Abstract
Retinitis pigmentosa (RP) is a label for a group of diseases caused by a large number of mutations that result in rod photoreceptor cell death followed by gradual death of cones. The mechanism of cone cell death is uncertain. Rods are a major source of oxygen utilization in the retina and, after rods die, the level of oxygen in the outer retina is increased. In this study, we used the rd1 mouse model of RP to test the hypothesis that cones die from oxidative damage. A mixture of antioxidants was selected to try to maximize protection against oxidative damage achievable by exogenous supplements; α-tocopherol (200 mg/kg), ascorbic acid (250 mg/kg), Mn(III)tetrakis (4-benzoic acid) porphyrin (10 mg/kg), and α-lipoic acid (100 mg/kg). Mice were treated with daily injections of the mixture or each component alone between postnatal day (P)18 and P35. Between P18 and P35, there was an increase in two biomarkers of oxidative damage, carbonyl adducts measured by ELISA and immunohistochemical staining for acrolein, in the retinas of rd1 mice. The staining for acrolein in remaining cones at P35 was eliminated in antioxidant-treated rd1 mice, confirming that the treatment markedly reduced oxidative damage in cones; this was accompanied by a 2-fold increase in cone cell density and a 50% increase in medium-wavelength cone opsin mRNA. Antioxidants also caused some preservation of cone function based upon photopic electroretinograms. These data support the hypothesis that gradual cone cell death after rod cell death in RP is due to oxidative damage, and that antioxidant therapy may provide benefit.
Keywords: oxidative damage, photoreceptors, retinal degenerations
Retinitis pigmentosa (RP) refers to a group of diseases in which a mutation results in death of rod photoreceptors followed by gradual death of cones. The diseases referred to as RP show a similar phenotype consisting of pigmented spots scattered throughout the retina, narrowed retinal vessels, and retinal sheen suggesting atrophy. At one time, it was felt that inflammation was important in the pathogenesis, and hence the term “retinitis” was paired with a descriptor for the most prominent feature of the phenotype, the scattered pigment, resulting in the term retinitis pigmentosa. It is now known that this phenotype results whenever there is widespread rod photoreceptor cell death. Although toxins or infections can occasionally cause widespread rod death, it occurs most commonly when there is a mutation in a gene that is selectively expressed in rods and, either through gain or loss of function, the mutation promotes rod cell death. Mutations in 36 different genes have been found to cause RP, and mutations in many more cause widespread rod cell death in association with syndromes that have extraocular manifestations (www.sph.uth.tmc.edu/RetNet/sum-dis.htm).
How does diffuse death of rods throughout the retina result in the distinctive phenotype that ophthalmologists recognize as RP? Rods have an intimate relationship, both structurally and functionally, with retinal pigmented epithelial (RPE) cells. Rods require RPE cells for visual transduction and survival, and although the death of rods does not cause the immediate demise of RPE cells, it sets in motion a series of changes that after a substantial delay, often several years, causes RPE cells to migrate into the retina and surround retinal blood vessels (1). It appears that the death of rods leads to a gradual change in retinal blood vessels that causes them to release a chemoattractant for RPE cells stimulating their transretinal migration. It is remarkable that the death of rods can have these remote effects long after they have departed.
The loss of rods has delayed effects on other cells in addition to vascular and RPE cells. After the death of rods, cone photoreceptors begin to die. Depletion of rods is responsible for night blindness, the first symptom of RP, but it is the death of cones that is responsible for the gradual constriction of the visual fields and eventual blindness. The mechanism of the gradual death of cones is one of the key unsolved mysteries of RP. It appears that cones depend upon rods for survival, and that once rods die, the death of cones is inevitable, although the rate of cone death can vary greatly even among siblings with the same disease-causing mutation.
There are three prevailing theories of why cones depend upon rods for their survival. One is that as rods die, they release toxic agents that kill cones (2). A second hypothesis is that microglia activated by ongoing rod death migrate to the photoreceptor layer and secrete toxic substances that kill cones (3). The third theory is that rods produce a survival factor that is required by cones (4). These theories cannot explain why cones often survive for years after all rods have disappeared or the tremendous variability in the timing of cone cell death among individuals.
Another possible explanation for the slowly progressive death of cones after the death of rods is oxidative damage. Rods are more numerous than cones and are metabolically active cells with a high level of oxygen consumption. Choroidal vessels are not subject to autoregulation by tissue oxygen levels and as death of rods occurs, the level of oxygen in the retina increases (5, 6). It has been postulated that the narrowing of retinal vessels, one of the characteristic features of the RP phenotype, is due to high levels of oxygen in the retina (7), because retinal vessels are capable of autoregulation and constrict when tissue levels of oxygen are high. Studies in mouse models of RP have demonstrated that after the death of rods, retinal vessels not only constrict, but they also regress, so that the vascular density in the retina is significantly reduced (8). We postulated that after the death of rods, hyperoxia causes down-regulation of the constitutive expression of VEGF and other survival factors for vascular cells, which results in pruning of retinal vessels. To test this hypothesis, we maintained adult mice in 75% oxygen for several weeks, which has been demonstrated to increase tissue levels of oxygen in the retina. We found that VEGF expression was reduced as predicted, but we also found something that was not predicted: the degeneration of photoreceptors (9, 10). A search of the literature demonstrated that this same observation had been made in 1955 (11), but apparently its significance was not recognized. It is a very important observation, because it dramatically emphasizes the potential deleterious effects (presumably from oxidative damage) of high oxygen levels to photoreceptors.
