Abstract
True primates appeared suddenly on all three northern continents during the 100,000-yr-duration Paleocene–Eocene Thermal Maximum at the beginning of the Eocene, ≈55.5 mya. The simultaneous or nearly simultaneous appearance of euprimates on northern continents has been difficult to understand because the source area, immediate ancestors, and dispersal routes were all unknown. Now, omomyid haplorhine Teilhardina is known on all three continents in association with the carbon isotope excursion marking the Paleocene–Eocene Thermal Maximum. Relative position within the carbon isotope excursion indicates that Asian Teilhardina asiatica is oldest, European Teilhardina belgica is younger, and North American Teilhardina brandti and Teilhardina americana are, successively, youngest. Analysis of morphological characteristics of all four species supports an Asian origin and a westward Asia-to-Europe-to-North America dispersal for Teilhardina. High-resolution isotope stratigraphy indicates that this dispersal happened in an interval of ≈25,000 yr. Rapid geographic dispersal and morphological character evolution in Teilhardina reported here are consistent with rates observed in other contexts.
Keywords: carbon isotope excursion, euprimates, omomyids
Primates of modern aspect (euprimates) make their first appearance in the fossil record during the Paleocene–Eocene Thermal Maximum (PETM) at the beginning of the Eocene epoch ≈55.5 mya. One genus, the omomyid Teilhardina, is the oldest primate known in both Asia (1, 2) and Europe (3). In North America, the first appearance of Teilhardina, also during the earliest Eocene, is contemporaneous with the appearance of another primate, the adapoid Cantius (4, 5). Four hypotheses have been proposed to explain this geographic distribution: (i) primates originated in Africa and dispersed through Europe and Greenland to reach North America (6); (ii) primates originated in North America and dispersed via the Bering route to reach Asia and via Greenland to reach Europe (7); (iii) primates originated in Africa or Asia and dispersed through North America to reach western Europe (4, 8); or (iv) primates originated in Asia and dispersed eastward to North America and westward to Europe (2, 9). One variation of hypothesis iv is that primates might have originated on India before it collided with the Asian plate near the Paleocene/Eocene (P/E) boundary, spreading into Asia after the collision (10). Here, we reevaluate these hypotheses using a combination of highly resolved ages for the earliest records of Teilhardina on each continent and new morphologic evidence from the Teilhardina species involved. We conclude that none of the existing hypotheses fits the pattern that has emerged.
Results
It has become possible to compare ages of first appearance of Asian, European, and North American earliest Eocene mammals only in the past 3 yr, with identification of the global PETM marked by the Paleocene–Eocene carbon isotope excursion (CIE) on all three northern continents (11–13). This CIE coincides with an episode of intense global warming lasting ≈100 thousand years (Kyr) (14, 15), and the starting point of the excursion defines the P/E boundary (16, 17). It was during the PETM that euprimates, perissodactyls, and artiodactyls first appeared across the Holarctic continents. Early in the CIE interval, δ13C values decreased to a minimum and then gradually increased. The spike corresponding to the minimum value is situated 8.5–15 Kyr after the P/E boundary (14).
