Abstract
Oxidative stress has long been linked to the pathogenesis of neurodegenerative diseases; however, whether it is a cause or merely a consequence of the degenerative process is still unknown. We show that mice deficient in Cu, Zn-superoxide dismutase (SOD1) have features typical of age-related macular degeneration in humans. Investigations of senescent Sod1−/− mice of different ages showed that the older animals had drusen, thickened Bruch’s membrane, and choroidal neovascularization. The number of drusen increased with age, and exposure of young Sod1−/− mice to excess light induced drusen. The retinal pigment epithelial cells of Sod1−/− mice showed oxidative damage, and their β-catenin-mediated cellular integrity was disrupted, suggesting that oxidative stress may affect the junctional proteins necessary for the barrier integrity of the retinal pigment epithelium. These observations strongly suggest that oxidative stress may play a causative role in age-related retinal degeneration, and our findings provide evidence for the free radical theory of aging. In addition, these results demonstrate that the Sod1−/− mouse is a valuable animal model to study human age-related macular degeneration.
Keywords: animal model, superoxide dismutase
Age-related macular degeneration (AMD) is the leading cause of legal blindness in humans in developed countries (1–5). AMD is characterized by a progressive degeneration of the macula, usually bilateral, leading to a severe decrease in vision and a central scotoma. The decrease in vision results either from retinal degeneration, called geographic atrophy (dry or nonexudative AMD), or from the secondary effects of choroidal neovascularization (CNV; wet or exudative AMD). An early sign of AMD is the appearance of drusen, which are extracellular deposits that accumulate below the retinal pigment epithelium (RPE) and are known to be risk factors for developing CNV (6–8).
Mouse models of AMD that manifest some of the features of human AMD have recently begun to appear (9–14); however, most of these mice have only some of the characteristics of human AMD (15). The severity of AMD in humans progresses with increasing age, finally resulting in extensive degeneration of the retina. Therefore, animal models that mimic the complex and progressive characteristics of AMD are needed to investigate the pathogenesis of AMD.
Oxidative stress, which refers to cellular or molecular damage caused by reactive oxygen species (ROS), has been implicated in many age-related diseases and aging itself (16, 17). ROS include free radicals, hydrogen peroxide, and singlet oxygen and are often the by-products of oxygen metabolism. The retina is particularly susceptible to oxidative stress because of its high consumption of oxygen, high concentration of polyunsaturated fatty acids, and exposure to light (18). A growing body of evidence suggests that cumulative oxidative damage may be responsible for AMD (18, 19); however, a causative link has not been definitively demonstrated (18).
To determine whether there is a causative role of oxidative stress in the pathogenesis of AMD, we first focused on the age-related changes in the eyes of mice deficient in oxidative stress scavengers. One of the main antioxidant systems in the retina is made up of three superoxide dismutase (SOD) isoenzymes that catalyze superoxide radical dismutation (20). Cu, Zn-SOD (SOD1) exists in the cytosol, Mn-SOD (SOD2) in the mitochondrial matrix, and extracellular SOD (SOD3) in the tissue interstitium as the secretory form (21). Because the amount and activity of SOD1 are highest among the three isoenzymes in the human retina (21), it seemed reasonable to hypothesize that the lack of SOD1 would accelerate age-related pathological changes in the human retina.
We have investigated the age-related changes of the retinas of Sod1−/− mice and found that these mice have many of the key elements of human AMD including drusen, thickened Bruch’s membrane, and CNV. Moreover, the RPE cells of these mice show signs of oxidative damage, and their junctional integrities are damaged with the disruption of β-catenin-mediated cell adhesions. Thus, our results suggest a causative role of oxidative stress in the pathogenesis of retinal degeneration and demonstrate the critical role of SOD1 in protecting the RPE from age-related degeneration.
Results
Drusen in Senescent Sod1−/− Mice.
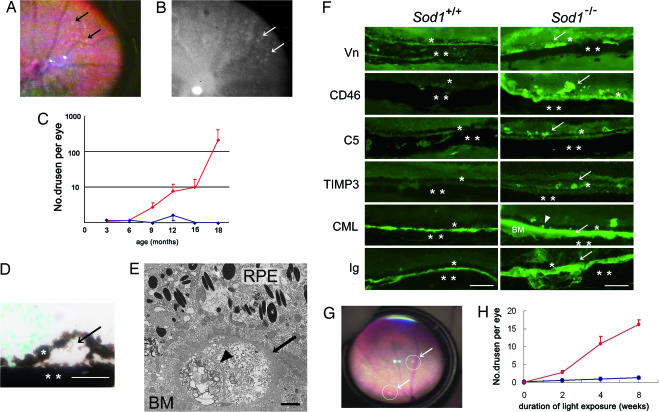
Fundus examinations were performed on 60 Sod1−/− mice (age, 3–18 months) and 50 age-matched wild-type mice. The fundi of Sod1−/− mice (n = 25) under 7 months of age appeared normal and indistinguishable from those of wild-type mice (n = 25). Mice older than 7 months showed white to slightly yellowish retinal deposits that appeared to be similar to drusen in humans (Fig. 1A). The drusen were hyperfluorescent during fluorescein angiography, as in humans (Fig. 1B), and the numbers increased with age (Fig. 1C). Drusen were detected in 31 of 36 eyes (86%) of Sod1−/− mice older than 10 months. In contrast, wild-type mice and heterozygous mice (Sod1+/−) showed very few, if any, drusen (Figs. 2A and 3 A, C, and E).