In a transgenic pig model of RP, we found unequivocal evidence of progressive oxidative damage in cones after the loss of rods (12); however, although the association of progressive oxidative damage with cone cell death is suggestive that cones die from oxidative damage, it does not constitute proof. To prove the hypothesis, it is necessary to demonstrate in a model of RP that protection from oxidative damage reduces cone cell death. We used a mouse model of RP to apply this definitive test to our hypothesis and herein report the results.
Results
Time Course of Cone Degeneration in rd1 Mice.
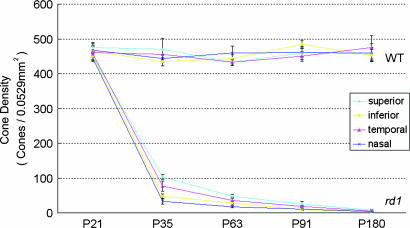
In mice homozygous for the rd1 mutation (rd1/rd1 mice), rod photoreceptor degeneration begins around postnatal day (P)10 and is almost completed by P21, whereas cone cell numbers are essentially normal at P21 and are down to undetectable levels by 2–4 months of age (13). The time course of cone cell loss was mapped in the retinas of rd1/rd1 mice in a C57BL/6 background and, because topographical differences have been noted in the rate of cone loss (14), the same 230 × 230-μm regions 1 mm superior, inferior, temporal, and nasal to the optic nerve were studied in each mouse (Fig. 8, which is published as supporting information on the PNAS web site). Cone density did not show any significant change in any of the four regions of the retina up to 6 months of age in wild-type C57BL/6 mice (Fig. 1). The number of cones in the retinas of rd1/rd1 mice was similar to that in wild-type mice at P21, but by P35, it was <25%. Cones continued to decrease over the next several months and were essentially eliminated between 3 and 6 months. Because P35 is within the time frame during which many cones are dying, and yet there are still ≈20% remaining, it was chosen as an endpoint for analyses.
Fig. 1.
The change in cone cell density over time in different regions of the retina in rd1 mice in a C57BL/6 genetic background. At several time points between P21 and P180, C57BL/6 mice, either wild-type or homozygous for the rd1 mutation (n = 6 for each at each time point), had cone cell density quantified in 0.0529-mm2 bins 1 mm superior, inferior, temporal, or nasal to the center of the optic nerve, as illustrated. Each point shows the mean (±SEM) calculated from measurements in six mice. In wild-type mice, there was no difference over time in cone cell density and no difference in different regions of the retina. In rd1 mice, there was a rapid decline in cone cell density between P21 and P35 and then a more gradual decline over the next 2 months. Cones were still detectable at P91 but not at P180. The rate of decline was greatest in the inferior and nasal parts of the retina and least in the superior part of the retina.
Increased Staining for a Marker of Oxidative Damage in Cones of rd1 Mice Is Reduced by Antioxidants.
Acrolein is a product of lipid peroxidation (15, 16) and is a biomarker that has been used to implicate oxidative damage in several diseases (17–20). Immunohistochemical staining of the retinas of rd1 mice with an antibody that specifically recognizes acrolein showed faint staining at P18 (Fig. 2, column 2, row 2), and by P35, there was a substantial increase in staining (column 2, row 3). There was colocalization of acrolein and peanut agglutinin (PNA), which labels cones (column 3, row 3) indicating there was oxidative damage in cones during this period of cone cell death. Systemic treatment of rd1 mice with a mixture of antioxidants including α-tocopherol, ascorbic acid, Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), and α-lipoic acid, essentially eliminated the staining for acrolein in P35 rd1 mouse retina, indicating that this regimen was able to markedly reduce oxidative damage (column 2, row 4).
Fig. 2.