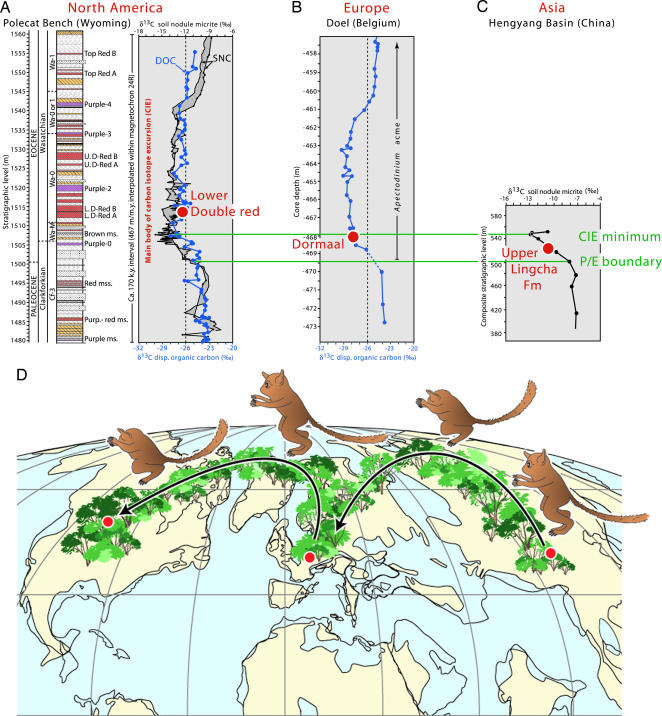
We correlated the CIE on the three northern continents and compared the stratigraphic positions and ages of the earliest records of Teilhardina on each continent (Fig. 1). The recently discovered Teilhardina asiatica from the Upper Lingcha Formation of China is from a level just above the P/E boundary, situated in the negative shift of the δ13C excursion but before the minimum value of the excursion (1,12). The type species Teilhardina belgica is known from Dormaal, just above the base of the fluviolagoonal Tienen Formation of Belgium (11). This base also lies within the negative shift of the δ13C excursion (18) and is estimated to be in an interval 4–10.5 Kyr after the P/E boundary (14). The early Eocene Willwood Formation of Wyoming has yielded five temporally successive Teilhardina species (19), of which Teilhardina brandti is the oldest. T. brandti, from the earliest Eocene (Wasatchian-0), was, until now, known from only one tooth (5), but several new specimens reported here reveal its phylogenetic importance. T. brandti and several other modern mammals (including artiodactyls and perissodactyls) first occur in a level that is situated above the minimum value of the δ13C excursion and that has an estimated age of 19–25 Kyr above the P/E boundary (14, 20, 21).
Fig. 1.
Paleogeographic map showing hypothetical migration routes of Teilhardina during the earliest Eocene. (A–C) Timing of migration is obtained by correlations of the δ13C excursion in North America (A), Europe (B), and Asia (C). T. brandti and other modern mammals first occur in the Lower Double Red (1,512 m) of Polecat Bench, Wyoming, which is situated above the minimum value of the δ13C excursion (spike at 1,507 m). The Lower Double (L.D-) Red has an estimated age of 19–25 Kyr above the P/E boundary, based on soil carbonate nodules (SNC) and dispersed (disp.) organic carbon (DOC) (14, 20, 21). U.D-, Upper Double; Purp., purple; Fm, formation. (D) We hypothesize that Teilhardina dispersed through continuous forest from southern Asia to Europe and from nothern Europe to North America during the first 25 Kyr of the PETM.
Thus T. asiatica, T. belgica, and T. brandti were almost contemporaneous, but the slight differences in their ages suggest that Teilhardina appeared first in Asia, dispersed from Asia to Europe at ≈5–12 Kyr after the P/E boundary, and reached North America no later than 25 Kyr after the P/E boundary. The entire dispersal of Teilhardina across the three northern continents, therefore, probably occurred within 15–25 Kyr. Such a high level of precision in correlating intercontinental biotic events is unprecedented for the Early Cenozoic and results from discovery of the global CIE. We cannot, as yet, constrain local first appearances statistically within the CIE interval, but the reported differences are corroborated by the character analyses below (Table 4, which is published as supporting information on the PNAS web site).
Dispersal rates of living mammals are in the range of 1–10 km/yr (22). The distance covered by Teilhardina would be ≈20,000 km. Thus, even if Teilhardina dispersed at the minimum rate documented in extant mammals, ≈1 km/yr, it could have covered this distance in 20 Kyr, which is consistent with the dispersal rate obtained by correlation of the CIE on the three northern continents.
We hypothesize that the primate Teilhardina, and probably basal genera of several other modern orders, dispersed east-to-west around the Northern Hemisphere at the beginning of the PETM. They migrated from South Asia to Europe, crossing the Turgai Straits (23–25), and then dispersed to North America via Greenland (26). Recent description of T. asiatica (1), together with new specimens of T. belgica (3) and T. brandti, reported here, make it possible to evaluate dental morphology of Teilhardina on all three continents in detail (Figs. 2–4) to determine whether their anatomical differences are consistent with this dispersal hypothesis. Our comparisons indicate that morphological evidence mirrors the stratigraphic sequence of species just described.
