Abstract
Erysipelothrix rhusiopathiae is a gram-positive bacterium that causes erysipelas in animals and erysipeloid in humans. We found two adhesive surface proteins of E. rhusiopathiae and determined the nucleotide sequences of the genes, which were colocalized and designated rspA and rspB. The two genes were present in all of the serovars of E. rhusiopathiae strains examined. The deduced RspA and RspB proteins contain the C-terminal anchoring motif, LPXTG, which is preceded by repeats of consensus amino acid sequences. The consensus sequences are composed of 78 to 92 amino acids and repeat 16 and 3 times in RspA and RspB, respectively. Adhesive surface proteins of other gram-positive bacteria, including Listeria monocytogenes adhesin-like protein, Streptococcus pyogenes protein F2 and F2-like protein, Streptococcus dysgalactiae FnBB, and Staphylococcus aureus Cna, share the same consensus repeats. Furthermore, the N-terminal regions of RspA and RspB showed characteristics of the collagen-binding domain that was described for Cna. RspA and RspB were expressed in Escherichia coli as histidine-tagged fusion proteins and purified. The recombinant proteins showed a high degree of capacity to bind to polystyrene and inhibited the binding of E. rhusiopathiae onto the abiotic surface in a dose dependent manner. In a solid-phase binding assay, both of the recombinant proteins bound to fibronectin, type I and IV collagens, indicating broad spectrum of their binding ability. It was suggested that both RspA and RspB were exposed on the cell surface of E. rhusiopathiae, as were the bacterial cells agglutinated by the anti-RspA immunoglobulin G (IgG) and anti-RspB IgG. RspA and RspB were present both in surface-antigen extracts and the culture supernatants of E. rhusiopathiae Fujisawa-SmR (serovar 1a) and SE-9 (serovar 2). The recombinant RspA, but not RspB, elicited protection in mice against experimental challenge. These results suggest that RspA and RspB participate in initiation of biofilm formation through their binding abilities to abiotic and biotic surfaces.
Bacteria display a number of cell surface proteins that are needed to inhabit particular ecological niches. In gram-positive pathogens, many surface proteins exhibit adhesive properties and hence play important roles in virulence (11, 23, 33). These proteins sometimes function as ligands to the receptors when the bacteria invade target cells and/or have specific affinity for host components, including immunoglobulins, plasma proteins, and extracellular matrix (ECM) such as collagen, fibronectin, and laminin for evasion from the host immune responses (11, 23, 33). As a common structural feature, these adhesive proteins contain a variety of repeat domains whose lengths vary from two to several hundred amino acid residues (23, 61). In many cases, the function of the repeat domains is not known; however, in a few cases, the repeat domains have been shown to be involved in recognition processes and constitute ligand-binding domains for other proteins or polysaccharides (23, 61). For example, Staphylococcus aureus protein A contains four or five repeats that can bind the Fc region of immunoglobulin G (IgG) (6, 59). Furthermore, the main ligand-binding domains of the fibronectin-binding proteins of Streptococcus dysgalactiae (FnBA and FnBB), S. aureus (FnBPA and FnBPB), Streptococcus pyogenes (F2 and SfbII), and Streptococcus equisimilis (FnB) have been localized in their repeat regions (21, 24, 27, 31).
There is also a group of adhesive surface proteins that contain tandem repeats beginning with the dipeptide Gly-Trp (GW modules). This includes Listeria monocytogenes surface protein (InlB) (13) and autolysin (Ami) (2), and staphylococcal autolysins (Atl, AtlE,and Aas) (18, 19, 36). It has been shown that the repeated GW modules constitute a binding domain for the teichoic acid or lipoteichoic acid polymers in the cell wall, and this association is important for displaying the proteins on the bacterial cell surface (3). The C-terminal domain of the L. monocytogenes Ami is composed of four tandem repeats containing eight GW modules, and the bacteria can bind to eukaryotic cells via this cell wall anchor domain (32). Although the ligand-binding domain has not been determined, Staphylococcus epidermidis AtlE containing three repeats with six GW modules has strong vitronectin-binding activity (18), suggesting that it may contribute to the colonization in vivo. Furthermore, AtlE can bind to a polystyrene surface, that is, AtlE functions in the first step of biofilm formation (18), suggesting that the GW modules of the autolysin may mediate bacterial attachment to abiotic surfaces.
Biofilms are sessile communities formed by bacteria attaching to abiotic and biotic surfaces (4, 7, 15, 37). Biofilm formation has been proposed to be one of the important virulence factors in many bacteria because biofilms facilitate bacterial colonization in vivo by impeding antibiotic penetration and impairing host defenses (4, 7). Development of the biofilm involves at least two steps: (i) rapid attachment of the bacteria to the surface (early adherence), followed by (ii) a more prolonged accumulation phase that involves cell proliferation and intercellular adhesion (37). Recently, surface proteins of S. aureus (Bap) (5) and Enterococcus faecalis (Esp) (58) have been proven to be involved in the first step of biofilm formation. The two proteins contain repeat domains and show some degree of sequence similarity in their overall alignments (58). E. faecalis Esp has been found in most of the clinical isolates (46), suggesting that Esp also plays an important role in the pathogenesis of infection. Thus, many gram-positive surface proteins containing repeat domains are adhesive to abiotic and/or biotic surfaces. However, the repeat domains display numerous patterns of amino acid sequences, and molecular analyses on the role of the repeat domains are quite limited. Therefore, further examples and analyses are needed to make a generalized statement relative to the binding ability of the repeat domains and their relationship to bacterial colonization.
Erysipelothrix rhusiopathiae is a rod-shaped, gram-positive bacterium that causes a variety of diseases in animals and humans (60). The organism is the causative agent of swine erysipelas with symptoms ranging from acute septicemia to chronic endocarditis and polyarthritis (60). Twenty-five serovars have been described for genus Erysipelothrix. DNA-DNA hybridization revealed that they could be divided into at least two distinct species: E. rhusiopathiae comprising serovars 1a, 1b, 2, 4, 5, 6, 8, 9, 11, 12, 15, 16, 17, 19, 21, and type N and Erysipelothrix tonsillarum comprising serovars 3, 7, 10, 14, 20, 22, and 23 (55). Moreover, serovars 13 and 18 are considered to be members of two separate and new species (55). Among them, only E. rhusiopathiae is pathogenic for pigs, and the strains of serovars 1 and 2 have been frequently isolated from diseased pigs (60). The major virulence factor of E. rhusiopathiae is the exopolysaccharide capsule that can protect the bacterial cell from host defenses, such as opsonophagocytosis (47) and intracellular killing by macrophages (48). To date, although three distinct surface proteins of the organism have been described, their roles in relation to adhesive property are entirely unknown (50).
In the present study, we found that E. rhusiopathiae possessed two novel genes, designated rspA (for rhusiopathiae surface protein) and rspB. We describe that a novel consensus repeat found in deduced RspA and RspB proteins is also conserved in fibronectin- and collagen-binding proteins of other gram-positive bacteria. Recombinant RaspA (rRspA) and rRspB were shown to participate in early adherence to an inert surface and binding to ECMs, suggesting that RspA and RspB constitute a novel class of cell surface components which were involved in the initial step of biofilm formation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The E. rhusiopathiae strains used were Fujisawa (serovar 1a) (47), Fujisawa-SmR (serovar 1a) (47), 422/1E1 (serovar 1b) (55), ATCC 19414T (serovar 2) (55), SE-9 (serovar 2) (55), Doggerscharbe (serovar 4) (55), Pécs 67 (serovar 5) (55), Tuzok (serovar 6) (55), Goda (serovar 8) (55), Kaparek (serovar 9) (55), IV12/8 (serovar 11) (55), Pécs 9 (serovar 12) (55), Pécs 3597 (serovar 15) (55), Tanzania (serovar 16) (55), 545 (serovar 17) (55), 2017 (serovar 19) (55), Bano 36 (serovar 21) (55), and MEW 22 (serovar N) (55). The Erysipelothrix tonsillarum strain used was ATCC 43339T (serovar 7) (55). The Escherichia coli strains used were JM109 (Toyobo, Tokyo, Japan), DH5α (Toyobo), XL1-Blue MRF′ (Stratagene, La Jolla, Calif.), XLOLR (Stratagene), and M-15 (Qiagen GmbH, Hilden, Germany). The Erysipelothrix strains were grown at 37°C in brain heart infusion (Difco Laboratories/Becton Dickinson, Sparks, Md.) supplemented with 0.1% Tween 80 (pH 7.6). Cultivation of E. coli strains was performed as described previously (49).
