Abstract
The MAGE gene family is characterized by a conserved domain (MAGE Homology Domain). A subset of highly homologous MAGE genes (group A; MAGE-A) belong to the chromosome X-clustered cancer/testis antigens. MAGE-A genes are normally expressed in the human germ line and overexpressed in various tumor types; however, their biological function is largely unknown. Here we present evidence indicating that MageA2 protein, belonging to the MAGE-A subfamily, confers wild-type-p53-sensitive resistance to etoposide (ET) by inducing a novel p53 inhibitory loop involving recruitment of histone deacetylase 3 (HDAC3) to MageA2/p53 complex, thus strongly down-regulating p53 transactivation function. In fact, enhanced MageA2 protein levels, in addition to ET resistance, correlate with impaired acetylation of both p53 and histones surrounding p53-binding sites. Association between MAGE-A expression levels and resistance to ET treatment is clearly shown in short-term cell lines obtained from melanoma biopsies harboring wild-type-p53, whereas cells naturally, or siRNA-mediated expressing low MAGE-A levels, correlate with enhanced p53-dependent sensitivity to ET. In addition, combined trichostatin A/ET treatment in melanoma cells expressing high MAGE-A levels reestablishes p53 response and reverts the chemoresistance.
p53 tumor-suppressor is a key transcription factor that controls cell proliferation, inducing growth arrest or apoptosis in response to different cellular stresses (1). Although inactivating mutations of p53 confer growth advantage and drug resistance in certain human cancers, mutated p53 is not frequently observed in other tumors such as bone, testis, or skin (melanoma) origin (2, 3). As an alternative to p53 mutation, a range of proteins have been described to target wild-type p53 (wt-p53) protein function through diverse mechanisms (4, 5). The most significant p53-targeting proteins have been essentially ascribed to those deregulated/overexpressed in tumor cells, thus providing mechanistic insights about how p53-governed pathways could misbehave.
One of the first isolated tumor-specific antigens was the melanoma antigen 1 (MageA1) (6). Since then, the MAGE gene family has extensively increased in complexity with the conservation of the typical signature domain (Mage Homology Domain; MHD). The MAGE family has been divided in two big subfamilies: MAGE-I and -II. The MAGE-I family consists of a large number of chromosome X-clustered genes (Mage A, B, and C groups). Most of them are relevant cancer/testis antigens (7) and therefore are rarely expressed in normal adult tissues except for testis and various human tumors (8, 9). MAGE-II family is not related to cancer, crowding together different proteins containing MHD with undefined chromosome clustering. In this work we focus on group A members of the MAGE-I subfamily, referred to hereafter as MAGE-A.
Emerging data suggest potential involvement of MAGE family proteins in modulating cell survival. Two MAGE-II members, Necdin and hNRAGE, oppositely modulate p53 functions (10, 11). In addition, several MAGE-A genes are activated very early in lung carcinogenesis (12), and some are overexpressed in ovarian cancer cell lines resistant to paclitaxel and doxorubicin (13). MAGE-A members such as MageA1, -A4, and -A11 have been involved in transcription regulation through specific binding to transcription complexes (14–16). It was recently reported that BORIS, a cancer/testis antigen discovered as a novel CTCF paralogous, is able to transcriptionally induce several MAGE-A genes (17) and potentially promote cell proliferation and transformation (18).
Here we report that MageA2 interacts and represses p53 activity by recruiting transcription repressors [histone deacetylases (HDACs)] to p53 transcription sites. The correlation between MAGE-A expression and resistance to apoptosis has been validated in short-term melanoma cell lines, where combined trichostatin A (TSA) and etoposide (ET) treatment restores the p53 response and reverts the chemoresistance of melanoma cells expressing high levels of MAGE-A.
Results
MAGE-A Proteins Repress p53 Function.
We first observed that MAGE-A proteins such as MageA1, -A2, or -A6 were able to repress p53 transactivation function by using a specific synthetic promoter (pG13-LUC) as reporter (Fig. 1A). Similarly, by using MageA2 as a representative MAGE-A gene and a panel of p53-responsive promoters, we verified its efficacy in down-regulating p53 activity (Fig. 1B). However, no difference in p53 protein levels was detected, as evaluated in the same lysates used for gene reporter (data not shown). We then determined the effect of MageA2 expression on endogenous p53-target genes. We established a HA–MageA2-inducible U2OS cell line (M20) under the control of ponasterone A (PonA). Although p53 accumulation after ET treatment was comparable in cells expressing or not expressing HA–MageA2, the endogenous levels of p53-targets, p21Waf-1 or Bax, were severely impaired in HA–MageA2-overexpressing cells (Fig. 1C). p21Waf-1 mRNA levels followed a similar kinetic (see Fig. 6 A and B, which is published as supporting information on the PNAS web site). Conversely, siRNA-mediated knockdown of endogenous MAGE expression in U2OS cells (MAGE-A transcripts detected by RT-PCR in U2OS cells are MageA1, -A2, -A3, -A4, and -A6; data not shown) resulted in a significant earlier accumulation of p53-target proteins after DNA damage (Fig. 1D). MageA2 expression correlated with resistance to ET treatment as assessed in M20 cells overexpressing MageA2 (Fig. 1E) and in U2OS cells transfected with specific siRNA for knocking down MAGE-A levels (Fig. 1F). No significant protection to ET treatment was seen in p53-null Saos-2 cells by MageA2 expression, which, however, efficiently inhibited cell death induced by p53 overexpression (Fig. 6C). These data support the notion that after DNA-damage the p53-dependent response is impaired in cells overexpressing MageA2.