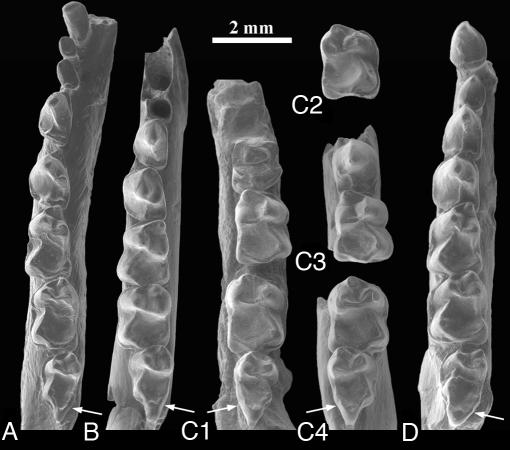
Fig. 2.
Earliest Eocene Teilhardina species: T. asiatica (A), T. belgica (B), T. brandti (C), and T. americana (D): lower teeth in occlusal view. Shown are IVPP V12357 (A), IRSNB M64 (B), UM 111434 (C1) (reversed in Fig. 3), UM 99031 (C2), USNM 493913 (C3) (reversed in Figs. 3 and 4), USNM 493914 (C4), and UW 6896 (D). Arrows indicate increasing breadth of hypoconulid lobe of M3.
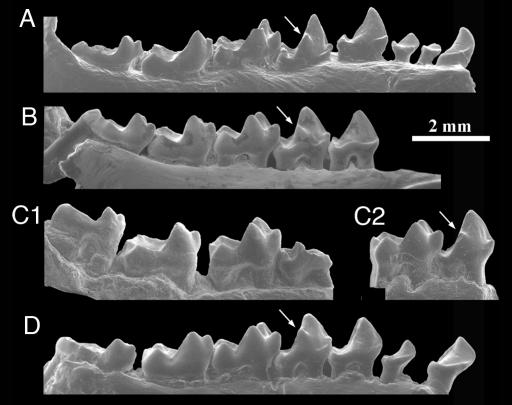
Fig. 3.
Earliest Eocene Teilhardina species, T. asiatica (A), T. belgica (B), T. brandti (C), and T. americana (D): lower teeth in lingual view. Arrows indicate progressive elevation of metaconid on P4. T. brandti shown are UM 111434 (reversed, C1) and USNM 493913 (reversed, C2).
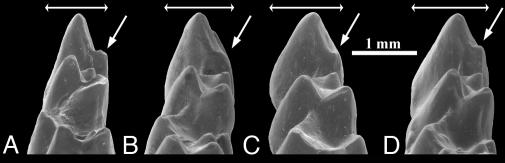
Fig. 4.
Earliest Eocene Teilhardina species, T. asiatica (A), T. belgica (B), T. brandti (C), and T. americana (D): last lower premolar (P4) in posterior view. Arrows indicate metaconid of P4, bars show increasing width of P4.
T. asiatica is morphologically most similar to T. belgica (1). These species share the following primitive characters: presence of P1, large canine, narrow cheek teeth, and weak labial cingulids, which, in combination, make them more primitive than any other omomyid. Neither species has any obvious autapomorphic features. But T. belgica is more derived and closer to American Teilhardina species in having stronger reduction of the first three premolars, a lower protoconid on P3–4–M1, a wider P4, and a squarer M2.
New specimens of T. brandti were collected in 2003 and 2004 from three separate areas of basal Eocene (Wasatchian-0) age in the Bighorn Basin, Wyoming (Figs. 2–4 and Table 1). The new specimens corroborate the validity of the species, and their broad geographic distribution across the basin shows that T. brandti is an index fossil of the Wasatchian-0 fauna (like the primitive artiodactyl Diacodexis ilicis and the perissodactyl Hyracotherium sandrae). T. brandti is morphologically intermediate between European T. belgica and North American T. americana. T. brandti is slightly larger than T. belgica and about the same size as T. americana (Table 5, which is published as supporting information on the PNAS web site). In several characters, T. brandti more closely resembles T. belgica: the lower metaconid on P4, the smaller and narrower hypoconulid lobe on M3, the tendency to have better defined hypoconulids on M1–2, the lower entoconid on M2, and the more open talonid notch between the entoconid and the trigonid (Fig. 3). At the same time, T. brandti resembles T. americana in having slightly wider cheek teeth because of basal inflation of the crown, a stronger ectocingulum, or both. In fact, labial cingulid development is intermediate between the two species: some specimens have little or no cingulum, as in T. belgica, whereas others have a moderately to well developed cingulum, as in T. americana.