DNA methods.
Restriction enzymes and other enzymes were purchased from Takara Shuzo Co., Ltd. (Tokyo, Japan). As otherwise mentioned, DNA manipulations were performed by standard procedures (44). Plasmid DNAs were isolated from E. coli by using a plasmid Miniprep kit (Promega, Madison, Wis.) according to the manufacturer's protocol. Genomic DNAs from Erysipelothrix strains were prepared by the method of Galán and Timoney (14).
The genomic DNA library of E. rhusiopathiae Fujisawa constructed in λZAP express vector (Stratagene) has already been described (49). The library was screened for immunoreactive phage clones as described previously (49). Immunoreactive plaques were purified, and the phagemids were rescued and used to infect E. coli XLOLR to obtain plasmid subclones as described previously (49).
PCR was performed by using a Perkin-Elmer thermal cycler model 9600 (PE Biosystems Japan, Tokyo, Japan). Takara Ex Taq polymerase and a Takara LA PCR kit were used for conventional and inverse PCR (35), respectively, according to the manufacturer's instructions. The synthetic oligonucleotide primers used for the inverse PCRs were L1 (5′-GCGACTGCCTTATTTTCCAA-3′) and R2 (5′-ATTGCGCGTAAAGTATGGGA-3′) for ClaI-digested fragment and H4-Comp (5′-CACGTTCCCCATTAGGTACTGGTG-3′) and H4 (5′-ACAGGTGTCGAACCAAGTCCAATC-3′) for HindIII-digested fragment. For construction of histidine (His)-tagged rRspA and rRspB proteins, the rspA and rspB fragments were amplified from the genomic DNA of E. rhusiopathiae Fujisawa by using SacI-RspA (5′-CCCCGAGCTCTTGTATGACTGGTATTCA-3′) plus H1 (5′-CACGTTCCCCATTAGGTACTGGTG-3′) and BamHI-RspB (5′-CCCCGGATCCGTTCTAATCGTTTCGGGTAT-3′) plus HindIII-RspB (5′-CCCCAAGCTTTTGTAACGGTTAATCCGTCT-3′), respectively. The rspA fragments were cut with SacI plus HindIII and cloned into the corresponding sites of pQE32 (Qiagen). The rspB fragments were cut with BamHI plus HindIII and cloned into the corresponding sites of pQE30 (Qiagen). Two sets of primers, RspAF (5′-TTGTATCGACTGGTATTCA-3′) plus L4 (5′-ATCACGAGACATACCGCCAA-3′) and RspBF (5′-ATCTTTACCCAATTCGACGT-3′) plus RspBR (5′-ATCAACCATAATCGACCCAT-3′), were designed for amplification of truncated rspA (positions 44 to 582) and rspB (positions 112 to 741) fragments, respectively, from genomic DNAs of various Erysipelothrix strains (numbers in parentheses indicate the positions with respect to the first nucleotide of each gene in E. rhusiopathiae Fujisawa).
Southern hybridization was performed as described previously (47). The truncated rspA and rspB fragments amplified from the genomic DNA of E. rhusiopathiae Fujisawa were labeled with DIG PCR labeling mixture (Roche Diagnostics GmbH, Mannheim, Germany) and used as probes.
DNA sequencing and data analysis.
Sequencing of cloned DNA fragments and PCR products were carried out by dye terminator chemistry with specifically designed primers on an Applied Biosystems model 373A automated DNA sequencer (PE Biosystems). The sequence data obtained were assembled with the Sequencher (version 3.1.1; Hitachi Software Engineering Co., Ltd., Yokohama, Japan) and analyzed with the GENETYX-MAC (version 7.3; Genetyx Corp., Tokyo, Japan). The deduced amino acid sequences obtained were searched for homology with databases by using BLAST network service available at the National Center for Biotechnology Information (Bethesda, Md.; http://www.ncbi.nlm.nih.gov).
Preparation of alkaline-extracted surface antigens.
Cell surface antigens of E. rhusiopathiae were extracted by alkaline treatment as described previously (16). Briefly, E. rhusiopathiae Fujisawa-SmR grown for 18 h was centrifuged and washed twice in distilled water. The bacteria were suspended 10 mM NaOH and incubated at 4°C with constant stirring for 18 h. After neutralization with hydrochloric acid, the bacterial suspension was centrifuged, and the supernatant obtained was concentrated by using a centrifugal filter device (Ultrafree-15 Biomax; Millipore Corp., Bedford, Mass.).
Preparation of rRsp proteins.
Purification of rRsp proteins was performed as described previously (49). Briefly, E. coli M-15 cells transformed with each recombinant plasmid described above were grown at 30°C and harvested. The bacterial cells were sonicated and unbroken cells and particulate material were removed by centrifugation. The cell extract was run on an nickel-nitrilotriacetic acid resin column (Qiagen GmbH), and the bound proteins were purified as described in the manufacturer's instructions. Fraction with rRsp was dialyzed against phosphate-buffered saline (PBS) and stored at −20°C until use.
Immunization of rabbits.
Immunization schedules described below were the same as described previously (49).
(i) Rabbit antisera against alkaline-extracted surface antigens.
Alkaline-extracted antigens in 2-ml aliquots emulsified with 2 ml of complete Freund’s adjuvant was used for immunization of rabbits (Japan SLC, Shizuoka, Japan). Sera obtained from the two rabbits were pooled and used for the immunoscreening.
(ii) Rabbit IgGs raised against rRsp proteins.
Each rRsp protein was further purified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gel regions containing the expected protein band were cut from the gel, ground, and mixed with phosphate-buffered saline (PBS), and the antigen emulsified with 2 ml of complete Freund’s adjuvant was used for immunization of rabbits (Japan SLC). The rabbit antiserum obtained had an anti-rRsp IgG titer of 1:51,200 (with an optical density endpoint of 1.0) as determined by an enzyme-linked immunosorbent assay by using rRspA or rRspB (1 μg/well) as the antigens. The IgG was purified through a column (HiTrap Protein A; Amersham Biosciences) according to the manufacturer's instructions. The purified IgGs were stored at −20°C until use.
SDS-PAGE.
SDS-PAGE was conducted with the discontinuous buffer of Laemmli (25) with a minigel (4% stacking gel, 12.5% running gel) (Bio-Rad, Tokyo, Japan), and the separated proteins were visualized by staining with Coomassie brilliant blue R-250.
Western blot analysis.
Proteins were subjected to SDS-PAGE as described above and blotted onto polyvinylidene difluoride membrane (Millipore Corp.). Unreacted sites were blocked with 5% (wt/vol) skim milk in PBS and incubated with anti-rRsp rabbit IgG antibodies (1 μg/ml) in the blocking solution. Blots were allowed to react with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (KPL, Gaithersburg, Md.). After subsequent washings with PBS containing 0.05% Tween 20 (T-PBS), blots were developed by using enhanced chemiluminescence (ECL Plus; Amersham Biosciences, Tokyo, Japan) according to the manufacturer's instructions and then exposed to Hyperfilm ECL (Amersham Biosciences).