A mixture of antioxidants prevents progressive lipid peroxidation in the retinas of rd1 mice between P18 and P35. rd1 mice were killed at P18 or divided into two groups and given daily injections between P18 and P35 of vehicle or vehicle containing a mixture of four antioxidants, α-tocopherol, MnTBAP, ascorbic acid, and α-lipoic acid. Ocular sections were stained with an antibody that specifically recognizes acrolein–protein adducts (a biomarker for lipid peroxidation) and a secondary FITC-labeled antibody (column 2), rhodamine-conjugated PNA that stains cones (column 1), and Hoechst, which stains all cell nuclei (column 4; to conserve space, the outer nuclear layer is labeled cones, but there are still some rods remaining at P18). All of the sections shown in column 2 were stained at the same time, and the results were identical in two mice from each group. There was some mild staining for acrolein in P18 rd1 mouse retina (column 2, row 2), and it was increased in P35 rd1 mouse retina (column 2, row 3). There was no detectable acrolein staining in cones at P18, but there was strong staining in remaining cones at P35 (columns 2 and 3, row 3) and increased staining in the inner nuclear layer (INL). Treatment with antioxidants between P18 and P35 resulted in a marked decrease in acrolein staining in rd1 mouse retina at P35 (column 2, row 4). The cones in vehicle-treated retinas from P35 rd1 mice are yellow in merged images (column 3, row 3), indicating oxidative damage in cones, and the lack of yellow staining in antioxidant-treated P35 rd1 mice (column 3, row 4) indicates that the oxidative damage in cones was substantially reduced by the antioxidant treatment.
Antioxidants Reduce Carbonyl Adducts in rd1 Mouse Retina.
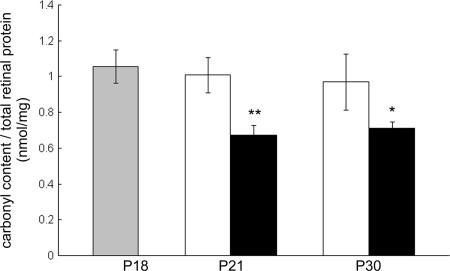
When proteins undergo oxidative damage, the most common modification is introduction of carbonyl groups into side chains (21), and ELISA for carbonyl adducts provides a quantitative measure of oxidative damage (22, 23). Starting at P18, rd1 mice were treated with the mixture of four antioxidants listed above or vehicle. Compared to those treated with vehicle, rd1 mice treated with antioxidants showed a significant reduction in carbonyl adducts per milligram of protein of retina at both P21 and P30 (Fig. 3). This confirms that the mixture of antioxidants was able to reduce oxidative damage in rd1 mouse retina.
Fig. 3.
A mixture of antioxidants significantly reduces carbonyl adducts on proteins in the retinas of rd1 mice. rd1 mice were killed at P18 or divided into two groups and given daily injections between P18 and P30 of vehicle or vehicle containing a mixture of four antioxidants, α-tocopherol, MnTBAP, ascorbic acid, and α-lipoic acid. The retina was dissected from one eye of each mouse, and retinal lysates were assayed for carbonyl adducts by ELISA (a quantitative measure of protein oxidation), as described in Materials and Methods. The bars show the mean (±SEM) carbonyl content (nmol per mg protein of retina) calculated from five mice. There was no significant difference in carbonyl content in the retinas of P18 rd1 mice and the retinas of rd1 mice treated with vehicle between P18 and P21 or between P18 and P30. The amount of carbonyl adducts on proteins in retinas of rd1 mice treated with antioxidants was significantly less than their corresponding vehicle control at P21 and P30 (∗, P < 0.02; ∗∗, P < 0.0005 by unpaired Student’s t test).
Antioxidants Reduce Cone Cell Death in rd1 Mouse Retina.
PNA selectively stains cone inner and outer segments, and examination of PNA-stained retinal flat mounts by confocal microscopy allows visualization of inner and outer segments and quantification of cone density (Fig. 4A). In rd1 mouse retina, cone inner segments are blunted and irregular when examined at low power, but at high power, individual inner segments can be distinguished and counted. However, low-power views provide a rapid means of judging cone density. At P21, cone density in the superior portion of rd1 retina appears similar in mice treated with the mixture of four antioxidants or vehicle starting at P18 (Fig. 4B, first column). By P35, cone density was markedly decreased in the superior retina of rd1 mice treated with vehicle but was only minimally decreased in the superior retina of rd1 mice treated with antioxidants (Fig. 4B, second column). Compared to vehicle-treated mice, antioxidant-treated mice also had greater cone density in the inferior, temporal, and nasal parts of the retina. Quantification of cone density showed no differences between antioxidant- and vehicle-treated mice in any of the four regions of the retina at P21, but by P35, antioxidant-treated rd1 mice showed significantly higher numbers of cones in each of the four regions (Fig. 4C).
Fig. 4.