Fig. 1.
MageA2 expression represses p53 function. (A) H1299 cells (p53-null) transfected with p53 or in combination with MageA1, -A2, and -A6 together with the p53-responsive promoter pG13LUC. Value corresponding to p53 transfection was reported to 100. (B) p53-specific reporter gene assay as in A using p53 and MageA2 with different p53-responsive promoters. (C) Western blot of M20 cells (HA–MageA2-inducible U2OS cells) expressing (+PonA) or not (No PonA) HA–MageA2. p53, Bax, and p21 protein levels were determined at the indicated time points after treatment with 10 μM ET. (D) Determination of p53, Bax, and p21 protein levels after ET treatment in U2OS cells transfected with control siRNA (siCont) or Mage siRNA (siMage). (E) Apoptosis determination by Annexin V assay coupled to FACS analysis (10,000 counted cells for at least three independent experiments). M20 cells expressing (+PonA) or not (No PonA) HA-MageA2 were treated with 20 μM ET for 36 h. (F) Similar experiment as in E, but performed in U2OS cells previously silenced with siRNA as indicated.
MageA2 Interacts with p53.
Interaction between endogenous p53 and MAGE-A proteins was detected in U2OS cells (Fig. 2A). A set of different p53 deletion mutants indicated that formation of MageA2/p53 complex in vivo required the p53-DNA-binding domain (p53DBD; amino acids 94–298), whereas the p53 transactivation domain (amino acids 1–98) and the p53-C-terminal domain (p53CTD; 298–393) were dispensable (Fig. 2 B and C). In vitro pulldown experiments showed that recombinant GST-p53 associated with in vitro translated (IVT) 35S-labeled MageA2 (Fig. 2D) and that GST–MageA2 bound specifically to the p53DBD (94–298) but not to p53CTD (298–393) or the N-terminal domain (p53NTD; 1–98) (Fig. 2E), suggesting a direct interaction between these proteins. In addition to p53, MageA2 associated and repressed p73 activity, whereas neither association nor repression was observed with p63 (Fig. 6 D and E), thus suggesting specificity of MageA2 for transcription factors in the p53 family and a direct link between protein–protein interaction and repression.
Fig. 2.
Interaction between MageA2 and p53. (A) Immunoprecipitation (IP) of endogenous p53/Mage protein complex in U2OS cells using anti-p53 polyclonal Ab or preimmune IgG. (B) IP in H1299 cells transfected with HA-MageA2 and the indicated p53 deletions. (C) IP assay similar to that in B but using p53 deletions expressing HA-tagged 94–298 (HA-p53DBD) and HA-p53 298–393 (HA-p53CTD). (D) In vitro binding assay using recombinant/purified GST and GST–p53 fusion protein incubated with IVT 35S-labeled HA–MageA2 (IVT MageA2). (E) In vitro binding assay using recombinant/purified GST and GST–MageA2 fusion protein incubated with IVT 35S-labeled domains of p53:p53NTD (1–98), p53DBD (94–298), and p53CTD (298–393) as indicated. ∗, Ig heavy chain; ∗∗, Ig light chain.
MageA2 Assembles HDAC3/p53 Protein Complex.