Fig. 1.
Senescent Sod1−/− mice showing drusen. (A) Fundus photograph from a 15-month-old Sod1−/− mouse. White and slightly yellowish deposits are evident (arrows). (B) Fluorescein angiogram showing hyperfluorescent areas corresponding to the sites of the drusen (arrows). (C) Plot of the number of drusen in Sod1−/− mice at 3–18 months of age, showing an increase in the number of drusen with age. Values for Sod1−/− are plotted in red, and those for Sod1+/+ are plotted in blue. (D) Dome-shaped deposit (arrow) between the RPE (∗) and choroid (∗∗) in a 12-month-old Sod1−/− mouse. (E) Electron micrograph of drusen (arrow) from a 12-month-old Sod1−/− mouse. Heterogeneous materials separated by a septum (arrowhead) are observed. The drusen appears to contain debris-like material of outer segments. BM, Bruch’s membrane. (F) Immunohistochemistry with markers for vitronectin (Vn), CD46, C5, TIMP3, CML, and Ig. Drusen and/or their surrounding tissues from 12-month-old Sod1−/− mice (Right) are positive for vitronectin, CD46, C5, TIMP3, CML, and Ig in contrast to the results for age-matched wild-type mice (Left). ∗, RPE; ∗∗, choroid; arrow, positive signal; arrowhead, positive signal of CML in RPE cell layer. (G) Fundus changes in a 5-month-old Sod1−/− mouse after continuous light exposure for 8 weeks (white fluorescent light of 10,000 lux). Drusen are present (circles and arrows). (H) The number of drusen increases with the length of light exposure in Sod1−/− mice, as compared with the minimal appearance of drusen in wild-type mice. Values for Sod1−/− are plotted in red, and those for Sod1+/+ are plotted in blue. (Scale bars: D and F, 50 μm; E, 2 μm.)
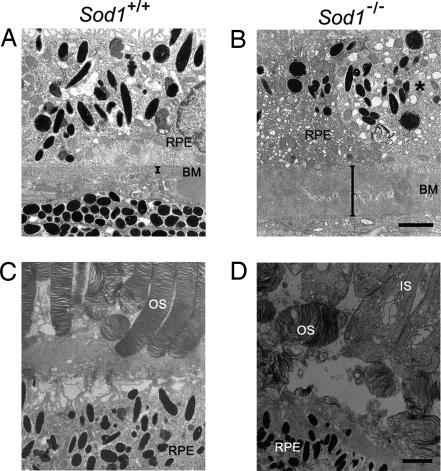
Fig. 2.
Degenerated RPE and thickened Bruch’s membrane in Sod1−/− mice. (A) Ultrastructure of Bruch’s membrane (BM) in a 12-month-old wild-type mouse. (B) Thickened Bruch’s membrane of an age-matched Sod1−/− mouse. RPE cells show marked vacuolization (∗). (C) Ultrastructure of the outer segments (OS) of the photoreceptor cells and RPE of a 12-month-old wild-type mouse. (D) Destruction of inner segments (IS), outer segments, and RPE in an age-matched Sod1−/− mouse. (Scale bars: B and D, 2 μm.)
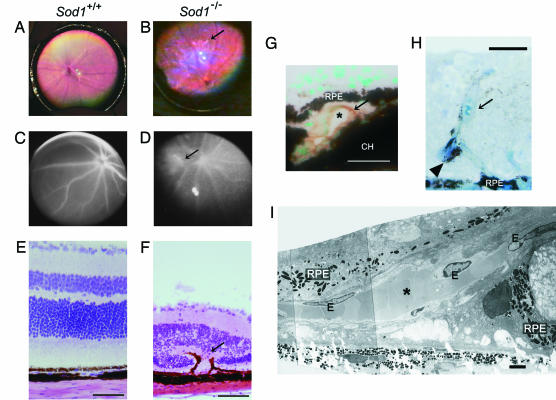
Fig. 3.