Antioxidants promote cone survival in rd1 mice. (A) Retinal sections or flat mounts stained with PNA show cone inner segments (IS) in P21 wild-type and rd1 mice. Retinal sections are counterstained with Hoechst to show the cell layers including the ganglion cell layer (GCL), the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), and the outer nuclear layer (ONL). The cone IS are markedly flattened in the retinas of rd1 mice compared to wild-type mice, and the ONL is reduced to a single layer of predominantly cone cell bodies. 3D reconstruction of confocal images of a PNA-stained retina from a wild-type mouse (column 1, row 3) shows normal cone IS. In rd1 mice, the cone IS are flattened, and their arrangement is somewhat disorganized (column 2, row 3). OS, outer segment. (B) At P18, rd1 mice started receiving daily injections of vehicle or vehicle containing a mixture of four antioxidants, α-tocopherol, MnTBAP, ascorbic acid, and α-lipoic acid. Mice were killed at P21 or P35, and one eye was used for retinal flat mounts, which were stained with PNA; the other retina was dissected and used for real-time RT-PCR (Fig. 5). Confocal images of cone inner segments in 0.0529-mm2 bins 1 mm superior, inferior, temporal, or nasal to the center of the optic nerve are shown. At P21, all of the images looked very similar, and so only those from the superior region of the retina of vehicle- and antioxidant-treated mice are shown. At P35, compared to vehicle-treated mice, antioxidant-treated rd1 mice appeared to have greater cone density in all four regions of the retina. (C) Quantification of cone density in each of the four 0.0529-mm2 bins for each retina was done as described in Materials and Methods. Each bar represents the mean (±SEM) calculated from measurements in 10 mice. There were no significant differences between vehicle- and antioxidant-treated mice at P21, but at P35, cone density was significantly higher in antioxidant-treated mice in all four regions of the retina. ∗, P < 1.0 × 10−6; ∗∗, P < 1.0 × 10−11; ∗∗∗, P < 1.0 × 10−12 by unpaired Student’s t test for difference from corresponding vehicle control.
Antioxidants Slow the Reduction in Medium-Wavelength Cone (m-Cone) and mRNA in rd1 Mouse Retina.
Cone outer and inner segments are compromised relatively early in the degeneration process, whereas cone cell bodies are maintained. The cell body is the site of mRNA synthesis, and therefore levels of mRNA for cone-specific genes should provide some assessment of the level of functioning of the cell bodies. At P21, there was no difference in the amount of m- or short-wavelength cone (s-cone) mRNA in the retinas of rd1 mice that had been treated with antioxidants compared to those treated with vehicle (Fig. 5). At P35, there was significantly more m-cone opsin mRNA in the retinas of rd1 mice that had been treated with antioxidants compared to those treated with vehicle, whereas the difference for s-cone opsin mRNA was less and was not statistically significant. This suggests that antioxidants help to preserve mRNA synthesis in the cell bodies of at least one population of cones.
Fig. 5.
Antioxidants reduce loss of cone-specific mRNAs in retinas of rd1 mice. At P18, rd1 mice started receiving daily injections of vehicle or vehicle containing a mixture of four antioxidants, α-tocopherol, MnTBAP, ascorbic acid, and α-lipoic acid. Mice were killed at P21 (n = 10 for each group) or P35 (n = 10 for each group), and one eye was used to isolate total retinal RNA. Real-time RT-PCR was done using primers specific for m- or s-cone opsin mRNA. Each bar represents the mean value (±SEM) calculated from 10 mice and normalized to the value for the P21 rd1 vehicle group, which was set to 1.00. Values for P21 rd1 antioxidant-treated mice were nearly identical to the corresponding P21 vehicle group for both mRNAs. At P35, the level of m-cone opsin mRNA was significantly higher in antioxidant- compared to vehicle-treated mice (∗, P < 0.01 by unpaired Student’s t test), but there was not a statistically significant difference in s-cone opsin mRNA levels between the two groups.
α-Tocopherol and α-Lipoic Acid Reduce Cone Cell Death in rd1 Mouse Retina.
We initially used a mixture of antioxidants to maximize our chances of seeing an effect if one existed. Because there was a robust effect with the mixture of antioxidants, we sought to determine whether any of the antioxidants, when used alone, had a detectable effect on cone survival. Each of the antioxidants was compared to administration of its own vehicle in separate independent experiments. At P35, rd1 mice treated with α-tocopherol in olive oil had significantly greater cone cell density than their comparison group of mice treated with only olive oil (Fig. 6A). In contrast, the use of MnTBAP or ascorbic acid had no significant effect (Fig. 6 B and C). The results with α-lipoic acid were interesting. Compared to control rd1 mice treated with 30% ethanol in PBS, cone density was significantly greater in three of four regions of the retina in rd1 mice treated with α-lipoic acid in 30% ethanol in PBS (Fig. 6D). This control group had somewhat lower cone densities than other groups, suggesting a potential deleterious effect of 30% ethanol in PBS; however, despite this, the results suggest that α-lipoic acid and α-tocopherol promote survival of cones after widespread death of rods.
Fig. 6.