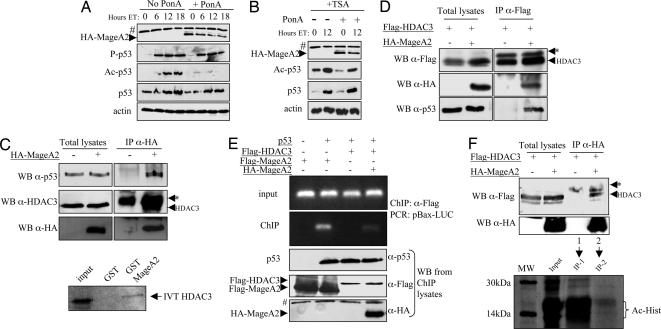
We analyzed the profile of p53 modifications in MageA2-inducible M20 cells after treatment with DNA-damaging agents. ET treatment caused rapid p53 phosphorylation (p53Ser-15) independent of MageA2 induction (compare No PonA vs. +PonA), whereas the p300-acetylation target, p53-Lys-382, was severely impaired in M20 cells overexpressing MageA2 (Fig. 3A). The use of HDAC inhibitor TSA counteracted the effect of MageA2 expression on p53 acetylation (Fig. 3B), suggesting that a balance between histone acetyltransferases (HATs)/HDACs activities could be affected in cells overexpressing MageA2. We therefore decided to investigate whether MageA2 could associate with HDACs, because the ability of MageA1 to recruit HDAC1 had been reported (14). Regulation of MageA2 expression as seen in M20 cells treated with PonA and MAGE-A knocked-down U2OS cells, correlated with similar changes in HDAC3, but not HDAC1, protein levels (data not shown). Therefore, we focused our studies on the relationship between MageA2 and HDAC3. HA–MageA2 was immunoprecipitated from transiently transfected 293T cells (which contain very low levels of MAGE-A proteins) where endogenous HDAC3 was found to form a complex with HA–MageA2. As expected, endogenous p53 coimmunoprecipitated with MageA2 (Fig. 3C Upper). Using recombinant GST-MageA2 and 35S-IVT HDAC3, a weak but reproducible interaction between MageA2 and HDAC3 was detected (Fig. 3C Lower). The possibility that MageA2 could recruit HDACs to p53 then was confirmed when we saw that Flag–HDAC3 associated with endogenous p53 preferentially when MageA2 was expressed (Fig. 3D). Moreover, by analyzing the ability of p53, MageA2, and HDAC3 to bind the Bax promoter in cotransfection/ChIP assay (see Fig. 7A, which is published as supporting information on the PNAS web site), we found that MageA2 was able to bind the Bax promoter only when p53 was transfected and that HDAC3 required p53/MageA2 complex to associate with this promoter (Fig. 3E). Importantly, HA–MageA2/Flag–HDAC3 complex immunoprecipitated from H1299 cells revealed strong deacetylase activity in vitro (Fig. 3F), indicating that MageA2 could assemble active HDAC3 to p53 complex as part of its repressive function. MageA1 and -A2 share almost 80% of protein identity, and as expected both can associate in vivo to HDAC1 (ref. 14; data not shown). Likewise, they are able to repress p53 activation as shown in Fig. 1A, suggesting that more than one MAGE-A member could inhibit p53 activity by recruiting class-I HDAC (HDAC1, -2, -3, and -8).
Fig. 3.
MageA2 recruits HDAC3 to p53. (A) Time-course experiment in M20 cells after addition of 10 μM ET. PonA was added 15 h before ET treatment. DO1 Ab was used for total p53, whereas anti-P-p53Ser15 (P-p53) and anti-Ac-p53Lys382 (Ac-p53) were used for specific phosphorylation and acetylation. (B) Similar to A but using 300 nM TSA plus ET treatment on M20 cells expressing or not expressing HA–MageA2. (C Upper) IP of transiently transfected 293T cells with HA–MageA2. (C Lower) In vitro binding assay using recombinant/purified GST and GST–MageA2 fusion protein incubated with IVT 35S-labeled HDAC3. (D) IP of transiently transfected 293T cells with HA–MageA2 and Flag–HDAC3 expression vectors. (E Upper) ChIP performed in H1299 cells transfected with pBax-Luc, p53, and Flag-tagged construct as indicated. Samples were immunoprecipitated (ChIP) or not (input) by using anti-Flag Ab, followed by pBax–Luc PCR amplification (see diagram in Fig. 7A). (E Lower) Control of the indicated transfections by Western blot. (F Upper) IP of transiently transfected H1299 cells with HA–MageA2 and Flag–HDAC3. Lanes 1 and 2 show the absence and presence of MageA2/HDAC3 complex, respectively. (F Lower) In vitro deacetylation assay of 14C-acetylated histones (Ac-Hist) by immunoprecipitated complex shown in Lanes 1 (IP-1) and 2 (IP-2) of Upper. Input indicates mock-treated 14C-acetylated histones. MW, 14C-labeled protein molecular mass; #, unspecific band; ∗, Ig heavy chain.
MageA2/HDAC3 Complex Can Associate with p53 on its DNA-Binding Sites, Inducing Histone Hypoacetylation in Melanoma Cells.
Overexpression of MAGE-A genes was originally found in melanoma, a human tumor highly refractory to chemotherapy despite harboring wt-p53 (3, 19). Therefore, we analyzed the p53-dependent response in two human short-term cell lines obtained from melanoma biopsies expressing different MAGE-A levels and harboring wt-p53 (20). MAGE-A levels were determined through gene-specific RT-PCR (21). 15392M and 13923M cells express very low and high levels of MAGE-A, respectively (Fig. 7B). Upon ET treatment, the p53 response as well as p53 acetylation were severely impaired in 13923M cells expressing high MAGE-A levels compared with 15392M cells expressing low MAGE-A levels (Fig. 4 A and B). Importantly, both acetylation as well as the p53 response to ET were restored, as determined by both p53 acetylation and p21/Bax protein levels, when TSA was used with ET (Fig. 4C).
