Abstract
α-Galactosylceramide (α-GalCer) is the prototype compound for studying the presentation of glycolipids on CD1d molecules to natural killer T (NKT) lymphocytes. A single i.v. dose of glycolipid triggers a cascade of events involving the production of several cytokines over the course of a day, a short-lived activation of NKT and natural killer (NK) cells, and a more prolonged adaptive T cell immune response if certain antigens are given together with α-GalCer. We find that a recently described analogue, α-C-galactosylceramide (α-C-GalCer), more potently induces these innate and adaptive immune responses in mice. α-C-GalCer acts as a more effective trigger for IL-12 and IFN-γ production, although it minimally elicits IL-4 and TNF-α release into the serum. Also, α-C-GalCer better mobilizes NKT and natural killer cells to resist B16 melanoma. To help understand these effects, we find that α-C-GalCer binds more stably to dendritic cells than α-GalCer and that dendritic cells loaded with α-C-GalCer induce larger and more long lasting NKT cell responses in vivo. When glycolipid is targeted to dendritic cells in spleen together with antigens in dying cells, such as irradiated tumor cells, α-C-GalCer is active as an adjuvant for T cell-mediated immunity at lower doses, just 20 ng per mouse, where it is also able to up-regulate the required CD40L costimulatory molecule on NKT cells. Therefore, α-C-GalCer represents a glycolipid that binds more stably to dendritic cells and acts as a more effective link between innate and adaptive immunity in vivo.
Keywords: immune therapy, maturation, natural killer T, CD40L
The glycolipid, α-galactosylceramide (α-GalCer), is a natural product of mollusks and is presented on CD1d molecules to the invariant Vα14+ T cell receptor on natural killer T (iNKT) cells (1). The presentation of glycolipid on CD1d molecules to iNKT cells results in a cascade of cellular events that is able to provide resistance to tumors. For example, α-GalCer-activated iNKT cells recognize endogenous glycolipids on tumor cells and kill them, e.g., human myeloma (2) and glioma (3). Dendritic cells (DCs), when they present α-GalCer to iNKT cells, produce IL-12, which mobilizes natural killer (NK) cells that also have antitumor effects (4). α-GalCer additionally acts as an adjuvant for adaptive T cell immunity (5). This adjuvant effect means that DCs, upon capturing tumor antigens, are able to differentiate or mature in response to iNKT cells and then induce Th1-type CD4+ and cytolytic CD8+ T cells (6–8). Given these outcomes for tumor immunity, it is important to identify analogues that influence the immune outcome of glycolipid administration, particularly to improve the clinical efficacy of CD1d-binding glycolipids.
Several previously uncharacterized glycolipid ligands for CD1d now have been identified. The iNKT cells recognize an endogenous ligand, lysosomal glycolipid isoglobotrihexosylceramide, such that mice genetically deficient in the lysosomal glycosphingolipid degrading enzyme β-hexosaminidase (Hexβ−/−) have severely reduced iNKT cell numbers (9). Another natural ligand is disialoganglioside GD3. This ligand is expressed on melanomas and is presented to iNKT cells when DCs process tumor cells (10). Microbial glycolipids also are presented by CD1d on hexβ−/− antigen-presenting cells, including α-glucuronosylceramide from the Sphingomonas cell wall (11, 12), α-galacturonosylceramides from Sphingomonas wittichii, and glycolipids from Borrelia burgdorferi, the Lyme disease spirochete (13).
CD1d-binding glycolipid analogs, including sphingolipids and sulfatide variants, also have been synthesized. These synthesized ligands induce mouse and human NKT cells to secrete IFN-γ and IL-4 (13). One of these glycolipids, α-C-galactosylceramide (α-C-GalCer; refs. 14 and 15), is much more active than α-GalCer for inducing resistance to coadministered tumor cells and malaria parasites. In the current study, we wanted to determine whether the more active glycolipid α-C-GalCer is a more potent inducer of DC function in various forms of innate and adaptive resistance.
Results
α-C-GalCer Is a Distinct and More Potent Inducer of Innate Responses in Mice.