Table 1.
List of specimens of T. brandti from earliest Eocene [Wasatchian (Wa)-0] of the Bighorn Basin, Wyoming
| ID number | Description | Location |
|---|---|---|
| UM 99031 | Holotype, M2 | SC-351, northern Bighorn Basin (5) |
| UM 111434 | Fragment of right dentary with P4–M3, the most complete specimen of T. brandti. The three molars are well preserved and not worn; only the trigonid of P4 is missing. The M2 is identical in size and morphology to the holotype | SC-67, northern Bighorn Basin |
| USNM 493913 | Right dentary fragment with P4–M1 | WW-108, southern Bighorn Basin |
| USNM 493914 | Right dentary fragment with M2–3 | WW-107, southern Bighorn Basin |
| USNM 521795 | Right dentary fragment with M2 | WW-75, southern Bighorn Basin |
| USNM 525543 | Left isolated P4, left isolated M1, left isolated M2 | WW-96, southern Bighorn Basin |
| USNM 525544 | Left isolated P4 and left isolated M2 | WW-84, southern Bighorn Basin |
| USNM 525545 | Left isolated M2 | WW-97, southern Bighorn Basin |
| USNM 525546 | Right dentary fragment with M2–3 | WW-96, southern Bighorn Basin |
| USNM 525621 | Right dentary fragment with M1–3 | WW-125, southern Bighorn Basin |
| USNM 525622 | Left dentary fragment with M2–3 | WW-96, southern Bighorn Basin |
All specimens listed are new except the holotype, and all have been found in association with Wa-0 mammalian markers such as D. ilicis, H. sandrae, and A. junnei. SC, Sand Coulee fossil locality, UM; UM, University of Michigan Museum of Paleontology; USNM, Department of Paleobiology, U.S. National Museum of Natural History (USNM), Smithsonian Institution, Washington, DC; WW, Willwood fossil locality, USNM.
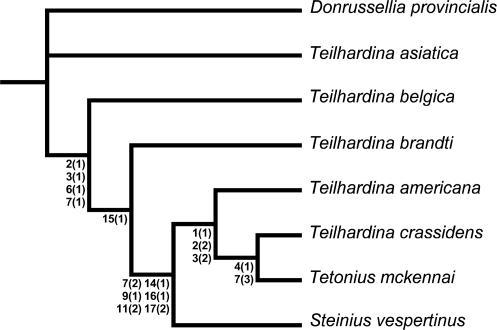
Whereas T. brandti is intermediate between T. belgica and T. americana, T. belgica is intermediate between T. asiatica and T. brandti (Tables 2 and 3). This morphocline suggests a basal omomyid lineage T. asiatica–T. belgica–T. brandti–T. americana, with a clear evolutionary gradient in dental characters. The Holarctic Teilhardina lineage is characterized by progressive reduction of the first three premolars, increasing elevation of the metaconid relative to the protoconid on P4, and widening of P4 and the molars (Fig. 4). Concomitantly, the medial incisor enlarged while the primitive caniniform canine became premolariform. Such gradual evolution was documented in endemic American lineages of omomyids, including Teilhardina (19, 27). This kind of dental modification probably reflects a shift in diet to a regimen richer in fruit and gums (28). The Teilhardina lineage is supported by an equally weighted parsimony analysis of 17 dental characters (Fig. 5). Only the omomyid Steinius vespertinus could modify the morphocline because S. vespertinus is more primitive than T. americana. However, Steinius occurs >1 million years later than T. americana (29) and could derive directly from a different Eurasian euprimate stock.
Table 2.