CNVs in Sod1−/− mice. (A) Fundus of a 15-month-old wild-type mouse. (B) Fundus of a 15-month-old Sod1−/− mouse showing exudative retinal changes (arrow) adjacent to the optic disk. (C) Fluorescein angiogram of the wild-type mouse reveals no abnormal findings. (D) Fluorescein angiogram reveals dye leakage (arrow) from the CNV in a 15-month-old Sod1−/− mouse. (E) Hematoxylin and eosin staining of a retinal section of a wild-type mouse. (F) Fibrovascular tissue (arrow) involving RPE in the thinned retina of the Sod1−/− mouse. A thinning of the outer nuclear layer can be seen. Hematoxylin and eosin staining. (G) CNV in a 12-month-old Sod1−/− mouse. CNV is present beneath the RPE, and the vascular cavity (∗) is surrounded by CD31-positive (diaminobenzidine) endothelial cells (arrow). Methyl green counterstaining was used. CH, choroid. (H) CNV with vessel cavity filled with erythrocytes (arrow) proliferates spirally into the sensory retina in a 12-month-old Sod1−/− mouse. RPE is attached within the intraretinal vascular structure (arrowhead). Toludine blue was used for staining. (I) Electron micrograph of CNV. CNV (∗) surrounded by endothelial cells (E) pass through a defect in Bruch’s membrane. RPE is severely disrupted. (Scale bars: E–H, 50 μm; I, 2 μm.)
Light microscopic examination of histological sections of the retinas of a 12-month-old Sod1−/− mouse showed dome-shaped deposits between the RPE and Bruch’s membrane (Fig. 1D). Heterogeneous materials were detected beneath the RPE on Bruch’s membrane by electron microscopy (Fig. 1E).
Markers of drusen, including vitronectin, a fluid-phase regulator binding the terminal component complex to regulate the control complex-mediated lysis (22); CD46, a membrane-bound regulator that facilitates inactivation of activated complement components (23); complement 5 (C5); tissue inhibitor of metalloproteinases 3 (TIMP3), a potent local inhibitor of matrix metalloproteinase regulating the rate of Bruch’s membrane turnover (24); carboxymethyl lysine (CML); and Igs were detected in the drusen by immunohistochemistry (Fig. 1F).
In a proteomics analysis by Crabb et al. (25), vitronectin, TIMP3, and CML were found in the drusen of human cadaver eyes with AMD. In the retinas of Sod1−/− mice, positive staining for CD46, C5, and Ig was observed to extend beyond the RPE, which would indicate that the inflammatory reactions occurred, not only at the site of the drusen, but also in the surrounding tissues, including the retina (Fig. 1F). These findings are consistent with recent findings that local inflammation involving Igs, complements, and complement-related genes may play a role in the pathogenesis of AMD in humans (26–29).
Induction of Drusen by Exposure to Light.
Because exposure to light (30, 31) and smoking (32) have been reported in epidemiological studies to be risk factors for AMD in humans, we attempted to induce drusen in young Sod1−/− mice by light exposure. Young Sod1−/− mice (age, 5 months) have normal retinal architecture and do not have any drusen. However, after constant white fluorescent light exposure (10,000 lux, equal to outdoor sunlight) for 24 h per day, drusen appeared (Fig. 1G), and their numbers increased with the duration of the light exposure (Fig. 1H)
Degeneration of RPE and Thickened Bruch’s Membrane in Sod1−/− Mice.
Degenerative changes of the RPE and photoreceptor cell layer are another hallmark of both the wet and dry types of AMD in humans (33). Degeneration of the RPE was observed in a 12-month-old Sod1−/− mouse by electron microscopy (Fig. 2A and B). Bruch’s membrane was markedly thickened (Fig. 2 A and B) with vacuolization of the RPE cells (Fig. 2B, asterisk) in a 12-month-old Sod1−/− mouse. The mean thickness of Bruch’s membrane in the eyes of 10- to 12-month-old Sod1−/− mice (n = 4) was 3.2 ± 1.5 μm in contrast to that in the wild-type (n = 4, 0.5 ± 0.2 μm, P < 0.05). Loss of the photoreceptor cell was also detected in 5 of 30 Sod1−/− mice (17%) that were examined histologically (representative case shown in Fig. 2 C and D). These alterations are consistent with histopathological findings in human eyes with AMD (33).
CNV in Sod1−/− Mice.
The presence of CNV is a hallmark of the wet type of AMD in which the neovascularization in the choroid extends into the RPE and subretinal space. We found that 5 of 60 Sod1−/− mice (8.3%) showed an exudative lesion by fundus examination (Fig. 3A and B), and 3 of 30 Sod1−/− mice older than 10 months (10%) had CNV by histological examination. The CNVs were confirmed by dye leakage during fluorescein angiography (Fig. 3 C and D). In contrast, the wild-type mice did not show CNV by fundus examination (n = 50) or histological examinations (n = 30).
Histological examination of the retinal sections containing the exudative lesion seen by ophthalmoscopy and area of dye leakage showed intraretinal fibrovascular tissues extending from the choroid into the retina (Fig. 3 E and F). In a 15-month-old Sod1−/− mouse, the CNV beneath the RPE can be seen to penetrate through Bruch’s membrane, and the blood vessel cavity was surrounded by cells positive for CD31, a marker for endothelial cells (Fig. 3G). A 16-month-old senescent Sod1−/− mouse had CNV penetrating into the sensory retina (Fig. 3H). Electron microscopy showed ruptures of the Bruch’s membrane and the RPE cell layer at the site of the CNV (Fig. 3I).