Fig. 4.
MageA2/HDAC complex deacetylates p53 and histones surrounding p53-binding sites. (A) DNA-damage response in melanoma cells expressing low (15392M) or high (13923M) MAGE-A levels, after addition of 20 μM ET. (B) p53 modifications in 15392M and 13923M melanoma cells after ET damage (20 μM). (C) Similar to B but using 300 nM TSA plus ET (20 μM) treatment. (D) ChIP assay followed by quantitative PCR using real-time PCR. Protein/DNA complexes were immunoprecipitated from 15392M and 13923M melanoma cells before and after ET treatment using the indicated Abs. AcH3, acetylated histone H3 Ab. Data are expressed as the ratio between damaged/undamaged ChIP values, indicating the enrichment of each protein to the indicated promoter after damage.
p53/MageA2/HDAC3 protein complex can be found assembled at p53-binding sites (Fig. 3E), suggesting that the inhibitory effect of such complex could be extended to other acetylation-dependent substrates critically involved in transcription regulation such as nucleosome histones. In fact, MageA2 was able to down-regulate the transcriptional activity of the nonacetylable p53 mutant, p539KR (22), both alone or when cotransfected with the histone acetyl transferase p300 (Fig. 7 C and D), strongly suggesting that histone hypoacetylation could represent the main mechanism used by MageA2 for targeting p53 activity.
Using 15392M and 13923M melanoma cells as models, we analyzed both endogenous protein/chromatin complexes as well as chromatin acetylation status by using a quantitative ChIP approach before and after DNA damage. As shown in Fig. 4D, p53 was similarly recruited to p21 or Bax promoter after ET treatment independently of MAGE levels, as evidenced by the ratio between damaged/undamaged ChIP value, thus confirming that MAGE-A expression does not affect the DNA-binding ability of p53. However, significant HDAC3 recruitment to p21 and Bax promoters after damage was clearly related to MAGE expression. On the contrary, histone H3 acetylation level increased more efficiently after damage in cells lacking MAGE when compared with cells expressing high MAGE levels. Addition of TSA caused a globally strong histone acetylation status in both cell strains, resulting in no difference (ratio next to 1) with respect to ET addition. Similar data were obtained when the MageA2-inducible M20 cell line was tested (Fig. 7E). All together our data support the notion that MAGE-A proteins could recruit HDACs to p53, causing hypoacetylation of histones surrounding p53-binding sites and of p53 protein itself, thus weakening the p53-dependent transactivation function.
Melanoma Cells Expressing MAGE-A Genes Are Refractory to DNA Damage-Induced Apoptosis.
ET treatment of 15392M and 13923M melanoma cells showed a dose-dependent induction of cell death in cell expressing low MAGE-A levels, whereas cells expressing high MAGE-A levels remained almost insensitive to such treatment. Importantly, addition of TSA (able to efficiently stimulate p53 function in cell expressing high levels of MAGE-A; see Fig. 4C) significantly sensitized 13923M cells to ET-induced apoptosis (Fig. 5A). Similar effects were observed in MageA2-inducible M20 cell line (see Fig. 8A, which is published as supporting information on the PNAS web site). Melanoma cell lines harboring mutant p53 (23) were found to be strongly resistant to ET/TSA treatment independently of MAGE-A expression as assessed in SK-Mel-28 (high MAGE-A expression) and BE cells (low MAGE-A expression) (Fig. 5B; MAGE-A and p53 levels for both lines are shown in Fig. 8B), supporting the hypothesis that MAGE-A proteins could exert their function by inhibiting wt-p53 function.
Fig. 5.
MAGE-A expression correlates with resistance to DNA-damage-induced apoptosis. (A) Apoptosis determination by subG1 by FACS in 15392M and 13923M cells. wt-p53 melanoma cells were treated with the indicated concentration of ET for 48 h. Where indicated, 300 nM TSA was added with ET. (B) Similar to A but using melanoma cell lines harboring mutant p53. (C Left) Apoptosis determination by subG1/FACS analysis in 13923M cells cotransduced with siRNA vector, pSR (empty), or pSRM (Mage knockdown), and pBabe (empty) or pBabe-EGFP-TNVp53OD (p53 inactivation) as shown. Cells were treated with 40 μM ET for the indicated time. (C Right) Determination of MAGE-A protein levels and expression of GFP-p53OD in 13923M-pSR and 13923M-pSRM cells using anti-MAGE and anti-GFP polyclonal Ab, respectively. (D) Short-term cell lines obtained from melanoma biopsies harboring wt-p53 were grouped depending on expression level of MAGE-A (see Fig. 7B). Melanoma cells expressing no or low MAGE-A levels (gray lines) and high MAGE-A levels (black lines) were treated with doses of ET as indicated. Cell viability was calculated by MTT assay 72 h after treatment. The experiment was repeated two times with similar results.