Characters used in phylogenetic analysis
| Character no. | Character | Description |
|---|---|---|
| 1 | Canine alveolus | (0) Large; (1) small; (2) smallest |
| 2 | P1 | (0) Present; (1) reduced; (2) absent |
| 3 | P3 shape | (0) Straight; (1) slightly procumbent; (2) procumbent |
| 4 | Length of P3–4 compared with M1–2 | (0) P3–4 not compressed; (1) P3–4 anteroposteriorly compressed |
| 5 | P3 paraconid | (0) Distinct cusp; (1) crestiform to absent |
| 6 | Protoconid on P3 to M1 | (0) High; (1) low |
| 7 | P4 shape | (0) Very narrow; (1) narrow; (2) wide; (3) very wide |
| 8 | P4 paraconid | (0) Well developed and distinct; (1) small; (2) very small to absent |
| 9 | P4 metaconid | (0) Low; (1) intermediate; (2) high |
| 10 | Postprotoconid crest on P4 and M1 | (0) Absent; (1) present |
| 11 | Anterior portion of the oblique crest on P4 | (0) Reaching the base of the trigonid wall; (1) running part way up the trigonid wall; (2) rising on the trigonid wall, higher than the top of the metaconid |
| 12 | Anterior portion of oblique crest on M1 | (0) Reaching the base of the trigonid wall; (1) running part way up the trigonid wall; (2) reaching the top of the metaconid |
| 13 | Labial cingulids on lower molars | (0) Weak or absent; (1) moderate; (2) strong |
| 14 | Hypoconulids on M1 and M2 | (0) Well defined; (1) weak |
| 15 | M2 shape | (0) Narrow; (1) somewhat square; (2) clearly square; (3) square and inflated |
| 16 | M2 entoconid | (0) Low; (1) high |
| 17 | Hypoconulid lobe on M3 | (0) Narrow; (1) intermediate; (2) wide |
Outgroup taxon was Donrussellia provincialis. All characters were ordered.
Table 3.
Character matrix used for phylogenetic analysis
| Genus and species | Position on matrix |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| Donrussellia provincialis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 1 | 0 | 1 | 0 | 1 |
| Teilhardina asiatica | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Teilhardina belgica | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| Teilhardina brandti | ? | ? | ? | ? | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0/1/2 | 0 | 2 | 0 | 1 |
| Teilhardina americana | 1 | 1/2 | 2 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Steinius vespertinus | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Teilhardina crassidens | 1 | 2 | 2 | 1 | 1 | 1 | 3 | 1 | 2 | 1 | - | 2 | 2 | 1 | 3 | 1 | 2 |
| Tetonius mckennai | 2 | 2 | 2 | 1 | ? | 1 | 3 | 1 | 1 | 0 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
Changes in omomyid species, together with increasing I1 size, were probably related to increasing gum-feeding and frugivory (28). P1 for T. americana (character 2) is either reduced (most specimens) or absent (at least one) (19). Low entoconid on M2 (character 16) can be objectively assessed by measuring the angle made by the entocristid and post-entocristid (part of postcristid), i.e., the crests running anteriorly and posterolabially from the entoconid: When viewed from above or obliquely from the labial side, this angle is always obtuse in T. belgica and T. brandti (resulting in a low entoconid) and acute in T. americana and T. crassidens (high entoconid). Labial cingulids (character 13) are variable in T. brandti.
Fig. 5.
Single most-parsimonious tree recovered in PAUP 4.0 b10 (34), derived from an equally weighted parsimony analysis of a matrix containing eight taxa and 17 dental characters (see Tables 3 and 5). Tree length = 36, consistency index excluding uninformative characters = 0.8286, retention index = 0.8286. Unequivocal character-state changes are indicated along each branch.
Discussion
Fossil evidence suggests that North American omomyids came from Europe via the Greenland bridge rather than directly from Asia across the Bering land bridge. This hypothesis is in agreement with other Euroamerican mammal lineages studied from the earliest Eocene. The best species correlations are between representatives of modern orders from the Wasatchian-0 fauna of the Willwood Formation of Wyoming and those from the Tienen Formation of Dormaal, Belgium. Indeed, the earliest North American artiodactyl D. ilicis is slightly larger and more derived than Diacodexis gigasei from Dormaal (8, 30). D. ilicis is considered to be at the base of the American dichobunoid radiation. In addition to the species D. gigasei–D. ilicis, other closely allied Euroamerican species pairs in which the European species is slightly more primitive include the proviverrine creodonts Arfia gingerichi–Arfia junnei and Prototomus minimus–Prototomus deimos (31) and several lipotyphlan species. These Euroamerican lineages do not exclude a migration route from Asia to North America via the Bering land bridge for other taxa. Because some typical Asian late Paleocene groups are present in the late Paleocene or early Eocene of North America and are unknown in Europe, they probably dispersed directly from Asia to North America, possibly during the late Paleocene. This scenario could be the case for representatives of some modern orders, such as rodents, some perissodactyls, and limnocyonine creodonts.