Oxidative Damage of RPE and Disruption of β-Catenin-Mediated Cellular Integrity of RPE in Sod1−/− Mice.
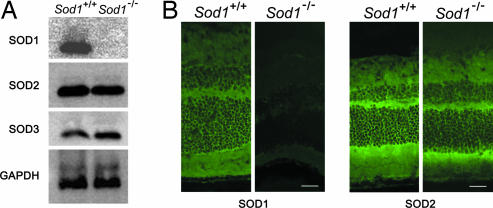
Western blot analysis showed a lack of expression of SOD1 protein in the eyes of Sod1−/− mice (n = 3). The expressions of SOD2 and SOD3 proteins in Sod1−/− mice did not differ significantly from those in wild-type mice (Fig. 4A). Histochemical analysis showed the diffuse expression of SOD1 in all layers of retina of wild-type mice and the complete absence in Sod1−/− mice, in contrast to its presence in SOD2 mice (Fig. 4B).
Fig. 4.
Expression of SOD1, SOD2, and SOD3 in the eyes of Sod1−/− mice. (A) Western blot analysis reveals the absence of SOD1 protein expression in the eyes of Sod1−/− mice. The protein levels of SOD2 (Mn-SOD) and SOD3 (extracellular SOD) appear to be the same in wild-type and Sod1−/− mice. (B) SOD1 and SOD2 expression in the retinas of wild-type and Sod1−/− mice. (Scale bars: 50 μm.)
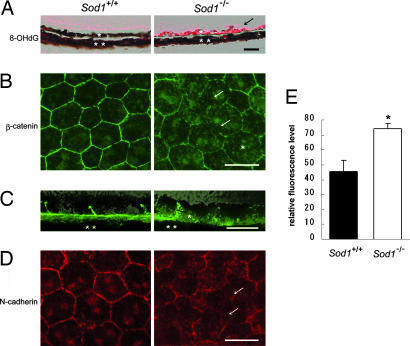
A marker of oxidative damage to DNA (34), 8-hydroxy-2′-deoxyguanosine (8-OHdG), was detected in the RPE of Sod1−/− mice but was minimally present in wild-type mice (Fig. 5A). CML, a marker of oxidative protein modification (35), was deposited in the thickened Bruch’s membrane (Fig. 1F) and RPE (Fig. 1F, arrowhead) in Sod1−/− mice, suggesting that the RPE and surrounding tissues had been damaged by oxidative processes in Sod1−/− mice.
Fig. 5.
Oxidatively damaged RPE and its disrupted β-catenin-mediated integrity in Sod1−/− mice. (A) 8-hydroxy-2′-deoxyguanosine (8-OHdG) was minimally present in the RPE of a 10-month-old wild-type mouse; however, it is markedly up-regulated in the RPE of a 10-month-old Sod1−/− mouse (arrow). ∗, RPE; ∗∗, choroid. (B) Irregular distribution of junctional proteins in RPE. β-Catenin is located at the cell walls of RPE cells in a flat-mount preparation of a 12-month-old wild-type mouse. The cytoplasmic level of β-catenin increased (∗), with reduced expression at the junctional sites (arrows). (C) A cross-section of the RPE of a 10-month-old wild-type mouse showing staining of the cell walls by β-catenin. An absence of β-catenin in the periphery and the increasing cytoplasmic level (∗) are observed in a 10-month-old Sod1−/− mouse. (D) Distribution of N-cadherin in a 12-month-old Sod1−/− mouse is severely disrupted, which is in contrast to the hexagonal expressions of N-cadherin in an age-matched wild-type mouse. Incomplete expression at the junctional sites can be seen (arrows). (E) Quantification of cytoplasmic fluorescence intensity of β-catenin in RPE cells by using the digitalized images of the flat mounts of the wild-type and the Sod1−/− mice. Randomized selected cells (n = 49) of each group are compared. Results are shown as mean ± SEM. ∗, Statistical difference between the two groups (P < 0.001, Student’s t test). (Scale bars: A, B, and D, 50 μm; C, 10 μm.)
To determine the effect of oxidative stress on the RPE, we examined whether the adherence junction proteins, which make up the barrier integrity of the RPE, were present in Sod1−/− mice (36). The location of β-catenin in the RPE of senescent Sod1−/− mice was studied in flat-mount preparations of the RPE. The presence of β-catenin was incomplete in the periphery of the RPE, indicating a disruption of the junctional integrity of the RPE (Fig. 5B, arrows) that is consistent with the light and electron microscopic observations (Figs. 1D, 2B, and 3F and I). The cytoplasmic level of β-catenin was higher in the RPE cells of a 10-month-old Sod1−/− mouse than in a wild-type mouse (Fig. 5C). Quantitative measurement of the cytoplasmic β-catenin level of the RPE cells of 12-month-old Sod1−/− mice and age-matched wild-type mice (n = 49, each group) revealed a 63.3% increase in the cytoplasmic level in the Sod1−/− mice (Fig. 5E, P < 0.001). These results are compatible with an in vitro study showing that oxidative stress changes the cellular distribution of β-catenin and that the cytoplasmic level of β-catenin increased after the exposure of hydroxyl peroxide in ARPE-19 cells, a human RPE cell line (36).