The direct relevance of MAGE-A levels and wt-p53 activity in resistance to DNA-damaging agents then was analyzed in 13923M cells. MAGE-A levels were regulated by pSR retrovirus vector transducing siRNA designed for targeting a highly conserved sequence of several MAGE-A genes (pSRMage). p53 activity was controlled by using pBabe-EGFP-TNVp53OD (24) retroviral vector (p53OD) encoding a p53 oligomerization domain peptide (amino acids 322–355) able to repress p53 function (Fig. 8C). 13923M cells were coinfected with pSR or pSRMage (Puro) and pBabe or pBabep53OD (Hygro) as indicated. Puromycin/hygromycin-resistant cells were selected. Significant increase in susceptibility to ET was observed in 13923M–pSRMage cells compared with control 13923M–pSR cells. This effect was clearly reduced when p53 activity was inactivated through p53OD (Fig. 5C), indicating that down-regulation of MAGE-A genes triggers p53-dependent cell death in ET-treated cells.
Finally, we compared survival properties of three human short-term cell lines obtained from melanoma biopsies expressing low MAGE-A levels and three similar strains expressing high MAGE-A levels, including 15392M and 13923M cells as reference (Fig. 7B). As indicated, all melanoma cells considered harbor wt-p53, as shown by their p53 genotype analysis (20). Melanoma cell strains were treated with different doses of ET, and survival was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay coupled to a multiwell scanning spectrophotometer. Data in Fig. 5D show a clear increased survival in cells expressing high MAGE-A levels when compared with cells expressing no/low MAGE-A levels, confirming that MAGE-A expression confers resistance to DNA damage-induced apoptosis in wt-p53 melanoma cells.
Discussion
p53 is an important tumor suppressor, playing a key role in maintaining genomic stability and controlling cell transformation. Thus, it is a common target for several viral oncoproteins such as HPV-E6, adenovirus E1B, and SV40 large T (25, 26). Besides the high mutation/inactivation rate of p53 as found in human cancers, p53 knockout mice are susceptible to various enhanced spontaneous and carcinogen-induced tumors (27).
p53 function has been shown to be tightly regulated through many different mechanisms dependent on specific modifications (28), subcellular localization (29–31), or the cell-cycle phase (32). Emerging data have highlighted the important role of HDACs in controlling p53 activity whereby the specificity of HDACs in regulating p53 transcription usually requires the assembling of protein complexes. For example, Mdm2, the major p53 regulator, as well as the oncogenic form of PML, PML-RAR, and the metastasis-associated MTA2/PID, have been shown to recruit HDACs to exert their negative control on p53 function (33–35). Here, we have provided evidence that MageA2/HDAC3 is a p53-repressing complex, where the tumor antigen MageA2 acts as p53–HDAC3 assembling protein, giving survival advantage to cells treated with DNA-damaging agents.
The effectiveness of deacetylation-mediated repression complex is based on the well known ability of HDACs to simultaneously affect both the specific transcription factor and the surrounding chromatin (36). p53/chromatin deacetylation has been shown to effectively repress p53 function. Our data suggest that deacetylation of histones surrounding p53-binding sites should represent the main mechanism by which MageA2 represses p53. Still, we cannot discard a contribution of endogenous p53 deacetylation because no cellular systems are available to evaluate the effects of MageA2 on endogenous unacetylable p53 mutant.
Little is known about the function of MAGE-A proteins. Presumably, the most conserved MAGE-A genes could share some related functions. Early activation of MAGE-A genes in cancer could be due to genomewide DNA hypomethylation, which is a frequently observed epigenetic event during carcinogenesis (37) and directly associated with induction of tumors in mice (38). Remarkably, it was recently reported that BORIS protein is able to induce epigenetic reprogramming (39), behaving as a potent activator for the expression of several MAGE-A genes (17). In this context, deregulated BORIS (found in numerous human cancers) could induce expression of MageA1, -A2, and/or -A6, possibly contributing to suppression of the p53 response. Correlation between MageA3/6 and MageC1 expression and the plasma–cell proliferating compartment of multiple myeloma was recently reported (40), pointing to a potential function of MAGE-A products in cell-cycle control of the clonogenic stem cell compartment.