Dispersal of the earliest Eocene primate Teilhardina and other modern mammals took place during the onset of the PETM and near the beginning of a marine transgression. This transgression followed a major eustatic lowering of sea level (11), which we infer exposed land bridges between the northern continents. The exceptionally high temperature at the beginning of the Eocene, combined with the existence of temporary land bridges, evidently permitted small subtropical mammals to cross high-latitude land bridges such as Greenland and Beringia. Because omomyids were strictly arboreal mammals, their rapid dispersal argues for the existence of a continuous evergreen forest belt at high latitudes during the PETM (32). This hypothesis is reinforced by recent studies showing evidence for a shift in the state of the climate system during this time, characterized by large increases in tropospheric humidity and enhanced cycling of carbon through terrestrial ecosystems (33).
Materials and Methods
This study is based on anatomical comparisons of new specimens of North American T. brandti with specimens of other species of Teilhardina (see Tables 1 and 5). Relative age of fossils is based on recent high-resolution carbon isotope stratigraphy (11, 12, 14).
Supplementary Material
Acknowledgments
We thank X. Ni and C. Li (Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China) for providing a cast of T. asiatica and allowing its illustration; R. Smith (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) for access to specimens from his collection; P. Missiaen for running the cladistic analysis; J. Cillis for producing scanning electron microscopy photographs at the Royal Belgian Institute of Natural Sciences; W. Sanders for fossil preparation; and G. Gunnell, P. Missiaen, E. Steurbaut, S. Zack, and two anonymous reviewers for helpful comments. New fossils of T. brandti were collected under permits from the U.S. Bureau of Land Management, with support from National Geographic Grant 7630-04 and National Science Foundation Grants EAR-0000941 (to K.D.R.) and EAR-0125502 (to P.D.G.). This paper is a contribution to Research Project MO/36/011, financially supported by the Belgian Federal Science Policy Office. K.D.R.’s collaboration was facilitated by a Forschungspreis from the Alexander von Humboldt Stiftung.
Abbreviations
- CIE
carbon isotope excursion
- Kyr
thousand years
- P/E
Paleocene/Eocene
- PETM
Paleocene–Eocene Thermal Maximum.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ni X., Wang Y., Hu Y., Li C. Nature. 2004;427:65–68. doi: 10.1038/nature02126. [DOI] [PubMed] [Google Scholar]
- 2.Ni X., Hu Y., Wang Y., Li C. Anthropol. Sci. 2005;113:3–9. [Google Scholar]
- 3.Smith T. Geologiska Foreningens i Stockholm Forhandlingar. 2000;122:148–149. [Google Scholar]
- 4.Gingerich P. D. Nature. 1986;320:319–321. [Google Scholar]
- 5.Gingerich P. D. Contrib. Mus. Paleontol. Univ. Mich. 1993;28:321–326. [Google Scholar]
- 6.Godinot M. Géobios Mém. Spéc. 1982;6:403–412. [Google Scholar]
- 7.Sloan R. E. In: Yochelson E. L., editor. Proc. N. Am. Paleontol. Conv; Lawrence, KS: Allen; 1970. pp. 427–453. [Google Scholar]
- 8.Gingerich P. D. Univ. Mich. Pap. Paleontol. 1989;28:1–97. [Google Scholar]
- 9.Beard K. C., Dawson M. R. Bull. Soc. Géol. France. 1999;170:697–706. [Google Scholar]