The distribution of N-cadherin was also altered in the RPE of Sod1−/− mice (Fig. 5D). The expression of junction adherence proteins, including β-catenin and N-cadherin, is necessary to maintain an epithelial phenotype. Increased cytoplasmic levels of β-catenin were observed in the RPE cells of Sod1−/− mice in vivo, suggesting that chronic oxidative damage to the RPE may change the location of β-catenin from cell walls to cytoplasm, which then leads to the destruction of cell junctions maintained by the cadherin–catenin complex.
Because RPE damage promotes CNV growth, oxidative stress to the RPE and the disruption of β-catenin-mediated integrity may possibly be one of the initial events in this AMD model.
Discussion
Our results indicated that the retinas of senescent Sod1−/− mice have many features of the retinas of patients with AMD and recapitulated well the key elements of the human pathology. We demonstrated that drusen accumulated with increasing age in these mice, and histological examination revealed that the appearance of the drusen was quite similar to those in humans. We also showed that the major components of human drusen were present in the drusen of Sod1−/− mice and that drusen could be induced in young Sod1−/− mice by exposure to light. These findings strongly suggest that oxidative stress plays a causative role in initiating drusen formation.
Current animal models of CNV mostly use laser injury to fracture Bruch’s membrane (37) or use viral transfection of angiogenic factors into RPE cells to induce CNVs (38). In our model, we used mice deficient in SOD1. The CNVs in our mice penetrated Bruch’s membrane, and fluorescein angiography showed that the CNVs had a spiraling pathway into the sensory retina.
Recently, it was reported that mice deficient in Ccl2−/− or Ccr2−/− had angiogenic leakage, implying that macrophage dysfunction can induce CNV (10). These mice had retinal degeneration and drusen-like structures that increased in number with age (10). Several other mouse models of AMD have been reported. However, according to the most recent review (15), only the Ccl2−/−, Ccr2−/−, and Sod1−/− mice have the characteristics of both drusen and CNV. Moreover, drusen can be induced by photooxidative stress in Sod1−/− mice, which seems to be compatible with the clinical finding that subjects with a longer duration of sunlight exposure are more susceptible to soft drusen (30). Because retinal degeneration after constant light exposure was similar in the wild-type and Sod1−/− mice (data not shown), the accumulation of drusen seems to be due to the increased oxidative stress to the RPE rather than to the photoreceptors.
Our results demonstrated that complex phenotypes similar to those of human AMD can be caused by deficiency of a single gene, and the movement of β-catenin to the cytoplasm may explain the destruction of the cellular integrity of RPE. This would then contribute to the formation of drusen and CNV. Because the SOD2 and SOD3 proteins are still present in Sod1−/− mice, SOD1 as a cytoplasmic scavenger of reactive oxygen species (ROS) may play a major role in protecting RPE cells from oxidative damages.
As in other neurodegenerative disorders of the CNS, the pathogenesis of human AMD has long been linked to oxidative stress, although its causative role has not been definitively determined. Indirect evidence that oxidative damage is associated with AMD comes from epidemiological studies showing that smoking and sunlight exposure significantly increase the risk of AMD (30–32). In addition, supplementation with antioxidants including vitamin C, vitamin E, β-carotene, copper, and zinc can slow the progression of the disease (39).
SOD1 is an abundant copper- and zinc-containing protein present in the cytosol, nucleus, peroxisomes, and mitochondrial intermembrane space. Its primary function is to act as an antioxidant enzyme, lowering the steady-state concentration of superoxide (20). Therefore, our results strongly suggest that oxidative stress is not merely an associated phenomenon but a primary cause of age-related retinal degeneration.
The relationship between SOD and AMD has been studied in humans. However, the activity of SOD in erythrocytes has not been correlated with AMD (40), and no clear relationship has yet been demonstrated in the analysis of the RPE of patients with AMD (41, 42). These studies seem not to be conclusive because the number of subjects is limited. Studies of SOD1 activity in the RPE involving larger numbers of patients are needed to determine the causative role of SOD1 in the pathogenesis of human AMD. It is also possible that some human AMD patients carry underexpressing mutations of the SOD1 gene.
Genetic polymorphism of the SOD2 gene, on the other hand, has been associated with AMD (43). Although a retinal thinning has been observed in mice deficient in SOD2, they die soon after birth and therefore are not suited for use in studies of long-term age-related retinal changes (44, 45).
In conclusion, we have demonstrated that the lack of SOD1 leads to the development of an animal recapitulating drusen, CNV, and RPE dysfunction, which could be a platform to validate therapies for age-related retinal degenerative disorders including AMD. Because β-catenin is a key molecule in constituting the integrity of the RPE cell layer, further research will be necessary to elucidate the means by which SOD1 deficiency interferes with the dynamics of β-catenin in the RPE.