Daily injections of α-tocopherol or α-lipoic acid between P18 and P35 promote cone survival in rd1 mice. Four independent experiments were done in which, starting at P18, rd1 mice were given daily injections of one of four antioxidants (n = 10 for each), and each of the four groups was compared to its own vehicle-injection group (n = 10 for each). The four antioxidants were α-tocopherol (200 mg/kg in olive oil; A), MnTBAP (10 mg/kg in PBS; B), ascorbic acid (250 mg/kg in PBS; C), and α-lipoic acid (100 mg/kg in PBS containing 30% ethanol; D). At P35, mice were killed, and the retina from one eye was flat-mounted and stained with PNA; cone density was quantified in 0.0529-mm2 bins 1 mm superior, inferior, temporal, or nasal to the center of the optic nerve using confocal microscopy as described in Materials and Methods. Each bar represents the mean (±SEM) calculated from values obtained from 10 mice. Mice treated with α-tocopherol showed significantly higher cone density in three of four regions of the retina (∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 5.0 × 10−5 by unpaired Student’s t test). Compared to their respective vehicle controls run in parallel, there was no significant difference in cone density in any region of the retina in mice treated with MnTBAP or ascorbic acid. Mice treated with α-lipoic acid had significantly higher cone densities in three of four regions of the retina compared to corresponding vehicle control mice (†, P < 0.05; ††, P < 0.01, by unpaired Student’s t test). The values for the α-lipoic acid vehicle control group were lower than those for the other three vehicle control groups, suggesting there may have been a deleterious effect for the PBS containing 30% ethanol vehicle.
Antioxidants Preserve Cone Function in rd1 Mice.
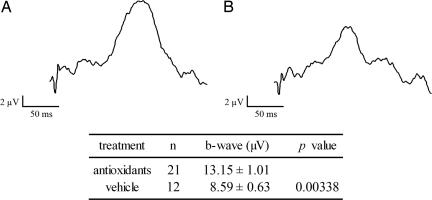
Starting at P18, rd1 mice were treated with the mixture of four antioxidants listed above or vehicle and at P27, photopic electroretinograms (ERGs) were performed by an investigator masked with respect to treatment group. Compared to those treated with vehicle, rd1 mice treated with antioxidants had a significantly greater mean b-wave amplitude (Fig. 7) indicating some preservation of cone function.
Fig. 7.
Antioxidants provide partial preservation of cone function in rd1 mice. rd1 mice were given daily injections between P18 and P27 of vehicle or vehicle containing a mixture of four antioxidants, α-tocopherol, MnTBAP, ascorbic acid, and α-lipoic acid. Photopic ERGs were done at P27, as described in Materials and Methods, with investigator masked with respect to treatment group. Representative wave forms are shown for the antioxidant (A) and vehicle groups (B), and measurements showed that mean b-wave amplitudes were significantly higher in the antioxidant group by unpaired Student’s t test.
Discussion
In previous studies, we have shown that placement of adult mice in 75% oxygen for ≥2 weeks causes extensive death of photoreceptors, demonstrating the potential severe negative consequences of oxidative damage in photoreceptors (9, 10, 24). Using oxygen electrodes, it has been shown that oxygen levels are significantly increased in the outer retina after rods degenerate (5). We have also shown in a transgenic pig model of RP that after disappearance of rods, there is progressive oxidative damage in cones during the period when cones are dying (12). In this study, we showed that in the rd1 mouse model of RP, staining for acrolein, a marker for oxidative damage to lipids, is not detectable in cones at P18 when rod degeneration is just being completed, but by P35, ≈2 weeks after the disappearance of most rods and in midst of cone cell death, remaining cones stain for acrolein. This suggests that in rd1 mice with RP, similar to transgenic pigs with RP, widespread loss of rods is followed by oxidative damage in cones.
We sought an antioxidant regimen that would have a high likelihood of substantially reducing oxidative stress in cones of rd1 mice. In various models, three agents, α-tocopherol, ascorbic acid, and α-lipoic acid, have been shown to reduce one or more types of oxidative stress (25–30). Previous work has suggested that these agents may have additive effects when used together (31). Another potent antioxidant that is adept at reducing oxidative damage in mitochondria, MnTBAP, does not cross the blood–brain or blood– retinal barriers under normal circumstances (32), but because the blood–retinal barrier is compromised in RP, MnTBAP was also added to the regimen. Daily systemic injections of the mixture starting at P18, the time of onset of cone cell death, reduced staining for acrolein in cones at P35 to undetectable levels, indicating that the treatment reduced oxidative damage in cones. Using a quantitative measurement of the amount of oxidative damage in the retina, ELISA for carbonyl adducts (22, 23), it was confirmed that the mixture of antioxidants was able to have the desired effect of reducing oxidative damage in the retina. This reduction of oxidative damage resulted in a significant increase in the number of surviving cones at P35, proving that oxidative damage contributes to cone cell death in the rd1 model of RP. The hypothesis was also supported by the demonstration that antioxidant-treated rd1 mice also had a significant increase in m-cone opsin mRNA levels at P35 compared to vehicle-treated mice. The lack of a significant difference in s-cone opsin mRNA suggests that s-cones are not benefited to the same extent as m-cones by the mixture of antioxidants that were used. A possible explanation is a difference in the endogenous oxidative damage defense system in the two cell types that makes s-cones more vulnerable to oxidative damage and less salvageable by exogenous antioxidants. We are currently investigating differences in the endogenous oxidative damage defense systems of rods and cones; doing this for cone subtypes is a much greater technical hurdle, but may also provide important information.