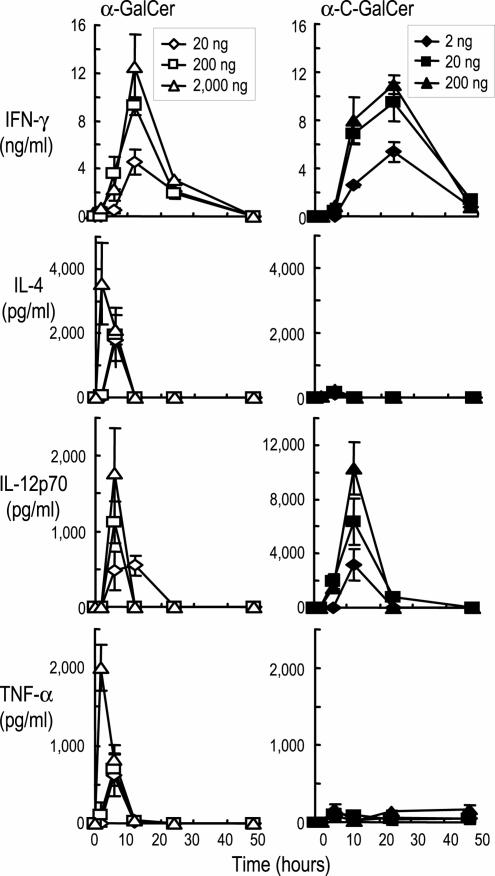
To understand the capacity of the new α-C-GalCer analogue to enhance resistance to malaria and tumors in mice (14), we analyzed several different types of innate and adaptive immune responses. First, we administered graded doses of each glycolipid i.v. and monitored the innate response in terms of elevations of serum cytokines (Fig. 1 Top). For induction of IFN-γ, the response to α-GalCer began to decrease at 20 ng per mouse, whereas for α-C-GalCer, 2 ng was the limiting dose (Fig. 1). Another distinction was that α-C-GalCer induced more prolonged production of IFN-γ and higher levels of IL-12 but did not induce IL-4 or TNF-α (Fig. 1). Thus, α-C-GalCer is a more active inducer of the innate production of cytokines than the prototype α-GalCer, and the innate response is qualitatively different.
Fig. 1.
α-C-GalCer is more potent than α-GalCer at inducing an innate cytokine response. Serum cytokines after graded doses of α-GalCer (Left) or α-C-GalCer (Right) i.v., measured by sandwich ELISAs. Data are means from four mice in two experiments.
Distinct CD11c+ DC and NK Cell Populations Produce IL-12 and IFN-γ After GalCer Stimulation.
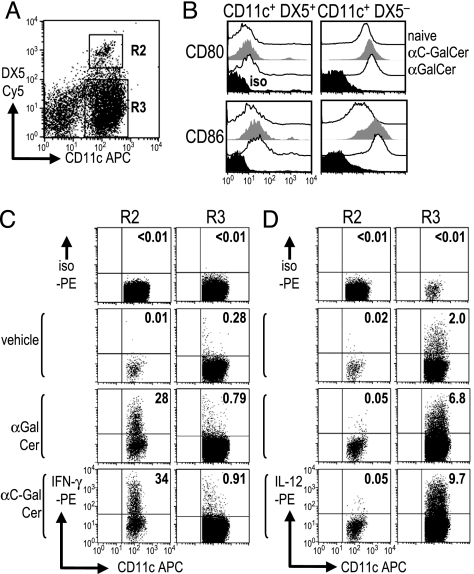
To determine the source of the serum cytokines in the experiments of Fig. 1, we examined two other responses that take place when DCs present glycolipids, i.e., the DCs start to produce IL-12, and this released cytokine, in turn, mobilizes IFN-γ-producing NK cells. We found that both of these events could be documented with FACS assays after administration of either α-C-GalCer or α-GalCer i.v. High expression of CD11c marks DCs (16, 17), but CD11c also can be expressed at intermediate levels on some NK cells (18–22). CD11c+ cells were isolated 6 h after glycolipid injection and were analyzed for surface markers. Two populations, CD11c+ DX5+ and CD11c+ DX5−, were distinguished within the T cell antigen receptor Vβ-negative fraction (Fig. 2A, R2 and R3). CD80/86 costimulatory molecules were expressed at much higher levels on CD11c+ DX5− cells (Fig. 2B), which were also MHC class II rich (data not shown), relative to CD11c+ DX5+ cells. Also, CD86 was up-regulated on the CD11c+ DX5− cells after injection of either glycolipid (Fig. 2B). This finding indicates that DX5− cells are DCs, whereas other CD11c+ cells are NK cells carrying the DX5 NK marker (18). When intracellular cytokines were assessed after 20 ng of α-C-GalCer or 2 μg of α-GalCer i.v., some of the CD11c+ DX5+ NK cells preferentially produced IFN-γ, not IL-12 (Fig. 2C Left). Reciprocally, a fraction of the CD11c+ DX5− DCs produced IL-12p40 but little or no IFN-γ (Fig. 2D Right). The IFN-γ producing population did not express B220 and MHC class II (data not shown) and, therefore, seems different from the recently described subset of IFN-γ-producing killer DCs (19, 20). Thus, IL-12 and IFN-γ are produced by distinct CD11c+ cells in spleen, the former by costimulatory DCs and the latter by NK cells activated by the DC–NKT interaction.
Fig. 2.
Distinct cytokine production by subsets of CD11c+ spleen cells. (A and B) CD11c+ cells were isolated from spleen 6 h after injection of vehicle, α-C-GalCer, or α-GalCer. After gating on TCRβ− cells, we distinguished Cy5 anti-DX5+ NK cells (R2) and DX5− DCs (R3) within APC-anti-CD11c+ cells (A) and measured CD80 and CD86 expression (B). (C and D) As in A, but the cells were cultured for 4 h in brefeldin A without other stimuli before staining the R2 and R3 populations for intracellular IFN-γ (C) or IL-12 (D).