Binding of rRsp proteins to an abiotic surface.
Purified rRsp proteins and bovine serum albumin (BSA; Pierce, Rockford, Ill.) were biotinylated with biotin N-hydroxysuccinimide ester (Sigma-Aldrich). After the confirmation of integrity of the biotinylated proteins by using Western blotting probed with HRP-conjugated streptavidin (Zymed Laboratories, Inc., San Francisco, Calif.), a 50 nM concentration of biotin-labeled rRsp proteins and BSA was serially diluted in PBS, and 100 μl of the protein solutions were distributed in wells of a sterile 96-well polystyrene microtiter plate (Becton Dickinson Labware, Franklin Lakes, N.J.). After a 5-min incubation at room temperature (RT), the wells were washed three times with T-PBS, and the wells were blocked with 5% skim milk for 30 min at RT. After a washing with T-PBS, HRP-conjugated streptavidin (Zymed Laboratories, Inc.) was added to the wells and incubated at RT for 1 h. The wells were washed three times with T-PBS, and the bound rRsp proteins were visualized by TMB Microwell Peroxidase Substrate System (KPL). The color reaction was stopped by addition of an equal volume of 1 M phosphoric acid, and the absorbance was measured at 450 nm with a microplate reader (MTP-120; Corona Electric, Ibaraki, Japan). A well to which rRsp proteins had not been added served as a control, and the value was subtracted from the experimental readings.
Inhibition of bacterial adherence to an abiotic surface by rRsp proteins.
E. rhusiopathiae Fujisawa-SmR grown for 18 h was centrifuged, washed, and suspended in PBS. Aliquots of 100 μl containing the bacterial suspension were added to 900 μl of various concentrations of rRsp protein solutions that were preincubated at RT for 3 min in the wells of a 24-well polystyrene plate (Becton Dickinson Labware) and incubated at 37°C for 30 min. After the wells were rinsed three times with PBS, the bacteria adhering to the surface were harvested through vigorous washings with 1 ml of PBS and spread onto agar plates. Bacterial adherence was quantified by viable-cell counts after the plates were incubated for 24 h. Each assay was repeated three times, and statistical significance was assessed by Student unpaired t test.
Binding of ECMs to immobilized rRsp proteins.
The ECMs used in the present study (type I collagen from calf skin, type IV collagen from human placenta, and bovine plasma fibronectin) were purchased from Nakarai Tesque (Kyoto, Japan). Various concentrations of rRsp protein in 50-μl solutions were placed in wells of 96-well polystyrene microtiter plates (MaxiSorp; Nalgen Nunc International Corp., Tokyo, Japan) and incubated at RT for 30 min. The wells were washed three times with T-PBS and blocked with 5% skim milk at RT for 30 min. The wells were then washed three times with T-PBS, and 50 μl of the ECM solutions (50 μg/ml), rabbit IgG (50 μg/ml) (Sigma-Aldrich), or PBS was added to the wells. The wells containing PBS that had been prepared for each concentration of each Rsp protein served as controls for background values. The rabbit IgG was served as a negative control that does not bind Rsp proteins. The plates were incubated at RT for 1 h and then washed three times with T-PBS. Monoclonal antibodies (MAbs [IgG1 isotype]; Sigma-Aldrich) against each ECM or rabbit IgG were added to the wells and incubated at RT for 1 h. After the wells were washed three times with T-PBS, 50 μl of HRP-conjugated rabbit anti-mouse IgG1 antibody (ICN Pharmaceuticals, Inc., Aurora, Ohio.) was added to the wells and incubated at RT for 1 h. Detection of bound antibodies was performed by using the TMB Microwell Peroxidase Substrate system (KPL) as described above, and background values were subtracted from the experimental readings. Each assay was performed in triplicate and repeated three times.
Bacterial agglutination assay.
E. rhusiopathiae Fujisawa-SmR grown for 18 h was centrifuged, washed, and suspended in PBS. Aliquots of 50 μl containing the bacterial suspension were mixed with 50 μl of either anti-rRspA IgG (20 μg/ml), anti-rRspB IgG (20 μg/ml), a nonspecific rabbit IgG (20 μg/ml; Sigma-Aldrich), or PBS in a 96-well round-bottom microtiter plate (Becton Dickinson Labware). The plate was incubated at 4°C for 18 h, and the agglutination was examined visually.
Protection experiments in mice.
Female mice (BALB/c; Japan SLC), 9 to 12 weeks old, were immunized subcutaneously with either 10 μg of rRspA or rRspB antigen in incomplete Freund’s adjuvant. Two weeks later, the mice were boosted intraperitoneally with 5 μg of the same antigens in complete Freund’s adjuvant. As a control, mice were immunized with PBS emulsified with Freund’s adjuvant by the same schedule. At 7 days after boosting, mice were challenged with ca. 30 CFU of E. rhusiopathiae Fujisawa-SmR and then were observed for 14 days. Statistical significance was assessed by chi-square analysis.
Nucleotide sequence accession number.
The nucleotide sequence determined in the present study has been deposited in the DDBJ/EMBL/GenBank databases under accession number AB052682.
RESULTS
Cloning and sequencing of rsp genes.
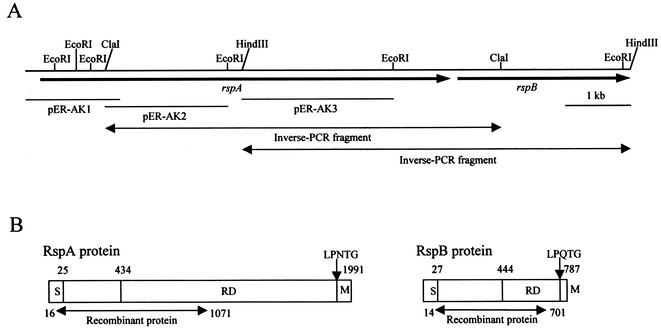
After an immunoscreening of the E. rhusiopathiae genomic library with the rabbit antisera raised against the alkaline-extracted surface antigens, 10 plaques that gave strong positive immunoreactions were selected, and the phagemids were rescued to create plasmid subclones. The cloned fragments were sequenced, and the deduced translation products of open reading frames (ORFs) were compared to amino acid sequences in databases by using the BLAST program. Among them, seven subclones contained a part of known gene, spaA.1, which encoded the major protective antigen located on the cell surface (49). The remaining three subclones, designated pER-AK1, pER-AK2, and pER-AK3, contain DNA regions encoding parts of unknown protein(s), where pER-AK1 and pER-AK2 overlapped (Fig. 1A). Inverse PCRs were used to amplify fragments containing the downstream DNA regions of pER-AK2 and pER-AK3, and amplified fragments were directly sequenced. The two DNA fragments obtained were found to overlap, and the entire DNA sequence determined was 9,140 bp. The sequence contained two putative ORFs with an intergenic space of 249 bp. The two ORFs were encoded on the same DNA strand, and the genes were designated rspA and rspB, as shown in Fig. 1A. The rspA and rspB genes were preceded by typical Shine-Dalgarno sequences located 10 to 15 bp upstream of the putative start codons. An inverted repeat sequence was identified 4 bp downstream of the stop codon of rspA. The G+C contents of rspA (39.4%) and rspB (36.2%) were within the range of that of E. rhusiopathiae total genome (35 to 40%) (55).
FIG. 1.