- 10.Krause D. W., Maas M. C. Geol. Soc. Am. Spec. Pap. 1990;243:71–105. [Google Scholar]
- 11.Steurbaut E., De Coninck J., Roche E., Smith T. Bull. Soc. Géol. France. 1999;170:217–227. [Google Scholar]
- 12.Bowen G. J., Clyde W. C., Koch P. L., Ting S., Alroy J., Tsubamoto T., Wang Y., Wang Y. Science. 2002;295:2062–2065. doi: 10.1126/science.1068700. [DOI] [PubMed] [Google Scholar]
- 13.Koch P. L., Zachos J. C., Gingerich P. D. Nature. 1992;358:319–322. [Google Scholar]
- 14.Magioncalda R., Dupuis C., Smith T., Steurbaut E., Gingerich P. D. Geology. 2004;32:553–556. [Google Scholar]
- 15.Farley K. A., Eltgroth S. F. Earth Planet. Sci. Lett. 2003;208:135–148. [Google Scholar]
- 16.Dupuis C., Aubry M.-P., Steurbaut E., Berggren W. A., Ouda K., Magioncalda R., Cramer B. S., Kent D. V., Speijer R. P., Heilmann-Clausen C. Micropaleontology. 2003;49(Suppl 1):41–59. [Google Scholar]
- 17.Gradstein F. M., Ogg J. G., Smith A. G., Bleeker W., Lourens L. J. Episodes. 2004;27:83–100. [Google Scholar]
- 18.Steurbaut E., Magioncalda R., Dupuis C., Van Sismaeys S., Roche E., Roche M. Geol. Soc. Am. Spec. Pap. 2003;369:291–317. [Google Scholar]
- 19.Bown T. M., Rose K. D. Paleontol. Soc. Mem. 1987;23:1–162. [Google Scholar]
- 20.Bowen G. J., Koch P. L., Gingerich P. D., Norris R. D., Bains S., Corfield R. M. Paleocene–Eocene Stratigraphy and Biotic Change in the Bighorn and Clarks Fork Basins, Wyoming. In: Gingerich P. D., editor. Univ. of Michigan Papers on Paleontology. Vol. 33. Univ. of Michigan: Ann Arbor; 2001. pp. 73–88. [Google Scholar]
- 21.Bains S., Norris R. D., Corfield R. M., Bowen G. J., Gingerich P. D., Koch P. L. Geol. Soc. Am. Spec. Pap. 2003;369:1–9. [Google Scholar]
- 22.Jaksic F. M., Iriarte J. A, Jiménez J. E., Martinez D. R. Biol. Inv. 2002;4:157–173. [Google Scholar]
- 23.Iakovleva A. I., Brinkhuis H., Cavagnetto C. Palaeogr. Palaeoclimatol. Palaeoecol. 2001;172:243–268. [Google Scholar]
- 24.Hooker J. J., Dashzeveg D. Geol. Soc. Am. Spec. Pap. 2003;369:479–500. [Google Scholar]
- 25.Godinot M., de Lapparent de Broin F. Deinsea. 2003;10:255–275. [Google Scholar]
- 26.McKenna M. C. In: Structure and Development of the Greenland–Scotland Ridge. Bott M. H., Saxov A., Talwani M., Thiede J., editors. New York: Plenum; 1983. pp. 351–399. [Google Scholar]
- 27.Rose K. D., Bown T. M. Nature. 1984;309:250–252. [Google Scholar]
- 28.Strait S. G. J. Vertebr. Paleontol. 2001;21:322–334. [Google Scholar]
- 29.Rose K. D., Bown T. M. Proc. Natl. Acad. Sci. USA. 1991;88:98–101. doi: 10.1073/pnas.88.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith R., Smith T., Sudre J. Bull. Inst. Roy. Sci. Nat. Belg. Sci. Terre. 1996;66:177–186. [Google Scholar]
- 31.Smith T., Smith R. Belg. J. Zool. 2001;131:117–135. [Google Scholar]
- 32.Tiffney B. H. Acta Univ. Carol. Geol. 2000;44:5–16. [Google Scholar]
- 33.Bowen G. J., Beerling D. J., Koch P. L., Zachos J. C., Quattlebaum T. Nature. 2004;432:495–499. doi: 10.1038/nature03115. [DOI] [PubMed] [Google Scholar]
- 34.Swofford D. L. PAUP*4.0B10. Sunderland, MA: Sinauer; 2003. [Google Scholar]
- 35.Rose K. D. J. Hum. Evol. 1995;28:231–244. [Google Scholar]
- 36.Strauss D. J., Sadler P. M. Math. Geol. 1989;21:411–427. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.