α-C-GalCer on DCs Is More Effective in Expanding Cytokine-Producing NKT Cells.
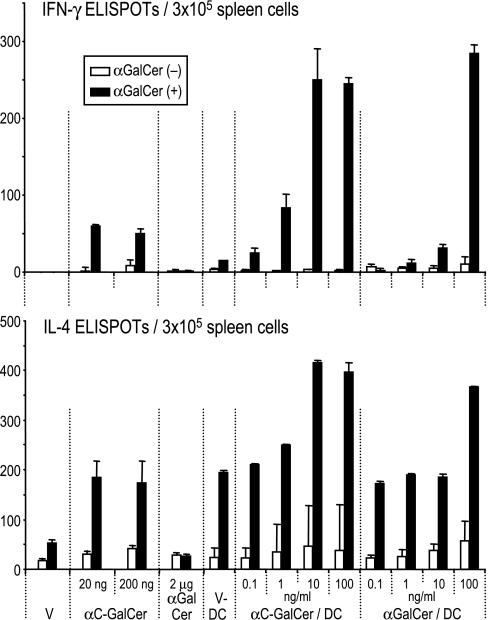
Our prior work found that α-GalCer, when loaded on DCs, elicited prolonged “priming” of NKT cells in spleen and liver for 2 or more days (23). We injected either free glycolipid or glycolipid-loaded DCs and looked 2 d later for primed NKT cells, i.e., cells that would respond to α-GalCer during a 16-h ELISPOT assay for IFN-γ or IL-4 (Fig. 3Upper and Lower, respectively). Under these conditions, the major primed, cytokine-secreting cells are known to be NKT cells (23). α-C-GalCer proved to be more potent than α-GalCer, either as a free drug or pulsed in graded doses onto DCs (Fig. 3). Even low doses of α-C-GalCer sufficed as a free drug at 20 ng per mouse. Also, 1–10 ng/ml α-C-GalCer, as opposed to 100 ng/ml α-GalCer, was sufficient to load DCs (Fig. 3). Therefore α-C-GalCer is a more potent glycolipid for enhancing NKT function via DCs in vivo.
Fig. 3.
Enhanced cytokine-producing NKT cells after injection of free glycolipid or glycolipid loaded DCs. Groups of mice (x axis) were immunized i.v. with vehicle (V), free glycolipid, or DCs that had been pulsed ex vivo with V, α-GalCer, or α-C-GalCer. Two days later, spleens were analyzed with or without α-GalCer (100 ng/ml) restimulation for 16 h, and IFN-γ or IL-4-ELISPOT-forming cells were counted.
More Stable Binding of α-C-GalCer to DCs and More Prolonged NKT Responses.
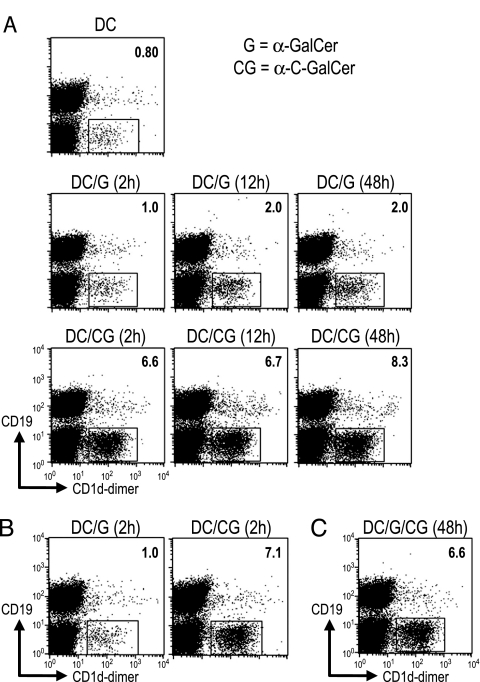
To better understand the potency of α-C-GalCer, we first applied α-GalCer or α-C-GalCer for graded times to the DCs (2, 12, and 48 h) and assayed NKT cell expansion 5 d after i.v. administration of the DCs to mice. α-GalCer was able to expand NKT cell numbers (measured as CD19− cells that bound CD1d-αGalCer tetramers) if added to DCs for 12 h but not 2 h, whereas α-C-GalCer loading onto DCs required just 2 h, and the expansion at day 5 was also much greater (Fig. 4A). This finding suggested that binding of α-C-GalCer to DCs was more stable than binding of α-GalCer. To test this possibility, we added glycolipid to DCs for 2 h, washed three times, and cultured the cells for an additional 2 d without glycolipid in the presence of granulocyte/macrophage colony-stimulating factor to sustain DC viability. These α-C-GalCer-pulsed DCs were able to expand NKT cell numbers to high levels 5 d after injection (Fig. 4B), indicating that the association of α-C-GalCer to DCs was stable. We also added both α-C-GalCer and α-GalCer for 48 h to the DCs, but α-GalCer did not compete, as evidenced by strong NKT cell expansion in vivo 5 d later (Fig. 4C). Thus, synthetic glycolipids can be developed that bind more stably to DCs and improve NKT cell expansion in vivo.