Schematic diagram of E. rhusiopathiae genomic region encoding rsp genes and the structural organization of rsp gene products. (A) rspA and rspB genes are represented by arrows. Horizontal solid lines, positions of the cloned fragments of the plasmids pER-AK1, pER-AK2, and pER-AK3; horizontal double-headed arrows, regions amplified by inverse PCRs. (B) S, signal peptide; RD, repeat domain; M, the membrane-spanning region and positively charged residues. Numbers indicate the positions of amino acids in the proteins.
Distribution of rsp genes in Erysipelothrix strains.
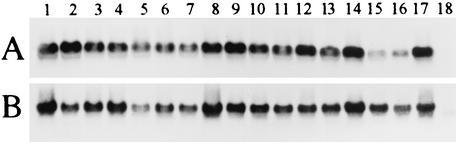
PCRs were performed to amplify the truncated rspA and rspB fragments with primers RspAF plus L4 and RspBF plus RspBR, respectively. DNA fragments of the expected sizes (539 and 630 bp for rspA and rspB, respectively) were amplified from the genomic DNAs of all of the E. rhusiopathiae strains used in the present study; however, no fragment was amplified from the genomic DNA of the E. tonsillarum strain (data not shown). The amplified rspA and rspB fragments were hybridized with rspA and rspB probes, respectively, in the subsequent Southern hybridization (Fig. 2). Sequence analyses of 489 bp of the internal sequences within the rsp gene fragments from the strains of serovars 2 (ATCC 19414T), 4, 19, and 21 showed at least 93.5 and 97.9% identities with rspA and rspB of Fujisawa strain, respectively (data not shown), suggesting that both genes were conserved in the E. rhusiopathiae strains.
FIG. 2.
Detection of rsp genes in Erysipelothrix strains by Southern hybridization. PCR-amplified rspA (A) and rspB (B) fragments were hybridized with rspA and rspB probes, respectively. Lanes: 1, Fujisawa; 2, 422/1E1; 3, ATCC 19414T; 4, SE-9; 5, Doggerscharbe; 6, Pécs 67; 7, Tuzok; 8, Goda; 9, Kaparek; 10, IV12/8; 11, Pécs 9, 12, Pécs 3597; 13, Tanzania; 14, 545; 15, 2017; 16, Bano 36; 17, MEW 22; 18, ATCC 43339T.
Amino acid sequence analysis of rsp gene products.
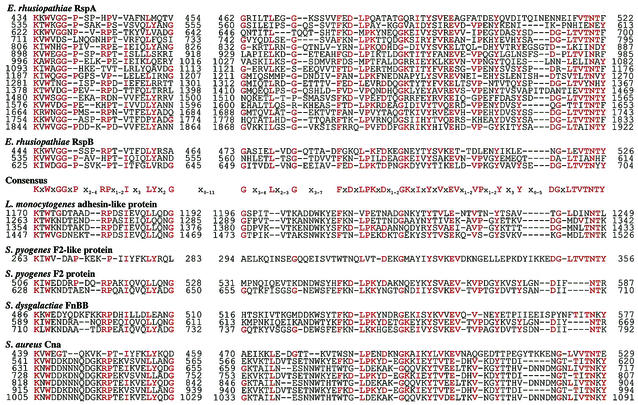
The rspA and rspB encoded proteins of 1,991 and 787 amino acids (aa), respectively. The deduced proteins contained putative N-terminal signal sequences, and thus the predicted molecular masses of the mature form of RspA and RspB were estimated to be 219 and 85 kDa, respectively. The RspA and RspB proteins showed global structural similarity to many adhesive surface proteins of gram-positive bacteria. Their protein structures can be divided into three distinct domains: (i) an N-terminal domain preceded by the signal sequence for secretion, (ii) a repeat domain, and (iii) a C-terminal domain with the LPXTG motif followed by a hydrophobic region and positively charged amino acids, which were necessary for translocation and anchoring to the cell wall (10) (Fig. 1B). They showed relatively low homology in their N-terminal domains (25% identity between aa 25 to 433 of RspA and aa 27 to 433 of RspB) and the repeat domains (40% identity between aa 1754 to 1922 of RspA and aa 443 to 618 of RspB). In the repeat domains, we found that the units of 78 to 92 aa were repeated 16 and 3 times in RspA and RspB, respectively. The repeat units constitute a novel consensus sequence starting with the amino acids Lys-X-Trp (KXW, where X is any amino acids) and designated KXW modules (Fig. 3). Although homology searches inquired by either whole length of the repeat domains or every one unit of the KXW modules showed a low degree of sequence homology (24 to 26% identity) to other adhesive surface proteins, visual alignment of the amino acid sequences through the KXW modules revealed the presence of the consensus repeats in the following proteins: S. aureus Cna (39), S. equisimilis FnB (27), S. dysgalactiae FnBB (26), L. monocytogenes adhesin-like protein (accession no. AL591976-29; protein-id CAC 98705.1), Listeria innocua adhesin-like protein (accession no. AL596165; protein-id CAC 95868.1), S. pyogenes PFBP (42), S. pyogenes protein F2, and F2-like protein (21, 53) (Fig. 2). Except for Cna, the consensus repeats have not been described before in these proteins. Cna has been shown to have three repeats of 187 aa in the domain called B (39). We found that each of the repeats could be divided into two units of the KXW modules and Cna included an additional unit in its N terminus, finally constituting seven units of the KXW modules, as shown in Fig. 3.
FIG. 3.
Alignment of deduced amino acid sequence of repeat domains of Rsp proteins with the corresponding sequences found in adhesive surface proteins of other gram-positive bacteria. The conserved sequences identified by BLAST searches are visually aligned. The consensus sequences representing the residues that appeared at least 15 times in these alignments are shown in red. The numbers indicate the positions of the amino acid residues in the proteins. Dashes, gaps in the aligned sequences. Origins of sequences (accession number): L. monocytogenes adhesin-like protein (AL591976-29, protein-id CAC 98705.1), S. pyogenes F2-like protein (AE009964-1, protein-id AAL96942.1), S. pyogenes F2 (U31980), S dysgalactiae FnBB (Z22151), S aureus Cna (M81736).
Similar to the repeat domains, the N-terminal domains of the Rsp proteins showed a low degree of sequence homology with the N-terminal regions of some collagen- and fibronectin-binding proteins, S. aureus Cna (39), E. faecalis Ace (41), S. equisimilis FnB (27), S. pyogenes F2 (21), and PFBP (42), and putative surface proteins of L. monocytogenes and L. innocua. Especially, among them, those of Cna and Ace have been shown to form a trench as the collagen-binding sites of these proteins (41, 54). The amino acid sequence of the collagen-binding domains within these N-terminal regions were therefore aligned with the corresponding regions of the Rsp proteins in order to highlight their similarity (Fig. 4). However, no significant similarity was found in the C-terminal domains of Rsp proteins.
FIG. 4.
Alignment of deduced amino acid sequence of the N-terminal regions of Rsp proteins with corresponding regions of collagen-binding proteins of S. aureus and E. faecalis. The alignment was performed by means of the program CLUSTAL W (version 1.8). Identical and conserved amino acids are shown in boldface. The regions that have been shown to form the putative collagen-binding trench on the surface of S. aureus Cna (39) are underlined. The numbers indicate the positions of the amino acid residues in the proteins. Origins of sequences (accession number): S. aureus Cna (M81736), E. faecalis (AF260872).
Expression and purification of rRsp proteins.
The rRsp proteins expressed in E. coli were purified as described in Materials and Methods and were subjected to SDS-PAGE (Fig. 5). Although the purified rRspA showed a single band in the gel, the purified rRspB occasionally showed several faint bands, as well as one major band (Fig. 5, lane 2), as have been observed in other surface proteins (9, 49). An increase in the smaller bands in the gels led to a reduction of the largest band (data not shown). When the purified rRspB was probed by Western blots with anti-rRspB rabbit IgG raised against the major band, all of the protein bands reacted with the antibody (data not shown), suggesting that partial degradation of the rRspB protein had occurred.