We were not optimistic that a single agent could provide enough antioxidant activity to have detectable effects. Thus, we were surprised to find that compared to paired vehicle controls, daily administration of either α-tocopherol or α-lipoic acid as monotherapy also resulted in a significant increase in cone cell number at P35 in rd1 mice. These data provide additional support for the hypothesis that oxidative damage is an important contributor to cone cell death in RP. It may be significant that both of these agents are lipid soluble, which may allow for more sustained levels and better access to appropriate cellular compartments.
The demonstration that oxidative damage contributes to cone cell death in RP has important clinical implications. It provides a therapeutic target that may apply to all (or most) RP patients. The enormous genetic heterogeneity among the diseases that constitute RP is a problem for the development of treatments that deal with primary genetic defects; any such treatments will apply to a very small fraction of patients with RP. If, regardless of the genetic defect that leads to rod-cell death, the same treatment can be used to prolong cone survival and function, the impact will be enormous. As long as cones survive, useful vision is possible and, although it would be ideal to salvage rods as well, it is not necessary, because patients can carry on relatively normal lives with only cone function. Treatments involving neurotrophic factors also have broad applicability and are important to pursue. It is likely that benefits will be additive or synergistic by reducing oxidative damage to cones while simultaneously increasing the threshold for apoptosis with neurotrophic factors.
Although this study validates oxidative damage as a therapeutic target in RP, much more is needed to translate this finding into a useful treatment for patients. Damage occurs rapidly and locally after reactive oxygen species are generated, and antioxidants must be present at the right time, correct cellular compartment, and appropriate levels to prevent the damage. Whether intermittent administration of exogenous antioxidants can have a significant impact in patients with RP is uncertain. Novel delivery approaches that incorporate sustained release may be useful. Combining this approach with other strategies designed to bolster components of the endogenous defense system directed against oxidative stress, as well as enhancing levels of survival factors, may be needed to provide meaningful benefit to patients with RP.
Materials and Methods
Injections with Antioxidants.
Mice were treated in accordance with the recommendations of the Association for Research in Vision and Ophthalmology. Litters of homozygous rd1/rd1 mice in a C57BL/6 background were given daily injections of a mixture of antioxidants (25–30) including α-tocopherol (200 mg/kg in olive oil), ascorbic acid (250 mg/kg in PBS), and α-lipoic acid (100 mg/kg in PBS/30% ethanol). All three were obtained from Sigma Aldrich (St. Louis, MO). A metalloporphyrin superoxide dismutase mimetic that protects against intracellular generation of reactive oxygen species, MnTBAP (refs. 33 and 34; 10 mg/kg in PBS; AG Scientific, San Diego, CA), was also injected.
Measurement of Cone Cell Density.
Wild-type C57BL/6 mice and those homozygous for the rd1 mutation were killed at several times after birth and, after removal of the cornea, iris, and lens, a small cut was made at 12:00 in the retina for future orientation. Eyecups were fixed in 4% paraformaldehyde for 1–2 h, and then the entire retina was carefully dissected away from the RPE, severed at the optic nerve, and removed from the eye. Retinas were placed in 10% normal goat serum in PBS for 30 min at room temperature (RT), incubated for 1 h at RT in 1:30 rhodamine-conjugated PNA (Vector Laboratories, Burlingame, CA) in PBS containing 1% normal goat serum, and flat-mounted with the photoreceptors facing upward. The retinas were examined with a Zeiss LSM 510 META confocal microscope (Carl Zeiss, Oberkochen, Germany) with a Zeiss Plan-Apochromat 20×/0.75-N.A. objective using an excitation wavelength of 543 nm to detect rhodamine fluorescence. Retinas were examined by oil immersion with a 63×/1.4-N.A. Zeiss Plan-Apochromat objective to evaluate cone morphology in detail. Images were acquired in the frame-scan mode. The number of cones present within four 230 × 230-μm (512 × 512 pixels) squares located 1 mm superior, temporal, inferior, and nasal to the center of the optic nerve was determined. For some eyes, retinal sections were cut, stained with PNA and Hoechst, and examined with the confocal microscope using excitation wavelengths of 543 nm (PNA) or 405 nm (Hoechst).
Real-Time RT-PCR.
In some experiments, one eye was used for measurement of cone density, and the fellow eye was used to measure levels of m- or s-cone opsin mRNA by real-time RT-PCR as described (35) using primers for m-cone opsin (forward: 5′-TCA TTT CCT GGG AGA GAT GG-3′ and reverse: 5′-AGG CCA TAA GGC CAG TAC CT-3′) or s-cone opsin (forward: 5′-GCC TCA GTA CCA CCT TGC TC-3′ and reverse: 5′-CTG GCG ATG AAG ACT GTG AA-3′). For normalization, cyclophilin was amplified (forward: 5′-CAG ACG CCA CTG TCG CTT T-3′ and reverse: 5′-TGT CTT TGG AAC TTT GTC TGC AA-3′).