Our work has focused on the mechanistic relationship among MageA2, p53, and HDAC3. However, the scenario on how the expression of MAGE-A proteins confer resistance to p53-dependent apoptosis should become more complex when the respective protein family members are considered. First, as we showed here, different MAGE-A proteins can repress both p53 and p73 function. Second, more than one class-I HDAC could be recruited by MAGE-A proteins (14) to p53-binding sites. Finally, most cancer cells coexpress several MAGE-A genes, thus increasing their ability to specifically regulate the p53 family members mainly involved in the DNA-damage response (i.e., p53 and p73). HDAC inhibitors therefore should be useful to counteract effects of massive MAGE-A expression at least in treatment of human melanoma.
Materials and Methods
Cells and Reagents.
H1299 (p53-null), U2OS (wt-p53), Saos-2 (p53-null), and 293T cell lines were cultured as described (17). M20 are HA–MageA2-inducible U2OS cells using the Ecdysone-Inducible Expression System (Invitrogen). M20 cells were induced by adding 5 μM PonA (Invitrogen). Status of p53 in short-term melanoma cell lines has been described (20). RT-PCR for specific MAGE-A transcripts detection was done as described (21). ET and TSA were from Sigma.
Plasmids, siRNA, and Transfections.
MageA1, -A2, and -A6 and p53 constructs expressing the p53NTD (1–98), p53DBD (94–298), and p53CTD (amino acids 298–393) were HA-tagged and cloned in pcDNA3 (Invitrogen). p53 His-94–393 and His-MageA2 were cloned in pcDNA3.1 (Invitrogen). Flag–HDAC3 was from C. Brancolini (University of Udine). For MAGE-A silencing, we used a pool of four siRNA, based on a highly conserved MageA2 sequence (SMARTpool; Dharmacon Research). This reagent was able to knock down all tested MAGE-A genes (MageA1, -A2, -A3, and -A6; data not shown). Transfection of siRNA was performed as described (41). Retroviral infection was performed through standard protocol by using pSuperRetro (pSR) vector (OligoEngine) encoding a sequence (5′-AACCAGCTATGTGAAAGTC-3′) (pSRMage) with 100% matching to several MAGE-A genes (A1, A2, A3, A4, A6, A7, A8, and A12) followed by puromycin selection. p53 activity was controlled by using pBabe-EGFP-TNVp53OD (24) followed by hygromycin selection.
Western Blot and Abs.
Western blot analysis was performed according to standard procedures by using the following primary Abs for p53: DO1, anti-p53 monoclonal (Santa Cruz Biotechnology), anti-acetyl p53 (Lys-382), and anti-phospho p53 (Ser-15) (16G8; Cell Signaling Technology). For MAGE-A detection, affinity-purified anti-MageA6 raised against GST-MageA6, anti-MAGE polyclonal Ab (Santa Cruz Biotechnology), or anti-Mage 57B monoclonal Ab (mAb) (a gift from G. Spagnoli, University of Basel, Basel, Switzerland) was used. Because of high homology between MAGE-A members, Mage polyclonal Abs are broadly reactive with almost all MAGE-A proteins. 57B anti-Mage monoclonal Abs do not recognize MageA1 but cross-react with many other MAGE-A proteins. Other polyclonal Abs were as follows: anti-actin (Sigma), anti-Bax (Cell Signaling Technology), anti-p21Waf1 (Santa Cruz Biotechnology), and anti-HDAC3 (Cell Signaling Technology). For tags, anti-HA 12CA5 mAb (Roche) and anti-Flag M2 mAb (Sigma) were used.
Determination of Luciferase and Cell Viability.
Luciferase activity was determined with the Dual Luciferase kit (Promega). Apoptosis was determined by subG1 or Annexin V staining (Annexin V-FITC; Sigma) as indicated using a FACS (FACSCalibur; Becton Dickinson). Cell viability was evaluated by MTT using a multiwell scanning spectrophotometer. (For details on these and other methods, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site).
Pulldown, IP, and Acetylation.
For in vitro binding assay, 35S-labeled proteins were IVT by using reticulocyte lysates (TNT system; Amersham Pharmacia) and incubated with purified recombinant GST-fused proteins immobilized on glutathione Sepharose 4B beads (Amersham Pharmacia). IP was performed as described (32). Histones (Sigma) were acetyleted by using recombinant p300 HAT domain and 14C Acetyl CoA.
ChIP.
Cells were crosslinked in 1% formaldehyde and harvested in RIPA-100 buffer (20 mM Tris·HCl, pH 7.5/100 mM NaCl/1 mM EDTA/0.5% Nonidet P-40/0.5% deoxycholate/0.1% SDS). Sonicated chromatin (0.5 Kb on average) (Bioruptor; Diagenode) was centrifuged. IP was performed overnight at 4°C. Abs used were as follows: for p53, PAb 240 and PAb 421 mAbs; for HDAC3, anti-HDAC3 (Cell Signaling Technology); for Mage, anti-Mage polyclonal Ab, anti-MAGE mAb (6C1), and anti-Mage 57B mAb; and for Ac-Histone H3, anti-acetylated Histone H3 polyclonal Ab (Upstate Biotechnology). DNA–protein complexes were recovered by 2-h incubation with protein A/G PLUS-Agarose (Santa Cruz Biotechnology). After DNA purification, quantitative PCR was performed on ABI PRISM 7000 PCR, by using TaqMan Universal PCR Master Mix (Applied Biosystems). Primers and probes were from Applied Biosystems, and amplification settings were as described (42).