FIG. 5.
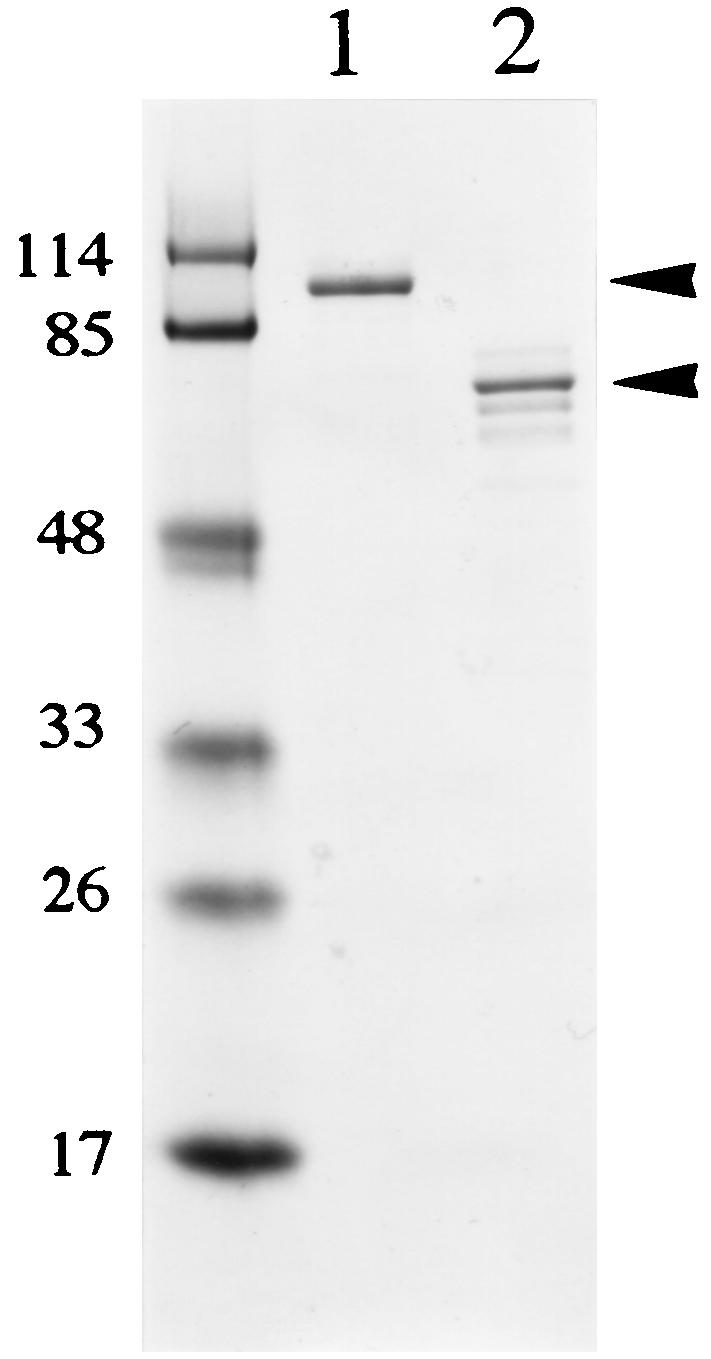
SDS-PAGE of rRsp proteins. rRspA and rRspB were expressed as His-tagged fusion proteins and purified on a nickel-nitrilotriacetic acid resin. The gel regions (arrowheads) containing either RspA or RspB were excised from the gels and used for immunization of rabbits. Lanes: 1, RspA; 2, RspB. Molecular size markers (in kilodaltons) are shown on the left.
Binding of rRsp proteins to polystyrene and inhibition of bacterial adherence.
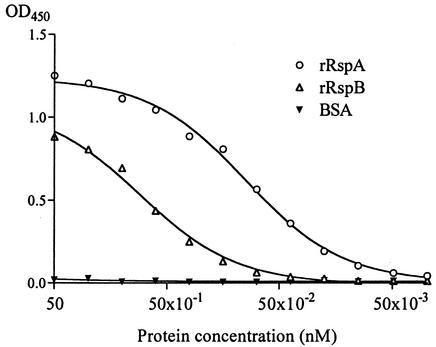
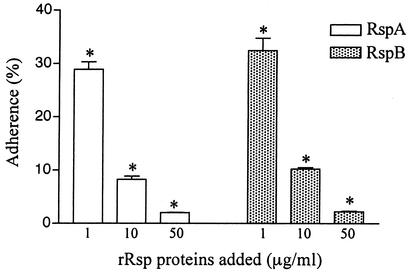
During the purification of rRsp proteins, we found that the purified proteins strongly bind to a polystyrene surface. Binding of bacterial cells or the cellular components to the polystyrene surface is considered to mimic the early adherence in developmental processes of biofilm in vitro. Therefore, we assayed the amounts of biotin-labeled rRsp proteins bound to the polystyrene surface. As shown in Fig. 6, rRsp proteins, especially rRspA, showed strong binding properties. The E. rhusiopathiae cells adhered by themselves to the polystyrene surface, and this adherence was inhibited by addition of either rRspA or rRspB in a dose-dependent manner (Fig. 7), suggesting that Rsp proteins were involved in the first step in biofilm formation.
FIG. 6.
Binding of rRsp proteins onto a polystyrene surface. Various concentrations of biotin-labeled rRsp protein or BSA solutions were added to the wells of 96-well polystyrene microtiter plate and incubated at RT for 5 min. After a washing step, HRP-conjugated streptavidin was added to the wells, and the bound rRsp proteins were determined. The absorbance values determined at 450 nm are the means from a representative experiment performed with triplicate samples.
FIG. 7.
Inhibition of adherence of E. rhusiopathiae Fujisawa-SmR to a polystyrene surface by rRsp proteins. The bacteria suspended in PBS were incubated for 30 min in the presence of various concentrations of rRspA or rRspB. After three rinses with PBS, the number of attached cells was quantitated. The values represent the percentages of cells bound (means ± standard errors) of triplicate wells. The results are representative of three independent experiments. Asterisks indicate a significant difference (P < 0.01) compared to the result obtained in the absence of each rRsp.
Binding of ECMs to immobilized rRsp proteins.
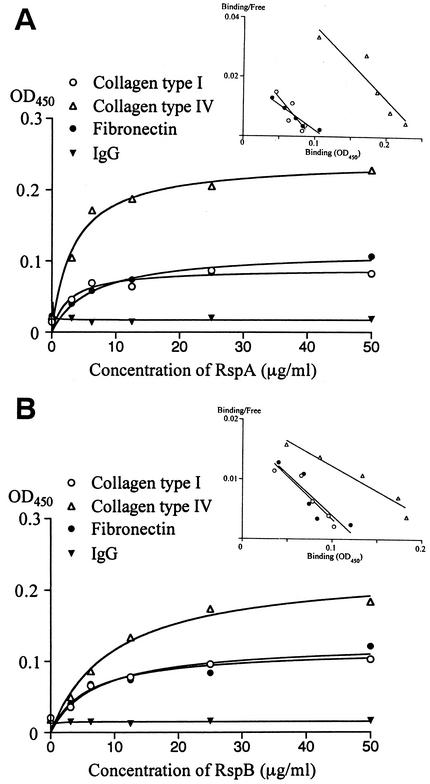
The avid binding properties of rRsp proteins to polystyrene plates hampered the examination of the binding of rRsp proteins to immobilized ECMs. Therefore, to examine the affinity between Rsp proteins and ECMs, we incubated ECMs with various amounts of immobilized rRsp and assayed the bound ECMs by using MAb to each ECM. Figure 8 illustrates that bindings of ECMs to Rsp proteins were rRsp concentration dependent and exhibited saturation kinetics. Scatchard analysis of ECM binding to Rsp proteins yielded linear plots, showing the specific binding of these ECMs to Rsp proteins.
