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 65:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziebuhr, W., V. Krimmer, S. Rachid, I. Löβner, F. Götz, and J. Hacker. 1999. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol. Microbiol. 32:345-356. [DOI] [PubMed] [Google Scholar]