Fig. 4.
More stable binding of α-C-GalCer to DCs. (A) Bone marrow-derived DCs were generated for 8 d in culture (see Materials and Methods) and exposed to α-GalCer (G) or α-C-GalCer (CG) during the last 2, 12, and 48 h. The cells were washed and injected i.v. to expand CD1d-GalCer dimer-binding NKT cells 5 d later in spleen. (B) As in A, but the DCs were loaded for 2 h with α-GalCer or α-C-GalCer, washed, cultured for 48 h in granulocyte/macrophage colony-stimulating factor-supplemented medium, and injected into mice to measure NKT cell numbers 5 d later. (C) As in B Right, but both glycolipids were added together for 48 h to DCs before induction of NKT cell expansion in vivo.
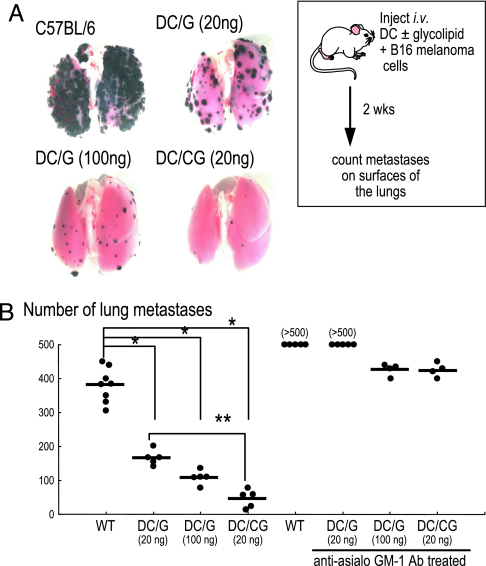
α-C-GalCer Is a More Potent Inducer of Innate Resistance to B16 Melanoma.
To test the in vivo efficacy of the innate response, we used a standard assay in which the establishment of metastases of MHC-class I low B16 melanoma cells is retarded (23). Lung metastases were evaluated 2 weeks after coadministration i.v. of DCs loaded with either glycolipid together with B16 melanoma cells. The α-C-GalCer-loaded DCs were more effective than α-GalCer-loaded DCs in reducing the size (Fig. 5A) and the number of lung metastases (Fig. 5B). When mice were depleted of NK cells by prior treatment with a polyclonal anti-asialo-GM1, the number of metastases increased relative to untreated controls, but again, DCs pulsed with α-GalCer induced some protection (Fig. 5B). This protection indicates that both NKT and NK cells are contributing to the resistance induced by α-C-GalCer-loaded DCs and that the α-C analogue is more potent than α-GalCer at mobilizing these innate mechanisms.
Fig. 5.
DCs loaded with glycolipids protect against lung metastases of B16 melanoma. Micrographs (A) and numbers of melanin-laden (black) metastases (B) in the lungs of groups of five to eight mice 14 d after B16 melanoma cells were administered i.v. together with DCs that had been exposed beforehand in vitro to either α-GalCer (G) or α-C-GalCer-(CG). We also tested mice depleted of NK cells by anti-asialo-GM1 treatment (∗, P < 0.005; ∗∗, P = 0.008).
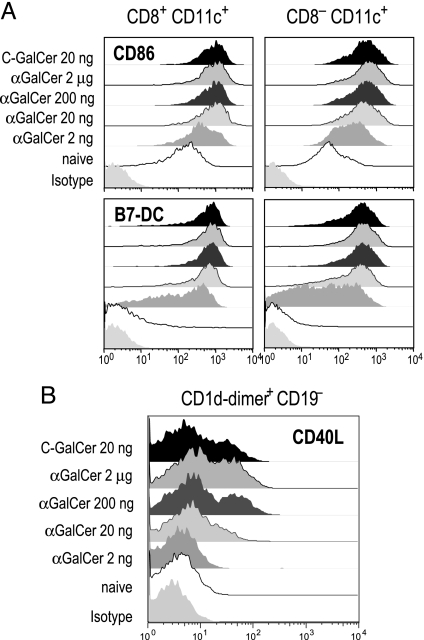
α-C-GalCer Actively Induces Costimulatory Molecules on DCs and NKT Cells.