FIG. 8.
Binding of ECMs to immobilized rRspA (A) and rRspB (B) proteins. Polystyrene microtiter plates were coated with various concentrations of rRsp. After a blocking step, 50 μg of ECMs, rabbit IgG, or PBS/ml was added to the immobilized proteins, and the bound ECM or rabbit IgG was detected by using MAbs. Background values (obtained by omitting ECMs or IgG) were subtracted to obtain specific bindings. Scatchard plot analyses of the specific bindings are shown as insets. Each point represents the average of triplicate determinations. The results are representative of three independent experiments.
Expression of native Rsp proteins.
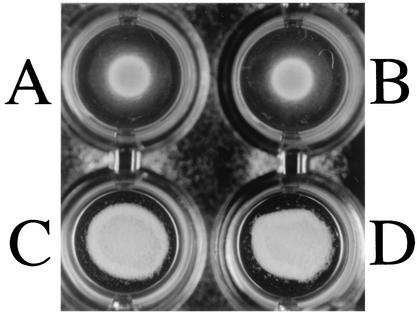
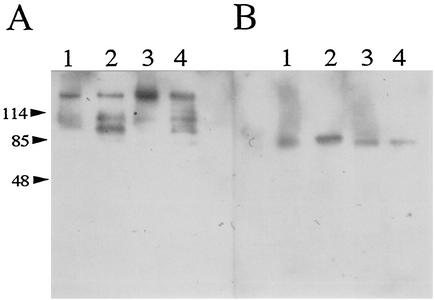
The bacterial cells of E. rhusiopathiae Fujisawa-SmR strain were strongly agglutinated by anti-rRspA and anti-rRspB IgGs (Fig. 9), indicating that the two Rsp proteins are located at the surface. The culture supernatants and the alkaline-extracted antigens of E. rhusiopathiae Fujisawa-SmR and SE-9 (serovar 2) were examined by Western blot analysis. The anti-rRspA IgG visualized a major band with a molecular mass of ca. 220 kDa, which was almost the same as the estimated molecular mass of RspA in both the culture supernatants and the alkaline-extracted antigens (Fig. 10A). In the lanes of the culture supernatants, several additional bands with lower molecular masses also appeared (Fig. 10A, lanes 2 and 4), suggesting that the smaller bands are likely partial degradation products or naturally processed forms of the native RspA protein. The anti-rRspB IgG visualized protein band of ca. 85 kDa, which was in agreement with the estimated molecular mass of RspB (Fig. 10B). These observations strongly suggest that RspA and RspB are not only exposed on the cell surface but also released in the supernatants, as were seen in other adhesive surface proteins of gram-positive bacteria (8, 49).
FIG. 9.
Bacterial agglutination. E. rhusiopathiae Fujisawa-SmR bacteria suspended in PBS were incubated at 4°C for overnight in the absence of rabbit IgG (A) and in the presence of nonspecific rabbit IgG (B), anti-rRspA rabbit IgG (C), and anti-rRspB rabbit IgG (D).
FIG. 10.
Detection of native Rsp proteins by Western blotting. Alkaline-extracted surface antigens and crude supernatant separated on SDS-PAGE were blotted onto polyvinylidene difluoride membranes and probed with anti-rRspA IgG (A) or anti-rRspB IgG (B). Lanes: 1, alkaline-extracted antigens of Fujisawa-SmR; 2, culture supernatant of Fujisawa-SmR; 3, alkaline-extracted antigens of SE-9; 4, culture supernatant of SE-9.
Protection experiments in mice.
All of the mice immunized with rRspB and those given PBS died within 7 days of challenge, whereas 15 of 40 mice (37.5%) that had been immunized with rRspA survived for 30 days after challenge (P < 0.001), indicating that the RspA is one of the protective antigens of E. rhusiopathiae.
DISCUSSION
We have demonstrated here the presence of two genes, rspA and rspB, encoding the novel adhesive proteins of E. rhusiopathiae. The two rsp genes were tandemly located, and both genes were proven to be expressed in the E. rhusiopathiae strains under the culture conditions used. The translated proteins, namely, RspA and RspB, showed a broad spectrum of binding activity for abiotic and biotic surfaces. Two or more of adhesive surface proteins are sometimes produced in a gram-positive bacterium, i.e., fibronectin-binding proteins of S. dysgalactiae (26), S. aureus (22, 51), and S. pyogenes (17, 21, 24, 42, 56, 57), fibrinogen-binding proteins of S. aureus (30, 34), IgG-binding proteins of S. pyogenes (38), and collagen-binding S-layer proteins of Lactobacillus crispatus (52). However, the two Rsp proteins demonstrated here are unique for all surface adhesive proteins described to date in that the two proteins expressed in one strain are directly involved in adherence to abiotic and biotic surfaces.
The Rsp proteins had a limited degree of sequence identity with each other and showed low homology to fibronectin and collagen-binding proteins of other gram-positive bacteria. Nevertheless, alignment of the amino acid sequences revealed that the Rsp proteins, together with S. aureus Cna, S. equisimilis FnB, S. dysgalactiae FnBB, adhesin-like proteins of L. monocytogenes and L. innocua, and S. pyogenes FBP, share the same consensus repeats, i.e., the KXW modules. The KXW modules found in these proteins display the same or a similar amino acid array at dispersed positions, but the conformity among them was obvious. From the alignment of the KXW modules shown in Fig. 3, one can predict that these proteins share a common ancestor and/or that the modules have the similar biological function. In this context, it was noteworthy that the KXW modules found in the Cna corresponded to the previously described repeat region, called the B domain, whose biological function has yet to be assigned (12). The number of repeats found in the Cna varies from one to four in different strains of S. aureus (12). This leads to the hypothesis that the number of KXW modules may also vary among many gram-positive bacteria, some of which are shown in Fig. 3.
The two Rsp proteins were proven to bind to biotic surfaces of type I and IV collagens and fibronectin. The N-terminal regions of the Rsp proteins contained the sequences that apparently coincided with those of the collagen-binding domains of S. aureus Cna (39) and E. faecalis Ace (41) (Fig. 4). This suggests that the N-terminal regions of Rsp proteins may constitute the collagen-binding domain. So far, repeat domains represented by the motif, ED(T/S)x9-10GGx3-4(I/V)DF (31), which were described for S. aureus (FnBPA and FnBPB) (22, 51), S. dysgalactiae (FnBA and FnBB) (26), and S. pyogenes (F1, F2, SfbI, SfbII, and PFBP) (17, 21, 24, 42, 56), have been shown to be important in fibronectin-binding properties. Furthermore, the other fibronectin-binding motifs, LAGESGET and HFDNx2P, have been described for Streptococcus equi subsp. zooepidemicus FNZ (28) and S. pyogenes F2 (21), respectively. Although the motifs exemplified above are not present in the Rsp proteins, the KXW modules found in S. pyogenes protein F2 partly overlaps with the fibronectin-binding domain named UFBD (21). This leads to the speculation that the KXW modules, or at least a part of them, participate in the fibronectin-binding properties, although no further experiments have been carried out in the present study.