Supplementary Material
Acknowledgments
We thank Gianni Del Sal and his group; Marina Lusic, Serena Zacchigna, and Marco Bestagno (International Center for Genetic Engineering and Biotechnology); and Leonard Girnita (Cancer Center Karolinska, Stockholm, Sweden). This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro, Ministero dell’Istruzione dell’Università e della Ricerca Grant MIUR-MM05187239_004, Consiglio Nazionale delle Ricerche-Genomica Funzionale SP4, and the Translational and Functional Onco-Genomics European Commission Sixth Framework Program (to C.S.).
Abbreviations
- ET
etoposide
- HDAC
histone deacetylase
- IP
immunoprecipitation
- IVT
in vitro translated
- PonA
ponasterone A
- TSA
trichostatin A
- wt
wild-type
- p53DBD
p53-DNA-binding domain
- p53CTD
p53-C-terminal domain
- p53NTD
p53-N-terminal domain
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Harris S. L., Levine A. J. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 2.Olivier M., Eeles R., Hollstein M., Khan M. A., Harris C. C., Hainaut P. Hum. Mutat. 2002;19:607–614. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 3.Albino A. P., Vidal M. J., McNutt N. S., Shea C. R., Prieto V. G., Nanus D. M., Palmer J. M., Hayward N. K. Melanoma Res. 1994;4:35–45. doi: 10.1097/00008390-199402000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Thomas M. C., Chiang C.-M. Mol. Cell. 2005;17:251–264. doi: 10.1016/j.molcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Michael D., Oren M. Curr. Opin. Genet. Dev. 2002;12:53–59. doi: 10.1016/s0959-437x(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 6.van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 7.Simpson A. J., Caballero O. L., Jungbluth A., Chen Y. T., Old L. J. Nat. Rev. Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 8.Ohman Forslund K., Nordqvist K. Exp. Cell Res. 2001;265:185–194. doi: 10.1006/excr.2001.5173. [DOI] [PubMed] [Google Scholar]
- 9.Chomez P., De Backer O., Bertrand M., De Plaen E., Boon T., Lucas S. Cancer Res. 2001;61:5544–5551. [PubMed] [Google Scholar]
- 10.Taniura H., Matsumoto K., Yoshikawa K. J. Biol. Chem. 1999;274:16242–16248. doi: 10.1074/jbc.274.23.16242. [DOI] [PubMed] [Google Scholar]
- 11.Wen C. J., Xue B., Qin W. X., Yu M., Zhang M. Y., Zhao D. H., Gao X., Gu J. R., Li C. J. FEBS Lett. 2004;564:171–176. doi: 10.1016/S0014-5793(04)00353-9. [DOI] [PubMed] [Google Scholar]
- 12.Jang S. J., Soria J. C., Wang L., Hassan K. A., Morice R. C., Walsh G. L., Hong W. K., Mao L. Cancer Res. 2001;61:7959–7963. [PubMed] [Google Scholar]
- 13.Duan Z., Duan Y., Lamendola D. E., Yusuf R. Z., Naeem R., Penson R. T., Seiden M. V. Clin. Cancer Res. 2003;9:2778–2785. [PubMed] [Google Scholar]
- 14.Laduron S., Deplus R., Zhou S., Kholmanskikh O., Godelaine D., De Smet C., Hayward S. D., Fuks F., Boon T., De Plaen E. Nucleic Acids Res. 2004;32:4340–4350. doi: 10.1093/nar/gkh735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai T., Itoh K., Higashitsuji H., Nagao T., Nonoguchi K., Chiba T., Fujita J. J. Biol. Chem. 2004;279:15505–15514. doi: 10.1074/jbc.M310437200. [DOI] [PubMed] [Google Scholar]
- 16.Bai S., He B., Wilson E. M. Mol. Cell. Biol. 2005;25:1238–1257. doi: 10.1128/MCB.25.4.1238-1257.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vatolin S., Abdullaev Z., Pack S. D., Flanagan P. T., Custer M., Loukinov D. I., Pugacheva E., Hong J. A., Morse H., III, Schrump D. S., et al. Cancer Res. 2005;65:7751–7762. doi: 10.1158/0008-5472.CAN-05-0858. [DOI] [PubMed] [Google Scholar]
- 18.Klenova E. M., Morse H. C., III, Ohlsson R., Lobanenkov V. V. Semin. Cancer Biol. 2002;12:399–414. doi: 10.1016/s1044-579x(02)00060-3. [DOI] [PubMed] [Google Scholar]
- 19.Satyamoorthy K., Chehab N. H., Waterman M. J., Lien M. C., El-Deiry W. S., Herlyn M., Halazonetis T. D. Cell Growth Differ. 2000;11:467–474. [PubMed] [Google Scholar]