To further compare the consequences of α-C-GalCer and α-GalCer on DC function, we assessed expression of costimulatory molecules on the main subsets of myeloid CD11c+ DCs in spleen, distinguished as CD8+ and CD8−. Although 2 μg of α-GalCer is typically used as an adjuvant in mice, we found that 20 ng was sufficient to induce a near-maximal up-regulation of CD86 and B7-DC costimulatory molecules and that 20 ng of α-C-GalCer and α-GalCer were equally effective by this criterion (Fig. 6A). We also verified that the effect of α-C-GalCer on the surface markers of DCs was more potent with the i.v. rather than i.m. and s.c. routes of administration (Fig. 8 A and B, which are published as supporting information on the PNAS web site), and that the effects depended on NKT cells, as assessed with mice genetically lacking CD1d and NKT cells (Fig. 8 C and D). However, when we examined the up-regulation of another essential costimulator, CD40L, on NKT cells (24), we found that 20 ng of α-C-GalCer was as effective as 2 μg of α-GalCer but that 20 ng of α-GalCer was close to inactive (Fig. 6B). Thus, by using 20 ng of glycolipid, one has a situation in which both compounds up-regulate the expression of B7 family costimulators, but only the newer α-C-GalCer up-regulates CD40L expression on NKT cells.
Fig. 6.
Up-regulation of costimulatory molecules by α-C-GalCer. (A) Up-regulation of CD86 (x axis) on CD8+ and CD8− CD11c+ DC subsets 16 h after injecting graded doses of α-GalCer or α-C-GalCer i.v. (B) Up-regulation of CD40L on CD1d-dimer+ CD19− NKT cells stained for CD40L-PE, CD19-FITC, and CD1d-dimer-biotin/streptavidin-APC 2 h after i.v. injection of α-GalCer or α-C-GalCer.
α-C-GalCer Is a More Effective Adjuvant for Adaptive T Cell Immunity to Tumors.
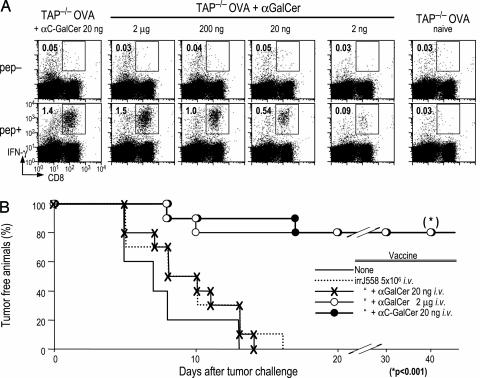
Because ligation of CD40 on DCs is essential for glycolipids to act as adjuvants for T cell immunity (24, 25), we examined the capacity of lower doses of glycolipid to induce adaptive immunity in two previously described systems. We first used a sensitive assay for presentation of cell-associated antigen. In this assay, dying splenocytes loaded with ovalbumin (OVA) are injected i.v. with graded doses of glycolipid. It was noted (6) that coadministration of 2 μg of α-GalCer led to the development of robust CD8+T cell response to the cell-associated OVA and that this induced immunity was the result of antigen presentation by DCs maturing in response to α-GalCer and NKT cells. Here we noted that 20 ng of α-C-GalCer induced a comparable response to 2 μg of α-GalCer. In contrast, lowering the dose of the parent compound α-GalCer to 20 ng reduced the T cell response, i.e., the response was only 42 ± 17% of the maximum observed with 2 μg of glycolipid (mean of three mice), and the response was just perceptible at 2 ng (Fig. 7A). Thus, 20 ng of α-C-GalCer acts as a more potent adjuvant than α-GalCer for the presentation of cell-associated antigens in mice, even though both induce high levels of CD86 costimulatory molecules on DCs at this dose.
Fig. 7.
DC-mediated T cell immunity induced by α-C-GalCer. (A) B6 mice were primed with 2 × 107 OVA-loaded, TAP−/− spleen cells and either graded doses of α-GalCer or 20 ng of α-C-GalCer i.v. as described in refs. 6 and 24. Seven days later, mice were assayed for IFN-γ-producing CD8+ T cells 6 h after restimulation with an MHC class I binding OVA peptide in brefeldin A. (B) Mice were immunized with 5 × 106 irradiated MHC class I negative J558 plasmacytoma cells and the indicated doses of glycolipid i.v., as described for this system of T cell-dependent protection against tumors (25). Two weeks later, groups of five mice were challenged s.c. with 1 × 106 MHC class I bearing J558 cells.
To extend the analysis to tumors, we injected irradiated J558 plasmacytoma cells i.v. rather than OVA-loaded splenocytes. It has been shown recently that α-GalCer is an effective adjuvant for immunization of protective antitumor T cells after uptake of irradiated MHC-negative tumor cells by DCs (25). As shown in Fig. 7B, mice that were given irradiated J558 tumor cells and either 20 ng of α-C-GalCer or 2 μg of α-GalCer, but not 20 ng of α-GalCer, developed resistance to challenge with MHC class I positive J558 tumor cells. These findings indicate that α-C-GalCer is an effective and more potent adjuvant that α-GalCer for adaptive resistance to tumor cells and that improved adjuvant function correlates with CD40L up-regulation on the NKT cells (Fig. 6B) and presentation of cell-associated antigen (Fig. 7A), but not on up-regulation of CD86 (Fig. 6A).