Methods
Mice.
Sod1−/− mice on a C57BL/6 background were generated as described (46), and wild-type C57BL/6 mice were purchased from The Jackson Laboratory. Genotyping was performed by PCR as described (46). All animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Visual Research.
Fundus Photography and Angiography.
Fundus examinations were performed with an indirect ophthalmoscope (Heine Optotechnik, Herrsching, Germany) and a 28-diopter lens by two masked examiners independently. Pupils were fully dilated with 0.5% tropicamide and 0.5% phenylephrine-HCL (Santen, Osaka), the fundus was photographed with a charge-coupled device (CCD) camera (CS5130; Tokyo Electronic Industry, Tokyo), and these photographs were stored as digitalized images. Angiograms were taken with a small animal fundus camera (RC2; Kowa, Tokyo, Japan), and 60 μl of 5% sodium fluorescein (Alcon Laboratories, Fort Worth, TX) per animal was injected into the tail vein.
Immunohistochemistry.
The enucleated mouse eyes were immersed immediately in optimal cutting temperature (OCT) compound (Lab-Tek) and frozen in dry ice–ethanol for immunohistochemical or hematoxylin and eosin staining. For fluorescent labeling, frozen sections (8 μm) were fixed in ice-cold acetone or 4% paraformaldehyde in PBS, blocked with rabbit or goat serum, and stained with rabbit Ab to human CD46 (Santa Cruz Biotechnology) (10), rabbit Ab to C5 (Abcam, Cambridge, MA), mouse Ab to mouse CML (TransGenic, Kumamoto, Japan), rabbit Ab to human TIMP3 (Lab Vision, Fremont, CA), goat Ab to human vitronectin (Santa Cruz Biotechnology) (47), rabbit Ab to 8-hydroxy-2′-deoxyguanosine (8-OHdG) (Nikken SEIL, Shizuoka, Japan), rabbit Ab to rat SOD1 (Stressgen Biotechnologies, Victoria, Canada), and rabbit Ab to human SOD2 (Stressgen Biotechnologies). The specificity of the selected Abs to mouse tissues was confirmed by Western blot analysis (C5, TIMP3, SOD1, and SOD2). The secondary Abs were anti-Ig Alexa Fluor 488 (Molecular Probes) to goat, mouse, or rabbit. For the immunostaining of 8-OHdG, images with positive reactions were merged with images from a light microscope.
Electron Microscopy.
For transmission electron microscopy (TEM), tissues were fixed in 2.5% glutaraldehyde and then postfixed in 1% OsO4. The fixed tissues were dehydrated and embedded in Quetol 812 and sectioned with a diamond knife. Quetol 812 sections (2 μm) were counterstained with toluidine blue and examined with a light microscope. Ultrathin sections (2 μm) were stained in 2% uranyl acetate and lead citrate and examined with an electron microscope (JEM-1200EX; JEOL).
Light Exposure.
Five-month-old Sod1−/− and wild-type mice were exposed to constant white fluorescent light (Toshiba Lighting and Technology, Tokyo) in a light box surrounded by mirrors (Tinker-N, Kyoto, Japan) for 24 h per day for 8 weeks. Fundus examination was performed every 2 weeks. The light intensity was 10,000 lux. Digitalized fundus images were recorded from the eyes of Sod1−/− (n = 25) and wild-type (n = 25) mice, and the number of drusen was counted by two examiners masked to the type of animal.
Western Blot Analysis.
Proteins extracted from the ocular tissues of 10- to 12-month-old Sod1−/− and wild-type mice were examined by Western blot using rabbit Abs to SOD1, SOD2, SOD3 (Stressgen Biotechnologies), and GAPDH (Biogenesis, Bournemouth, U.K.).
Flat-Mount Preparations and Digital Images.
The enucleated eyes from 12-month-old Sod1−/− mice and age-matched wild-type mice (n = 4, each group) were sectioned at the equator, and the anterior half, including the lens and vitreous, was discarded. The retinas were carefully peeled from the eyecup and optic nerve by using specialized scissors and forceps under a biomicroscope (SMZ-10; Nikon). The posterior eye segment containing the sclera and choroid was dissected into quarters by four radial cuts, mounted, and air-dried. Flat mounts were examined with a fluorescence microscope (BX51; Olympus, Melville, NY). The flat mounts were stained with mouse monoclonal Ab to β-catenin (Bioscience Laboratories, Bozeman, MT) or N-cadherin (Bioscience Laboratories), preconjugated with anti-mouse Ig Alexa Fluor 488 or 594 (Molecular Probes). Fluorescein images of β-catenin were analyzed after normalization to the background, and the degree of fluorescence of the cytoplasm of each cell was calculated from average pixel intensities by using commercial software (Adobe Photoshop CS; Adobe Systems, San Jose, CA).
Statistical Analyses.
All data are presented as means ± SEM, and the differences were analyzed with Student’s t test. P values <0.05 were considered statistically significant.