- 20.Daniotti M., Oggionni M., Ranzani T., Vallacchi V., Campi V., Di Stasi D., Torre G. D., Perrone F., Luoni C., Suardi S., et al. Oncogene. 2004;23:5968–5977. doi: 10.1038/sj.onc.1207780. [DOI] [PubMed] [Google Scholar]
- 21.van Baren N., Brasseur F., Godelaine D., Hames G., Ferrant A., Lehmann F., Andre M., Ravoet C., Doyen C., Spagnoli G. C., et al. Blood. 1999;94:1156–1164. [PubMed] [Google Scholar]
- 22.Ard P. G., Chatterjee C., Kunjibettu S., Adside L. R., Gralinski L. E., McMahon S. B. Mol. Cell. Biol. 2002;22:5650–5661. doi: 10.1128/MCB.22.16.5650-5661.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girnita L., Girnita A., Brodin B., Xie Y., Nilsson G., Dricu A., Lundeberg J., Wejde J., Bartolazzi A., Wiman K. G., et al. Cancer Res. 2000;60:5278–5283. [PubMed] [Google Scholar]
- 24.Gostissa M., Morelli M., Mantovani F., Guida E., Piazza S., Collavin L., Brancolini C., Schneider C., Del Sal G. J. Biol. Chem. 2004;279:48013–48023. doi: 10.1074/jbc.M310801200. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani F., Banks L. Oncogene. 2001;20:7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- 26.Pipas J. M., Levine A. J. Semin. Cancer Biol. 2001;11:23–30. doi: 10.1006/scbi.2000.0343. [DOI] [PubMed] [Google Scholar]
- 27.Attardi L. D., Donehower L. A. Mutat. Res. 2005;576:4–21. doi: 10.1016/j.mrfmmm.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Bode A. M., Dong Z. Nat. Rev. Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 29.Geyer R. K., Yu Z. K., Maki C. G. Nat. Cell Biol. 2000;2:569–573. doi: 10.1038/35023507. [DOI] [PubMed] [Google Scholar]
- 30.Boyd S. D., Tsai K. Y., Jacks T. Nat. Cell Biol. 2000;2:563–568. doi: 10.1038/35023500. [DOI] [PubMed] [Google Scholar]
- 31.Monte M., Benetti R., Collavin L., Marchionni L., Del Sal G., Schneider C. J. Biol. Chem. 2004;279:11744–11752. doi: 10.1074/jbc.M311123200. [DOI] [PubMed] [Google Scholar]
- 32.Monte M., Benetti R., Buscemi G., Sandy P., Del Sal G., Schneider C. J. Biol. Chem. 2003;278:30356–30364. doi: 10.1074/jbc.M302902200. [DOI] [PubMed] [Google Scholar]
- 33.Ito A., Kawaguchi Y., Lai C. H., Kovacs J. J., Higashimoto Y., Appella E., Yao T. P. EMBO J. 2002;21:6236–6245. doi: 10.1093/emboj/cdf616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Insinga A., Monestiroli S., Ronzoni S., Carbone R., Pearson M., Pruneti G., Viale G., Appella E., Pelicci P., Minucci S. EMBO J. 2004;23:1144–1154. doi: 10.1038/sj.emboj.7600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo J., Su F., Chen D., Shiloh A., Gu W. Nature. 2000;408:377–381. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]
- 36.de Ruijter A. J., van Gennip A. H., Caron H. N., Kemp S., van Kuilenburg A. B. Biochem. J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrlich M. Oncogene. 2002;21:5400–5413. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 38.Gaudet F., Hodgson J. G., Eden A., Jackson-Grusby L., Dausman J., Gray J. W., Leonhardt H., Jaenisch R. Science. 2003;300:489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- 39.Loukinov D. I., Pugacheva E., Vatolin S., Pack S. D., Moon H., Chernukhin I., Mannan P., Larsson E., Kanduri C., Vostrov A. A., et al. Proc. Natl. Acad. Sci. USA. 2002;99:6806–6811. doi: 10.1073/pnas.092123699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jungbluth A. A., Ely S., DiLiberto M., Niesvizky R., Williamson B., Frosina D., Chen Y. T., Bhardwaj N., Chen-Kiang S., Old L. J., et al. Blood. 2005;106:167–174. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- 41.Elbashir S. M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser M. D., Iggo R. D. Proc. Natl. Acad. Sci. USA. 2002;99:95–100. doi: 10.1073/pnas.012283399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.