Discussion
α-GalCer, when presented on CD1d molecules of DCs to NKT cells, induces several responses that can be protective against tumors, as most recently described for experimental hematologic tumors in mice (25). New analogues of α-GalCer are being synthesized, and one of these, the C-glycoside analogue of α-GalCer (α-C-GalCer), was found to be more potent in helping mice to defend against mouse malaria and B16 melanoma (14). To understand the basis for the improved adjuvant effects of α-C-GalCer, we have studied its capacity to stimulate several innate and adaptive immune responses, particularly at the level of DCs.
When we compared the effects of the two synthetic glycolipids given i.v., the α-C-GalCer was more active than α-GalCer, and it induced a more prolonged IFN-γ and IL-12 innate response, without IL-4 or TNF-α (Fig. 1). The IFN-γ and IL-12 were produced by NK and DCs, respectively (Fig. 2). Previously, we had reported that α-GalCer induced IFN-γ production by DCs (24), but we overlooked the fact that the CD11c marker can be expressed by both DCs and activated NK cells. When we loaded the two synthetic glycolipids onto DCs ex vivo and then injected the cells, the α-C-GalCer-pulsed DCs also were able to induce a stronger and more prolonged IFN-γ-producing NKT response (Fig. 3). This finding, at least in part, can be attributed to the more stable association of α-C-GalCer with DCs, relative to α-GalCer. Likewise α-C-GalCer was more effective at inducing adaptive protective T cell-mediated immunity to cell-associated antigens including tumor cells, which are captured by DCs in vivo (Fig. 7). α-C-GalCer, like the parent α-GalCer, required glycolipid-presenting CD1d molecules and NKT cells, because DCs from CD1d−/− or Jα18−/− mice did not show signs of maturation. Therefore, α-C-GalCer is more potent than α-GalCer in allowing DCs to activate both innate and adaptive limbs of immunity in mice.
We also noted that the i.v. route was the most effective for both glycolipids, possibly because this route allowed access of glycolipid to DCs in the spleen, where there are relatively large numbers of NKT cells available for interaction with DCs. A single dose of 20 ng of α-C-GalCer was sufficient to set into motion the many innate and adaptive responses that we measured. A recent study evaluated intranasal injection of α-GalCer, which activated pulmonary IL-4-producing NKT cells along with the development of airway hyperreactivity (26, 27). To induce antigen-specific T cell immunity with α-GalCer through the oral route, Silk et al. (8) gave 8 μg of α-GalCer together with OVA antigen. Our data indicate that the type of glycolipid and the route of injection markedly influence the efficacy of glycolipids as adjuvants.
We found a significant lack of correlation between the maturation of DCs, as monitored by increased expression of B7 family costimulatory molecules, and the capacity of the DCs to elicit immunity. Even at a low dose, 20 ng of α-GalCer per mouse, the DCs up-regulated CD86 and B7-DC molecules (Fig. 6), but the same mice were unable to induce strong T cell responses to cell-associated antigens (Fig. 7). The induction of T cell immunity in this system is known to require an interaction of CD40L with CD40 on the DCs (7, 24), and up-regulation of CD40L expression on NKT cells correlated much better with the capacity of a given dose of glycolipid to act as an adjuvant (Fig. 6B). In keeping with our prior studies (24), the new data reiterate that the up-regulation of CD86 alone is insufficient to allow antigen-presenting DCs to initiate immunity. Other molecules, in this case a CD40–CD40L interaction, are essential.
Our finding that a glycolipid analogue can selectively promote Th1 type innate and adaptive responses is germane to using these compounds to enhance resistance to certain intracellular infections and tumors. IFN-γ producing NKT cells are more valuable for resistance to experimental tumors (23). Chang et al. recently found that mature DCs loaded with α-GalCer induce a prolonged expansion of NKT cells in patients with advanced cancer (28). The results from our current study indicate that CD1d analogues can be designed that are more effective in generating an immunostimulatory DC–NKT interaction. Clinical research will be needed to pursue the efficacy of these new glycolipids in cancer patients.
Materials and Methods
Mice.
Specific pathogen-free C57BL/6 (B6), BALB/c, and TAP−/− B6 female mice at 6–7 weeks were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice deleted of Jα281 (Jα18) and CD1d genes were generously provided by M. Taniguchi (The Institute of Physical and Chemical Research, Yokohama, Japan) and Luc Van Kaer (Vanderbilt University, Nashville, TN). Mice were studied in compliance with relevant laws and institutional guidelines.
Reagents.