ELISA for Carbonyl Protein Adducts.
Retinal homogenates in 10 mM Tris, pH 7.2/0.5% Triton X-100/50 mM NaCl/1 mM EDTA with a proteinase inhibitor mixture tablet (Roche) were centrifuged at 16,000 × g for 10 min at 4°C. Protein concentrations of supernatants were determined using the BCA protein assay kit (BioRad, Hercules, CA), adjusted to 4 mg/ml by dilution with TBS, and protein carbonyl content was determined by ELISA as described (22, 23).
Immunohistochemistry.
Ten-micrometer frozen sections of mouse eyes were dried at room temperature, and antigen retrieval and immunofluorescent staining were done as described (12) by using rabbit antiacrolein antibody (1:150; Cell Sciences, Canton, MA) or 1:30 rhodamine-conjugated PNA and 1:150 goat anti-rabbit IgG conjugated with FITC (Jackson ImmunoResearch, West Grove, PA). Sections were counterstained for 3 min at room temperature with Hoechst and viewed with a Zeiss LSM 510 META confocal microscope with a Zeiss Plan-Apochromat 20× objective lens. The excitation wavelength was set at 405 nm for Hoechst, 488 nm for FITC, and 543 nm for rhodamine. Images were acquired in the frame-scan mode, and stack size was 460.7 × 460.7 μm (512 × 512 pixels).
Recording of ERGs.
ERGs were recorded with an Espion ERG Diagnosys machine (Diagnosys, Littleton, MA) as described (24). Mice were adapted for 10 min to a background of white light at an intensity of 30 cd/m2, and then photopic ERGs were performed with a background intensity of 10 cd/m2. Mice were anesthetized with i.p. injection of Avertin (Aldrich, Milwaukee, WI) and topical administration of 0.5% proparacaine hydrochloride (Alcon Labs, Forth Worth, TX). Pupils were dilated with 1% tropicamide (Alcon Labs) and 2.5% phenylephrine (Bausch and Lomb, Tampa, FL). Mice were placed on a pad heated to 39°C, and platinum loop electrodes were placed on each cornea after application of Gonioscopic prism solution (Alcon Labs). A reference electrode was placed s.c. in the anterior scalp between the eyes, and a ground electrode was inserted into the tail. The head of the mouse was held in a standardized position in a ganzfeld bowl illuminator that ensured equal illumination of the eyes. Recordings for both eyes were made simultaneously with electrical impedance balanced. Sixty photopic measurements were taken at 1.40 log cd·s/m2, and the average value was recorded.
Supplementary Material
Acknowledgments
This work was supported by the Macula Vision Foundation, Dr. and Mrs. William Lake, and Mrs. Susan Meyers. P.A.C. is the George S. and Dolores Dore Eccles Professor of Ophthalmology.
Abbreviations
- RP
retinitis pigmentosa
- RPE
retinal pigmented epithelial/epithelium
- PNA
peanut agglutinin
- Pn
postnatal day n
- MnTBAP
Mn(III)tetrakis (4-benzoic acid) porphyrin
- m-cone
medium-wavelength cone
- s-cone
short-wavelength cone
- ERG
electroretinogram.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Li Z.-Y., Possin D. E., Milam A. H. Ophthalmology. 1995;102:805–816. doi: 10.1016/s0161-6420(95)30953-0. [DOI] [PubMed] [Google Scholar]
- 2.Ripps H. Exp. Eye Res. 2002;74:327–336. doi: 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- 3.Gupta N., Brown K. E., Milam A. H. Exp. Eye Res. 2003;76:463–471. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 4.Leveillard T., Mohand-Said S., Lorentz O., Hicks D., Fintz A.-C., Clerin E., Simonutti M., Forster V., Cavusoglu N., Chalmel F., et al. Nat. Genet. 2004;36:755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- 5.Yu D. Y., Cringle S. J., Su E. N., Yu P. K. Invest. Ophthalmol. Visual Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- 6.Yu D. Y., Cringle S. J., Valter K., Walsh N., Lee D., Stone J. Invest. Ophthalmol. Visual Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]
- 7.Noell W. K. Adv. Exp. Med. Biol. 1977;77:175–178. doi: 10.1007/978-1-4899-5010-9_14. [DOI] [PubMed] [Google Scholar]
- 8.Blanks J. C., Johnson L. V. J. Comp. Neurol. 1986;254:543–553. doi: 10.1002/cne.902540407. [DOI] [PubMed] [Google Scholar]