The Rsp proteins bound to a polystyrene surface and adherence of E. rhusiopathiae to the abiotic surface was inhibited by addition of each Rsp protein. Biofilm formation takes place in two steps: attachment of the bacteria to target surfaces and growth-dependent accumulation of bacteria in multilayered cell clusters (37). The polystyrene assay has been utilized for studying bacterial biofilms, and the adherence to the polystyrene surface in a short time is considered to reproduce the initiation of biofilm formation (15, 37). Although there was no publication on the occurrence of biofilm formation in Erysipelothrix species, E. rhusiopathiae formed surface-attached, sedimentary thick films on surfaces when the bacteria were grown in 1-liter culture in flasks (our unpublished observation). Therefore, our findings substantially indicate that the two Rsp proteins are directly involved in the first step of biofilm formation.
To date, several cell surface proteins involved in the biofilm formation have been described in gram-positive bacteria. In Staphylococcus species, the autolysin (AtlE) (18), accumulation-associated protein (AAP) (20, 45), and the biofilm-associated protein (Bap) (5) were proven to participate in biofilm formation. In E. faecalis, Esp (58), which shows sequence similarity to S. aureus Bap, has been shown to bind to a polystyrene surface, indicating that the protein participate in the first step of biofilm formation. The S. epidermidis AtlE (18), the S. aureus Bap (5), and E. faecalis Esp (58) have been shown to bind to an abiotic surface. Among them, Bap and Esp have been shown to play an important role in the pathogenesis of infection (5, 46, 58). Taken together, the present results imply that, in general, the bacterial surface proteins that bind to abiotic surfaces can also bind to biotic surfaces and play an important role in in vivo colonization. At present we do not know whether the binding of Rsp proteins to both biotic and abiotic surfaces is caused by the same mechanism(s) of ligand interactions and whether the KXW modules are involved in binding to the abiotic surface. Since the Rsp proteins share the KXW modules with fibronectin- and collagen-binding proteins of other gram-positive bacteria, examination of whether these surface proteins also play a role in the first step of biofilm formation might help us to find clues for the questions.
The Rsp proteins contained the C-terminal cell wall-anchoring region with an LPXTG motif, and the bacterial agglutination assay indicated that the two proteins were located on the cell surface. Furthermore, Western blotting analyses showed that Rsp proteins were also present in the culture supernatant. In particular, the RspA protein in the supernatant might have been cleaved by proteolysis. In pathogenic bacteria, proteolysis of surface molecules is sophisticatedly regulated at the cell surface, and the released forms of these surface proteins could promote bacterial colonization in host (1, 40). We have previously found that SpaA.1, a major cell surface and protective antigen of E. rhusiopathiae, was also present in the supernatant (49). The SpaA.1 has repetitive sequences containing the GW modules. Although each GW module of SpaA.1 consists of only 20 aa (29), they are similar to those of the Streptococcus pneumoniae CbpA that binds to cytokine-activated human lung epithelial cells and endothelial cells (43), implying that SpaA.1 may also exhibit adhesive function. Assuming that E. rhusiopathiae expresses such a proteolytic enzyme, the enzyme may release Rsp proteins and SpaA.1 in response to specific signals under a certain condition, and the released forms of the molecules may contribute to the bacterial colonization in vivo.
We showed that rspA and rspB were present in all known serovars of E. rhusiopathiae. This suggests that the two rsp genes are present among most prevailing strains. Furthermore, we have found that at least RspA was expressed in all of the E. rhusiopathiae strains tested (unpublished observations). Together with the fact that the immunization with RspA, but not with RspB, could elicit protection in mice against lethal challenge, the RspA is an attractive candidate for a cross-protection vaccine for all of the serovars. However, at present we cannot explain the difference in protective activity between RspA and RspB, and we do not know which domain of RspA is involved in the protection. Therefore, a clear understanding of the role of the Rsp proteins or KXW modules in protection at molecular level will enable us to design new types of microbial anti-infective therapy by targeting the Rsp proteins.
REFERENCES
- 1.Berge, A., and L. Björck. 1995. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J. Biol. Chem. 270:9862-9867. [DOI] [PubMed] [Google Scholar]
- 2.Braun, L., S. Dramsi, P. Dehoux, H. Bierne, G. Lindahl, and P. Cossart. 1997. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol. 25:285-294. [DOI] [PubMed] [Google Scholar]
- 3.Cossart, P., and R. Jonquieres. 2000. Sortase, a universal target for therapeutic agents against gram-positive bacteria? Proc. Natl. Acad. Sci. USA 97:5013-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 5.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deisenhofer, J. 1981. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-Å resolution. Biochemistry 20:2361-2370. [PubMed] [Google Scholar]
- 7.Donlan, R. M., and J. W. Costerton. 2002. Biofilm: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dramsi, S., C. Kocks, C. Forestier, and P. Cossart. 1993. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature, and the pleiotropic activator prfA. Mol. Microbiol. 9:931-941. [DOI] [PubMed] [Google Scholar]
- 9.Fischetti, V. A., K. F. Jones, B. N. Manjula, and J. R. Scott. 1984. Streptococcal M6 protein expressed in Escherichia coli: localization, purification, and comparison with streptococcal-derived M protein. J. Exp. Med. 59:1083-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischetti, V. A., V. Pancholi, and O. Schneewind. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol. Microbiol. 4:1603-1605. [DOI] [PubMed] [Google Scholar]
- 11.Fischetti, V. A. 2000. Surface proteins on gram-positive bacteria, p. 11-24. In V. A. Fischetti et al. (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 12.Foster, T. J., and M. Höök. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 13.Gaillard, J., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 14.Galán, J. E., and J. F. Timoney. 1990. Cloning and expression in Escherichia coli of a protective antigen of Erysipelothrix rhusiopathiae. Infect. Immun. 58:3116-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 16.Groschup, M. H., K. Cussler, R. Weiss, and J. F. Timoney. 1991. Characterization of a protective protein antigen of Erysipelothrix rhusiopathiae. Epidemiol. Infect. 107:637-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanski, E., and M. Caparon. 1992. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 89:6172-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heilmann, C., M. Hussain, G. Peters, and F. Götz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 19.Hell, W., H. G. Meyer, and S. G. Gatermann. 1998. Cloning of aas, a gene encoding a Staphylococcus saprophyticus surface protein with adhesive and autolytic properties. Mol. Microbiol. 29:871-881. [DOI] [PubMed] [Google Scholar]
- 20.Hussain, M., M. Herrmann, C. von Eiff, F. Perdreau-Remington, and G. Peters. 1997. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect. Immun. 65:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffe, J., S. Natanson-Yaron, M. G. Caparon, and E. Hanski. 1996. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains. Mol. Microbiol. 21:373-384. [DOI] [PubMed] [Google Scholar]
- 22.Jänsson, K., C. Signäs, H. P. Muller, and M. Lindberg. 1991. Two different genes encode fibronectin-binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur. J. Biochem. 202:1041-1048. [DOI] [PubMed] [Google Scholar]
- 23.Kehoe, M. A. 1994. Cell-wall-associated proteins in gram-positive bacteria, p. 217-261. In J.-M. Ghuysen and R. Hakenbeck (ed.), Bacterial cell wall. Elsevier, Amsterdam, The Netherlands.