Acknowledgments
We thank Yuka Kondo, Manabu Hirasawa, Yutaka Ohta, Ei-ichiro Nagasaka, and Masao Yoshikawa for technical assistance; Yumi Takanashi, Naoko Takayasu, and Junko Moriya for assisting with histology; Minoru Seki for angiography; Murat Dogru, Yoji Takano, Catherine Oshima, and Duco Hamasaki for reviewing the manuscript; Yasushi Inaida for financial support; and Makoto Suematsu for critical comments in interpretation of data.
Abbreviations
- AMD
age-related macular degeneration
- CNV
choroidal neovascularization
- RPE
retinal pigment epithelium
- SOD
superoxide dismutase
- C5
complement 5
- CML
carboxymethyl lysine.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sommer A., Tielsch J. M., Katz J., Quigley H. A., Gottsch J. D., Javitt J. C., Martone J. F., Royall R. M., Witt K. A., Ezrine S. N. Engl. J. Med. 1991;325:1412–1417. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 2.Smith W., Assink J., Klein R., Mitchell P., Klaver C. C., Klein B. E., Hofman A., Jensen S., Wang J. J., de Jong P. T. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 3.Attbo K., Mitchell P., Smith W. Ophthalmology. 1996;103:357–364. doi: 10.1016/s0161-6420(96)30684-2. [DOI] [PubMed] [Google Scholar]
- 4.VanNewkirk M. R., Weih L., McCarty C. A., Taylor H. R. Ophthalmology. 2001;108:960–967. doi: 10.1016/s0161-6420(01)00554-1. [DOI] [PubMed] [Google Scholar]
- 5.Oshima Y., Ishibashi T., Murata T., Tahara Y., Kiyohara Y., Kubota T. Br. J. Ophthalmol. 2001;39:367–372. doi: 10.1136/bjo.85.10.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird A. C., Bressler N. M., Bressler S. B., Chisholm I. H., Coscas G., Davis M. D., de Jong P. T., Klaver C. C., Klein B. E., Klein R., et al. Surv. Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 7.Smiddy W. E., Fine S. L. Ophthalmology. 1984;91:271–277. doi: 10.1016/s0161-6420(84)34309-3. [DOI] [PubMed] [Google Scholar]
- 8.Wang J. J., Foran S., Smith W., Mitchell P. Arch. Ophthalmol. 2003;121:658–663. doi: 10.1001/archopht.121.5.658. [DOI] [PubMed] [Google Scholar]
- 9.Hahn P., Qian Y., Dentchev T., Chen L., Beard J., Harris Z. L., Dunaief J. L. Proc. Natl. Acad. Sci. USA. 2004;101:13850–13855. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambati J., Anand A., Fernandez S., Sakurai E., Lynn B. C., Kuziel W. A., Rollins B. J., Ambati B. K. Nat. Med. 2003;11:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida T., Ohno-Matsui K., Ichinose S., Sato T., Iwata N., Saido T. C., Hisatomi T., Mochizuki M., Morita I. J. Clin. Invest. 2005;115:2793–2800. doi: 10.1172/JCI24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malek G., Johnson L. V., Mace B. E., Saloupis P., Schmechel D. E., Rickman D. W., Toth C. A., Sullivan P. M., Bowes Rickman C. Proc. Natl. Acad. Sci. USA. 2005;102:11900–11905. doi: 10.1073/pnas.0503015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rackoczy P. E., Zhang D., Robertson T., Barnett N. L., Papadimitriou J., Constable I. J., Lai C.-M. Am. J. Pathol. 2002;161:1515–1524. doi: 10.1016/S0002-9440(10)64427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng J., Mata N. L., Azarian S. M., Tzekov R. T., Birch D. G., Travis G. H. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 15.Rakoczy P. E., Yu M. J. T., Nusinowitz S., Chang B., Heckenlively J. R. Exp. Eye Res. 2006;82:741–752. doi: 10.1016/j.exer.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Andersen J. K. Nat. Med. 2004;10:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 17.Harman D. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 18.Beatty S., Koh H. H., Henson D., Boulton M. Surv. Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 19.Hahn P., Milam A. H., Dunaief J. L. Arch. Ophthalmol. 2003;121:1099–1105. doi: 10.1001/archopht.121.8.1099. [DOI] [PubMed] [Google Scholar]
- 20.Valentine J. S., Doucette P. A., Potter S. Z. Annu. Rev. Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 21.Behndig A., Svensson B., Marklund S. L., Karlsson K. Invest. Ophthalmol. Vis. Sci. 1998;39:471–475. [PubMed] [Google Scholar]