α-C-GalCer was synthesized as described in ref. 15. One milligram of α-C-GalCer was dissolved in DMSO, and a required aliquot was then dissolved in PBS buffer, warmed to 50°C, and sonicated for a few minutes. α-GalCer was kindly provided by the Pharmaceutical Research Laboratory, Kirin Brewery (Gunma, Japan) and diluted in PBS (6). LPS-free OVA was from Seikagaku Corp. (Tokyo, Japan). The following monoclonal antibodies (mAbs) were from BD Pharmingen (San Diego, CA): FITC-conjugated anti-CD4; FITC- or PE-conjugated anti-CD8α; APC-conjugated anti-CD11c; biotinylated-isotype control; and anti-CD40, -CD80, -CD86, and -CD119/IFN-γR. Biotinylated mAbs were detected with streptavidin-APC. To identify cells producing cytokines as part of the innate response to glycolipid, splenic CD11c+ cells were labeled with PE-Cy7 anti-CD11c and APC-anti-DX5 (18) to distinguish DX5− classical or conventional DCs from DX5+ NK cells. To identify the newly described IFN-γ-producing killer DX5+ DCs, we used expression of MHC class II and B220 (FITC antibodies), which are lacking from typical DX5+ DC NK cells (19, 20).
Stability of Glycolipid Binding to Bone Marrow-Derived DCs.
Bone marrow-derived DCs were generated with granulocyte/macrophage colony-stimulating factor for 8 d as described in ref. 29. DCs were pulsed with α-GalCer, α-C-GalCer, or both for 2–48 h. LPS also was added at day 7 for the last 16 h to drive DC maturation. Two to five days after injection of the DCs into mice i.v., spleen suspensions were analyzed for NKT, NK, or conventional T cells (6, 23).
Flow Cytometric Assay for Cytokine Production by CD11c+ Spleen Cells.
As described in ref. 6, splenocytes were released by teasing and treatment with collagenase D (Roche Diagnostics, Indianapolis, IN). CD11c+ cells were selected with anti-CD11c coated magnetic beads and cultured with brefeldin A (Pharmingen) for 4 h. The cells were incubated with 2.4G2 mAb to block FcγR; washed; incubated for 30 min with FITC anti-CD8, Cy5 anti-DX5, and APC anti-CD11c at room temperature; then washed; permeabilized (Cytofix/Cytoperm Plus, Pharmingen); stained with PE-conjugated anti-IFN-γ (XMG1.2) or anti-IL-12 mAbs for 15 min at room temperature; and analyzed with a FACS Calibur (Becton Dickinson, San Jose, CA) and either CellQuest (BD Biosciences, Mountain View, CA) or FlowJo (Tree Star, San Carlos, CA) software.
T Cell Immunity.
Mice were injected with 2 × 107 OVA pulsed and osmotically shocked, syngeneic, TAP−/− splenocytes as described in ref. 30. Seven days later, 5 × 106 spleen cells were cultured for 6 h in 24-well plates with 1 μM OVA257–264 peptide (for CD8+ T cells) or 2 μM OVA323–339 peptide (for CD4+ T cells) in the presence of brefeldin A to accumulate IFN-γ intracellularly. Cells were incubated with 2.4G2 mAb, then FITC anti-CD4 or -CD8 for 20 min at room temperature, and then permeabilized and stained for IFN-γ as above.
Tumor Protection Experiments.
Irradiated J558 plasmacytoma cells (5 × 106 100 Gy) together with glycolipid were coadministered to C57BL/6 mice as described in ref. 25. Two weeks later, groups of five mice were challenged s.c. with 1 × 106 J558 cells.
Statistical Analysis.
The statistical significance of differences between the experimental groups was determined by the Mann–Whitney exact rank sum test. Differences in tumor protection of each group were analyzed by using the log-rank test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Judy Adams for help in preparing the manuscript. This work was supported by National Institutes of Health Grants CA84512 and AI 051573.