- 9.Yamada H., Yamada E., Hackett S. F., Ozaki H., Okamoto N., Campochiaro P. A. J. Cell. Physiol. 1999;179:149–156. doi: 10.1002/(SICI)1097-4652(199905)179:2<149::AID-JCP5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Yamada H., Yamada E., Ando A., Esumi N., Bora N., Saikia J., Sung C.-H., Zack D. J., Campochiaro P. A. J. Am. Pathol. 2001;159:1113–1120. doi: 10.1016/S0002-9440(10)61787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noell W. K. Fed. Proc.; 1955. pp. 107–108. [Google Scholar]
- 12.Shen J., Yan X., Dong A., Petters R. M., Peng Y.-W., Wong F., Campochiaro P. A. J. Cell. Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 13.Carter-Dawson L. D., LaVail M. M., Sidman R. L. Invest. Ophthalmol. Visual Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- 14.LaVail M. M., Matthes M. T., Yasumura D., Steinberg R. Exp. Eye Res. 1997;65:45–50. doi: 10.1006/exer.1997.0308. [DOI] [PubMed] [Google Scholar]
- 15.Benedetti A., Comporti M., Esterbauer H. Biochim. Biophys. Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 16.Esterbauer H., Schaur R. J., Zollner H. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 17.Ando Y., Brannstrom T., Uchida K., Nyhlin N., Nasman B., Suhr O., Yamashita T., Olsson T., El Salhy M., Uchino M., Ando M. J. Neurol. Sci. 1998;156:172–176. doi: 10.1016/s0022-510x(98)00042-2. [DOI] [PubMed] [Google Scholar]
- 18.Calingasan N. Y., Uchida K., Gibson G. E. J. Neurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- 19.Uchida K., Kanematsu M., Sakai K., Matsuda T., Hattori N., Mizuno Y., Suzuki D., Miyata T., Noguchi N., Niki E. Proc. Natl. Acad. Sci. USA. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoritaka A., Hattori N., Uchida K., Tanaka M., Stadtman E. R., Mizuno Y. Proc. Natl. Acad. Sci. USA. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine R. L. Free Radic. Biol. Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- 22.Buss H., Chan T. P., Sluis K. B., Domigan N. M., Winterbourn C. C. Free Radic. Biol. Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 23.Lu L., Hackett S. F., Mincey A., Lai H., Campochiaro P. A. J. Cell. Physiol. 2006;206:119–125. doi: 10.1002/jcp.20439. [DOI] [PubMed] [Google Scholar]
- 24.Okoye G., Zimmer J., Sung J., P., G., Deering T., Nambu N., Hackett S. F., Melia M., Esumi N., Zack D. J., Campochiaro P. A. J. Neurosci. 2003;23:4164–4172. doi: 10.1523/JNEUROSCI.23-10-04164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao X., Phillis J. W. Free Radic. Biol. Med. 1995;23:365–370. doi: 10.3109/10715769509065257. [DOI] [PubMed] [Google Scholar]
- 26.Somani S. M., Husain K., Whitworth C., Trammell G. L., Malafa M., Rybak L. P. Pharmacol. Toxicol. 2000;86:234–241. doi: 10.1034/j.1600-0773.2000.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 27.Nespereira B., Perez-Ilzarbe M., Fernandez P., Fuentes A. M., Paramo J. A., Rodriguez J. A. Atherosclerosis. 2003;171:67–73. doi: 10.1016/j.atherosclerosis.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Kowluru R. A., Odenbach S. Diabetes. 2004;53:3233–3238. doi: 10.2337/diabetes.53.12.3233. [DOI] [PubMed] [Google Scholar]
- 29.Passage E., Norreel J. C., Noack-Fraissignes P., Sanguedolce V., Pizant J., Thirion X., Robaglia-Schlupp A., Pellissier J. F., Fontes M. Nat. Med. 2004;10:396–401. doi: 10.1038/nm1023. [DOI] [PubMed] [Google Scholar]
- 30.McDonald S. R., Sohal R. S., Forster M. J. Free Radic. Biol. Med. 2005;38:729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Stoyanovsky D. A., Goldman R., Darrow R. M., Organisciak D. T., Kagan V. E. Curr. Eye Res. 1995;14:181–189. doi: 10.3109/02713689509033513. [DOI] [PubMed] [Google Scholar]
- 32.Melov S., Schneider J. A., Day B. J., Hinerfeld D., Coskun P. E., Mirra S. S., Crapo J. D., Wallace D. C. Nat. Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- 33.Day B. J., Shawen S., Liochev S. I., Crapo J. D. J. Pharmacol. Exp. Ther. 1995;275:1227–1232. [PubMed] [Google Scholar]
- 34.Day B. J., Crapo J. D. Toxicol. Appl. Pharmacol. 1996;140:94–100. doi: 10.1006/taap.1996.0201. [DOI] [PubMed] [Google Scholar]
- 35.Shen J., Yang X., Xiao W. H., Hackett S. F., Sato Y., Campochiaro P. A. FASEB J. 2006;20:723–725. doi: 10.1096/fj.05-5046fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.