- 22.Hageman G. S., Mullins R. F., Russell S. R., Johnson L. V., Anderson D. H. FASEB. J. 1999;13:477–484. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- 23.Johnson L. V., Leitner W. P., Staples M. K., Anderson D. H. Exp. Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 24.Kamei M., Hollyfield J. G. Invest. Ophthalmol. Vis. Sci. 1999;40:2367–2375. [PubMed] [Google Scholar]
- 25.Crabb J. W., Miyagi M., Gu X., Shadrach K., West K. A., Sakaguchi H., Kamei M., Hasan A., Yan L., Rayborm M. E., et al. Proc. Natl. Acad. Sci. USA. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hageman G. S., Luthert P. J., Victor Chong N. H., Johnson L. V., Anderson D. H., Mullins R. F. Prog. Retin. Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 27.Haines J. L., Hauser M. A., Schmidt S., Scott W. K., Olson L. M., Gallins P., Spencer K. L., Kwan S. Y., Noureddine M., Gilbert L. R., et al. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 28.Edwards A. O., Ritter R., III, Abel K. J., Manning A., Panhuysen C., Farrer L. A. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 29.Klein R. J., Zeiss C., Chew E. Y., Tsai J. Y., Sackler R. S., Haynes C., Henning A. K., SanGiovanni J. P., Mane S. M., Mayne S. T., et al. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruickshanks K. J., Klein R., Klein B. E., Nondahl D. M. Arch. Ophthalmol. 1993;115:514–518. doi: 10.1001/archopht.1993.01090040106042. [DOI] [PubMed] [Google Scholar]
- 31.Wenzel A., Grimm C., Samardzija M., Reme C. E. Prog. Retin. Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Vingerling J. R., Hoffman A., Grobbee D. E., de Jong P. T. Arch. Ophthalmol. 1996;114:1193–1196. doi: 10.1001/archopht.1996.01100140393005. [DOI] [PubMed] [Google Scholar]
- 33.Green R. G., Harlan J. B. In: Age-Related Macular Degeneration. Berger J. W., Fine S. L., Maguire M. G, editors. St. Louis: Mosby; 1999. pp. 81–154. [Google Scholar]
- 34.Kasai H., Chung M. H., Jones D. S., Inoue H., Ishikawa H., Kamiya H., Ohtsuka E., Nishimura S. J. Toxicol. Sci. 1991;16:95–105. doi: 10.2131/jts.16.supplementi_95. [DOI] [PubMed] [Google Scholar]
- 35.Ishibashi T., Murata T., Hangai M., Nagai R., Horiuchi S., Lopez P. F., Hinton D. R., Ryan S. J. Arch. Ophthalmol. 1998;116:1629–1632. doi: 10.1001/archopht.116.12.1629. [DOI] [PubMed] [Google Scholar]
- 36.Bailey T. A., Kanuga N., Romero I. A., Greenwood J., Luthert P. J., Cheetham M. E. Invest. Ophthalmol. Vis. Sci. 2004;45:675–684. doi: 10.1167/iovs.03-0351. [DOI] [PubMed] [Google Scholar]
- 37.Semkova I., Peters S., Welsandt G., Janicki H., Jordan J., Schraermeyer U. Invest. Ophthalmol. Vis. Sci. 2003;44:5349–5354. doi: 10.1167/iovs.02-0732. [DOI] [PubMed] [Google Scholar]
- 38.Schwesinger C., Tee C., Rohan R. M., Joussen A. M., Fernandez A., Meyer T. N., Poulaki V., Ma J. J., Redmond T. M., Liu S., et al. Am. J. Pathol. 2001;158:1161–1172. doi: 10.1016/S0002-9440(10)64063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Age-Related Eye Disease Study Research Group. Arch. Ophthalmol. 2001;119:1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delcourt C., Cristol J. P., Leger C. L., Descomps B., Papoz L. Ophthalmology. 1999;106:215–222. doi: 10.1016/s0161-6420(99)90059-3. [DOI] [PubMed] [Google Scholar]
- 41.Liles M. R., Newsome D. A., Oliver P. D. Arch. Ophthalmol. 1991;109:1285–1288. doi: 10.1001/archopht.1991.01080090111033. [DOI] [PubMed] [Google Scholar]
- 42.Frank R. N., Amin R. H., Puklin J. E. Am. J. Ophthalmol. 1999;127:694–709. doi: 10.1016/s0002-9394(99)00032-x. [DOI] [PubMed] [Google Scholar]
- 43.Kimura K., Isashiki Y., Sonoda S., Kakiuchi-Matsumoto T., Ohba N. Am. J. Ophthalmol. 2000;130:769–773. doi: 10.1016/s0002-9394(00)00552-3. [DOI] [PubMed] [Google Scholar]
- 44.Li Y., Huang T. T., Carlson E. J., Melov S., Ursell P. C., Olson J. L., Noble L. J., Yoshimura M. P., Berger C., Chen P. H., et al. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 45.Sandbach J. M., Coscun P. E., Grossniklaus H. E., Kokoszka J. E., Newman N. J., Wallace D. C. Invest. Ophthalmol. Vis. Sci. 2001;42:2173–2178. [PubMed] [Google Scholar]
- 46.Matzuk M. M., Dionne L., Guo Q., Kumar T. R., Lebovitz R. M. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- 47.Teesalu T. T., Hinkkanen A. E., Vaheri A. Am. J. Pathol. 2001;159:2227–2237. doi: 10.1016/S0002-9440(10)63073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]