Abbreviations
- α-GalCer
α-galactosylceramide
- α-C-GalCer
α-C-galactosylceramide
- DC
dendritic cell
- iNKT
invariant natural killer T
- NK
natural killer
- NKT
natural killer T
- OVA
ovalbumin.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Taniguchi M., Harada M., Kojo S., Nakayama T., Wakao H. Annu. Rev. Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 2.Dhodapkar M. V., Geller M. D., Chang D. H., Shimizu K., Fujii S., Dhodapkar K. M., Krasovsky J. J. Exp. Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhodapkar K. M., Cirignano B., Finlay J. L., Steinman R. M. Int. J. Canc. 2004;109:893–899. doi: 10.1002/ijc.20050. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura H., Iwakabe K., Yahata T., Nishimura S., Ohta A., Ohmi Y., Sato M., Takeda K., Okumura K., Van Kaer L., et al. J. Exp. Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez-Aseguinolaza G., Van Kaer L., Bergmann C. C., Wilson J. M., Schmieg J., Kronenberg M., Nakayama T., Taniguchi M., Koezuka Y., Tsuji M. J. Exp. Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujii S., Shimizu K., Smith C., Bonifaz L., Steinman R. M. J. Exp. Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermans I. F., Silk J. D., Gileadi U., Salio M., Mathew B., Ritter G., Schmidt R., Harris A. L., Old L., Cerundolo V. J. Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 8.Silk J. D., Hermans I. F., Gileadi U., Chong T. W., Shepherd D., Salio M., Mathew B., Schmidt R. R., Lunt S. J., Williams K. J., et al. J. Clin. Invest. 2004;114:1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou D., Mattner J., Cantu C., III, Schrantz N., Yin N., Gao Y., Sagiv Y., Hudspeth K., Wu Y. P., Yamashita T., et al. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 10.Wu D. Y., Segal N. H., Sidobre S., Kronenberg M., Chapman P. B. J. Exp. Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattner J., Debord K. L., Ismail N., Goff R. D., Cantu C., Zhou D., Saint-Mezard P., Wang V., Gao Y., Yin N., et al. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 12.Kinjo Y., Wu D., Kim G., Xing G. W., Poles M. A., Ho D. D., Tsuji M., Kawahara K., Wong C. H., Kronenberg M. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 13.Wu D., Xing G. W., Poles M. A., Horowitz A., Kinjo Y., Sullivan B., Bodmer-Narkevitch V., Plettenburg O., Kronenberg M., Tsuji M., et al. Proc. Natl. Acad. Sci. USA. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmieg J., Yang G., Franck R. W., Tsuji M. J. Exp. Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G., Schmieg J., Tsuji M., Franck R. W. Org. Lett. 2004;6:4077–4080. doi: 10.1021/ol0482137. [DOI] [PubMed] [Google Scholar]
- 16.Metlay J. P., Witmer-Pack M. D., Agger R., Crowley M. T., Lawless D., Steinman R. M. J. Exp. Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowley M. T., Inaba K., Witmer-Pack M. D., Gezelter S., Steinman R. M. J. Immunol. Methods. 1990;133:55–66. doi: 10.1016/0022-1759(90)90318-p. [DOI] [PubMed] [Google Scholar]
- 18.Pillarisetty V. G., Katz S. C., Bleier J. I., Shah A. B., Dematteo R. P. J. Immunol. 2005;174:2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- 19.Chan C. W., Crafton E., Fan H. N., Flook J., Yoshimura K., Skarica M., Brockstedt D., Dubensky T. W., Stins M. F., Lanier L. L., et al. Nat. Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 20.Taieb J., Chaput N., Menard C., Apetoh L., Ullrich E., Bonmort M., Pequignot M., Casares N., Terme M., Flament C., et al. Nat. Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 21.Schleicher U., Hesse A., Bogdan C. Blood. 2005;105:1319–1328. doi: 10.1182/blood-2004-05-1749. [DOI] [PubMed] [Google Scholar]
- 22.Homann D., Jahreis A., Wolfe T., Hughes A., Coon B., van Stipdonk M. J., Prilliman K. R., Schoenberger S. P., von Herrath M. G. Immunity. 2002;16:403–415. doi: 10.1016/s1074-7613(02)00290-x. [DOI] [PubMed] [Google Scholar]
- 23.Fujii S., Shimizu K., Kronenberg M., Steinman R. M. Nat. Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 24.Fujii S., Liu K., Smith C., Bonito A. J., Steinman R. M. J. Exp. Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu K., Idoyaga J., Charalambous A., Fujii S., Bonito A., Mordoh J., Wainstok R., Bai X. F., Liu Y., Steinman R. M. J. Exp. Med. 2005;202:1507–1516. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer E. H., Goya S., Akbari O., Berry G. J., Savage P. B., Kronenberg M., Nakayama T., Dekruyff R. H., Umetsu D. T. Proc. Natl. Acad. Sci. USA. 2006;103:2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hachem P., Lisbonne M., Michel M. L., Diem S., Roongapinun S., Lefort J., Marchal G., Herbelin A., Askenase P. W., Dy M., et al. Eur. J. Immunol. 2005;35:2793–2802. doi: 10.1002/eji.200535268. [DOI] [PubMed] [Google Scholar]
- 28.Chang D. H., Osman K., Connolly J., Kukreja A., Krasovsky J., Pack M., Hutchinson A., Geller M., Liu N., Annable R., et al. J. Exp. Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inaba K., Romani N., Schuler G., Mirza A., Steinman R. M. In: Current Protocols in Immunology. Coligan J. E., Kruisbeek A. M., Margulies D. H., Shevach E. M., Strober W., editors. New York: Greene & Wiley; 1997. Chapter 3: Section I, Unit 3.7.7–3.7.15. [Google Scholar]
- 30.Iyoda T., Shimoyama S., Liu K., Omatsu Y., Maeda Y., Takahara K., Akiyama Y., Steinman R. M., Inaba K. J. Exp. Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.