- 24.Kreikemeyer, B., S. R. Talay, and G. S. Chhatwal. 1995. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol. Microbiol. 17:137-145. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Lindgren, P., H. J. McGavin, C. Signäs, B. Guss, S. Gurusiddappa, M. Höök, and M. Lindberg. 1993. Two different genes coding for fibronectin-binding proteins from Streptococcus dysgalactiae: the complete nucleotide sequences and characterization of the binding domains. Eur. J. Biochem. 214:819-827. [DOI] [PubMed] [Google Scholar]
- 27.Lindgren, P. E., C. Signäs, L. Rantamäki, and M. Lindberg. 1994. A fibronectin-binding protein from Streptococcus equisimilis: characterization of the gene and identification of the binding domain. Vet. Microbiol. 41:235-247. [DOI] [PubMed] [Google Scholar]
- 28.Lindmark, H., K. Jacobsson, L. Frykberg, and B. Guss. 1996. Fibronectin-binding protein of Streptococcus equi subsp. zooepidemicus. Infect. Immun. 64:3993-3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makino, S. i., K. Yamamoto, S. Murakami, T. Shirahata, K. Uemura, T. Sawada, H. Wakamoto, and Y. Morita. 1998. Properties of repeat domain found in a novel protective antigen, SpaA, of Erysipelothrix rhusiopathiae. Microb. Pathog. 25:101-109. [DOI] [PubMed] [Google Scholar]
- 30.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 31.McGavin, M. J., S. Gurusiddappa, P. E. Lindgren, M. Lindberg, G. Raucci, and M. Höök. 1993. Fibronectin receptors from Streptococcus dysgalactiae and Staphylococcus aureus: involvement of conserved residues in ligand binding. J. Biol. Chem. 268:23946-23953. [PubMed] [Google Scholar]
- 32.Milohanic, E., R. Jonquières, P. Cossart, P. Berche, and J. L. Gaillard. 2001. The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol. Microbiol. 39:1212-1224. [DOI] [PubMed] [Google Scholar]
- 33.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ní Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Höök, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 35.Ochman, H., A. S. Gerber, and D. L. Hartl. 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120:621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oshida, T., M. Sugai, H. Komatsuzawa, Y. M. Hong, H. Suginaka, and A. Tomasz. 1995. Staphylococcus aureus autolysin that has an N-acetylmuramoyl-l-alanine amidase domain and an endo-β-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc. Natl. Acad. Sci. USA 92:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 38.Otten, R. A., R. Raeder, D. G. Heath, R. Lottenberg, P. P. Cleary, and M. D. Boyle. 1992. Identification of two type IIa IgG-binding proteins expressed by a single group A streptococcus. J. Immunol. 148:3174-3182. [PubMed] [Google Scholar]
- 39.Patti, J. M., H. Jonsson, B. Guss, L. M. Switalski, K. Wiberg, M. Lindberg, and M. Höök. 1992. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J. Biol. Chem. 267:4766-4772. [PubMed] [Google Scholar]
- 40.Rasmussen, M., and L. Björck. 2002. Proteolysis and its regulation at the surface of Streptococcus pyogenes. Mol. Microbiol. 43:537-544. [DOI] [PubMed] [Google Scholar]
- 41.Rich, R. L., B. Kreikemeyer, T. R. Owens, S. LaBrenz, S. V. Narayana, G. M. Weinstock, B. E. Murray, and M. Höök. 1999. Ace is a collagen-binding MSCRAMM from Enterococcus faecalis. J. Biol. Chem. 274:26939-26945. [DOI] [PubMed] [Google Scholar]
- 42.Rocha, C. L., and V. A. Fischetti. 1999. Identification and characterization of a novel fibronectin-binding protein on the surface of group A streptococci. Infect. Immun. 67:2720-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Schumacher-Perdreau, F., C. Heilmann, G. Peters, F. Gotz, and G. Pulverer. 1994. Comparative analysis of a biofilm-forming Staphylococcus epidermidis strain and its adhesion-positive, accumulation-negative mutant M7. FEMS Microbiol. Lett. 117:71-78. [DOI] [PubMed] [Google Scholar]
- 46.Shankar, V., A. S. Baghdayan, M. M. Huycke, G. Lindahl, and M. S. Gilmore. 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 1999:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimoji, Y., Y. Yokomizo, T. Sekizaki, Y. Mori, and M. Kubo. 1994. Presence of a capsule in Erysipelothrix rhusiopathiae and its relationship to virulence for mice. Infect. Immun. 62:2806-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimoji, Y., Y. Yokomizo, and M. Mori. 1996. Intracellular survival and replication of Erysipelothrix rhusiopathiae within murine macrophages: failure of induction of the oxidative burst of macrophages. Infect. Immun. 64:1789-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimoji, Y., Y. Mori, and V. A. Fischetti. 1999. Immunological characterization of a protective antigen of Erysipelothrix rhusiopathiae: identification of the region responsible for protective immunity. Infect. Immun. 67:1646-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimoji, Y. 2000. Pathogenicity of Erysipelothrix rhusiopathiae: virulence factors and protective immunity. Microbes Infect. 2:965-972. [DOI] [PubMed] [Google Scholar]
- 51.Signäs, C., G. Raucci, K. Jönsson, P. E. Lindgren, G. M. Anantharamaiah, M. Höök, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sillanpaa, J., B. Martinez, J. Antikainen, T. Toba, N. Kalkkinen, S. Tankka, K. Lounatmaa, J. Keranen, M. Hook, B. Westerlund-Wikstrom, P. H. Pouwels, and T. K. Korhonen. 2000. Characterization of the collagen-binding S-layer protein CbsA of Lactobacillus crispatus. J. Bacteriol. 182:6440-6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smoot, J. C., K. D. Barbian, J. J. Van Gompel, L. M. Smoot, M. S. Chaussee, G. L. Sylva, D. E. Sturdevant, S. M. Ricklefs, S. F. Porcella, L. D. Parkins, S. B. Beres, D. S. Campbell, T. M. Smith, Q. Zhang, V. Kapur, J. A. Daly, L. G. Veasy, and J. M. Musser. 2002. Genome sequence and comparative microarray analysis of serotype M18 group A streptococcus strains associated with acute rheumatic fever outbreaks. Proc. Natl. Acad. Sci. USA 99:4668-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Symersky, J., J. M. Patti, M. Carson, K. House-Pompeo, M. Teale, D. Moore, L. Jin, A. Schneider, L. J. DeLucas, M. Höök, and S. V. Narayana. 1997. Structure of the collagen-binding domain from a Staphylococcus aureus adhesin. Nat. Struct. Biol. 4:833-838. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi, T., T. Fujisawa, Y. Tamura, S. Suzuki, M. Muramatsu, T. Sawada, Y. Benno, and T. Mitsuoka. 1992. DNA relatedness among Erysipelothrix rhusiopathiae strains representing all twenty-three serovars and Erysipelothrix tonsillarum. Int. J. Syst. Bacteriol. 42:469-473. [DOI] [PubMed] [Google Scholar]
- 56.Talay, S. R., P. Valentin-Weigand, P. G. Jerlstrom, K. N. Timmis, and G. S. Chhatwal. 1992. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect. Immun. 60:3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terao, Y., S. K., E. Kunitomo, J. Murakami, I. Nakagawa, and S. Hamada. 2001. Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol. Microbiol. 42:75-86. [DOI] [PubMed] [Google Scholar]
- 58.Toledo-Arana, A., J. Valle, C. Solano, M. J. Arrizubieta, C. Cucarella, M. Lamata, B. Amorena, J. Leiva, J. R. Penadés, and I. Lasa. 2001. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 67:4538-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uhlen, M., B. Guss, B. Nilsson, S. Gatenbeck, L. Philipson, and M. Lindberg. 1984. Complete sequence of the staphylococcal gene encoding protein A: a gene evolved through multiple duplications. J. Biol. Chem. 259:1695-1702. [PubMed] [Google Scholar]
- 60.Wood, R. L. 1999. Erysipelas, p. 419-430. In A. D. Leman et al. (ed.), Diseases of swine. Iowa State University Press, Ames, Iowa.
- 61.Wren, B. W. 1991. A family of clostridial and streptococcal ligand-binding proteins with conserved C-terminal repeat sequences. Mol. Microbiol. 5:797-803. [DOI] [PubMed] [Google Scholar]